Safety and Efficacy of Salt Restriction Across the Spectrum of Heart Failure
Abstract
1. Introduction
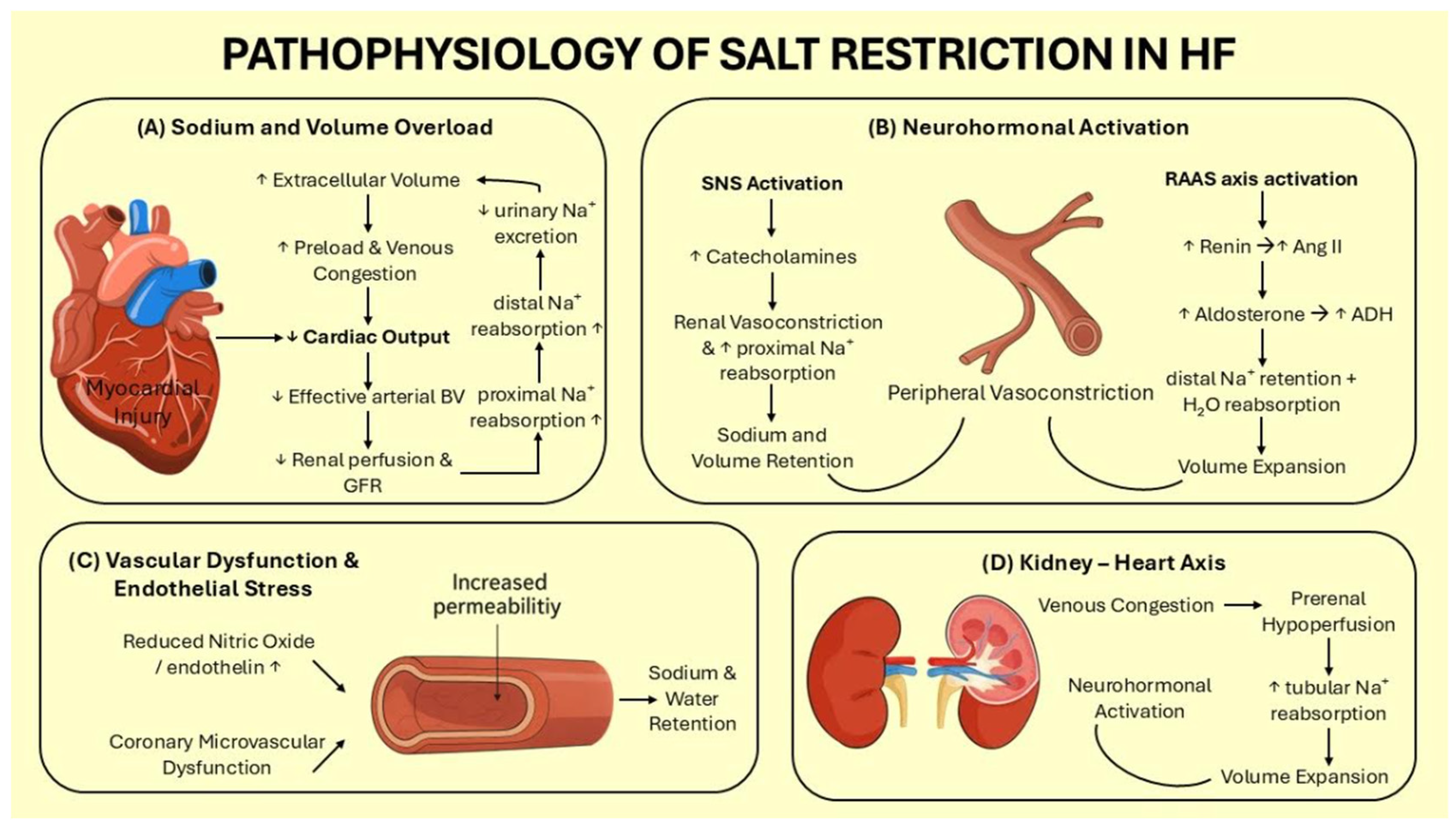
2. Pathophysiological Basis of Sodium Restriction in Heart Failure
2.1. Sodium and Volume Overload
2.2. Neurohormonal Activation
2.3. Vascular Dysfunction & Endothelial Stress
2.4. Kidney–Heart Axis
3. Historical Perspectives and Guideline Evolution
4. Clinical Evidence Across the Spectrum of Heart Failure
5. Risks and Controversies
5.1. Excessive Sodium Restriction Risks
5.2. Heterogeneity of Response
5.3. Challenges in Monitoring Sodium Intake
6. Public Health and Nutritional Considerations
6.1. Population-Wide Sodium Reduction
6.2. Caloric Adequacy, Protein Intake, and Micronutrient Needs
6.3. Importance of Dietary Counseling, Shared Decision-Making and Cardio-Nutrition Teams
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADH | Antidiuretic hormone |
| ADHF | Acute decompensated heart failure |
| ANP | Atrial natriuretic peptide |
| BNP | B-type natriuretic peptide |
| BP | Blood pressure |
| CKD | Chronic kidney disease |
| CO | Cardiac output |
| CVD | Cardiovascular disease |
| EF | Ejection fraction |
| ESC | European Society of Cardiology |
| NO | Nitric oxide |
| NYHA | New York Heart Association |
| HF | Heart failure |
| HFrEF | HF with reduced ejection fraction |
| HFpEF | HF with preserved ejection fraction |
| QoL | Quality of life |
| RAAS | Renin–angiotensin–aldosterone system |
| RCT | Randomized controlled trials |
| SDM | Shared decision-making |
| SGLT2 | Sodium glucose co-transporter 2 |
| SNS | Sympathetic nervous system |
References
- Takeda, A.; Martin, N.; Taylor, R.S.; Taylor, S.J. Disease Management Interventions for Heart Failure. Cochrane Database Syst. Rev. 2019, 2019, CD002752. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. SGLT-2 Inhibitors in Patients with Heart Failure: A Comprehensive Meta-Analysis of Five Randomised Controlled Trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef]
- Schwinger, R.H.G. Pathophysiology of Heart Failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.P.; Nagatomo, Y.; Kawai, F.; Kitai, T.; Mizuno, A. Fluid Restriction for Patients with Heart Failure: Current Evidence and Future Perspectives. J. Pers. Med. 2024, 14, 741. [Google Scholar] [CrossRef] [PubMed]
- Konerman, M.C.; Hummel, S.L. Sodium Restriction in Heart Failure: Benefit or Harm? Curr. Treat. Options Cardiovasc. Med. 2014, 16, 286. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Damman, K.; Dhont, S.; Banerjee, D.; Bayes-Genis, A.; Cannata, A.; Chioncel, O.; Cikes, M.; Ezekowitz, J.; Flammer, A.J.; et al. Dietary Sodium and Fluid Intake in Heart Failure. A Clinical Consensus Statement of the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2024, 26, 730–741. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. Correction to: 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Zabarovskaja, S.; Hage, C.; Linde, C.; Daubert, J.C.; Donal, E.; Gabrielsen, A.; Mellbin, L.; Lund, L.H. Adaptive cardiovascular hormones in a spectrum of heart failure phenotypes. Int. J. Cardiol. 2015, 189, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Stachteas, P.; Nasoufidou, A.; Patoulias, D.; Karakasis, P.; Karagiannidis, E.; Mourtzos, M.A.; Samaras, A.; Apostolidou, X.; Fragakis, N. The Role of Sodium-Glucose Co-Transporter-2 Inhibitors on Diuretic Resistance in Heart Failure. Int. J. Mol. Sci. 2024, 25, 3122. [Google Scholar] [CrossRef]
- de la Espriella, R.; Cobo, M.; Santas, E.; Verbrugge, F.H.; Fudim, M.; Girerd, N.; Miñana, G.; Górriz, J.L.; Bayés-Genís, A.; Núñez, J. Assessment of Filling Pressures and Fluid Overload in Heart Failure: An Updated Perspective. Rev. Esp. Cardiol. (Engl. Ed.) 2023, 76, 47–57. [Google Scholar] [CrossRef]
- Miller, W.L. Fluid Volume Overload and Congestion in Heart Failure. Circ. Heart Fail. 2016, 9, e002922. [Google Scholar] [CrossRef]
- Nijst, P.; Verbrugge, F.H.; Grieten, L.; Dupont, M.; Steels, P.; Tang, W.H.W.; Mullens, W. The Pathophysiological Role of Interstitial Sodium in Heart Failure. J. Am. Coll. Cardiol. 2015, 65, 378–388. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Mantzouranis, E.; Dimitroglou, Y.; Mavroudis, A.; Tsioufis, K. Fluid and Salt Balance and the Role of Nutrition in Heart Failure. Nutrients 2022, 14, 1386. [Google Scholar] [CrossRef] [PubMed]
- Triebel, H.; Castrop, H. The Renin Angiotensin Aldosterone System. Pflug. Arch. 2024, 476, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Guyton, A.C.; Cowley, A.W., Jr.; Young, D.B.; Coleman, T.G.; Hall, J.E.; Declue, J.W. Integration and Control of Circulatory Function. Int. Rev. Physiol. 1976, 9, 341–385. [Google Scholar] [PubMed]
- Young, D.B.; Pan, Y.; Guyton, A.C. Control of Extracellular Sodium Concentration by Antidiuretic Hormone-Thirst Feedback Mechanism. Am. J. Physiol. 1977, 232, R145–R149. [Google Scholar] [CrossRef]
- Hall, P.; Goldsmith, S.R. The Role of Vasopressin in Congestive Heart Failure. Cleve Clin. J. Med. 2006, 73, S19–S23. [Google Scholar] [CrossRef]
- Angermann, C.E.; Ertl, G. Natriuretic Peptides—New Diagnostic Markers in Heart Disease. Herz 2004, 29, 609–617. [Google Scholar] [CrossRef]
- Cadnapaphornchai, M.A.; Gurevich, A.K.; Weinberger, H.D.; Schrier, R.W. Pathophysiology of Sodium and Water Retention in Heart Failure. Cardiology 2001, 96, 122–131. [Google Scholar] [CrossRef]
- Drera, A.; Rodella, L.; Brangi, E.; Riccardi, M.; Vizzardi, E. Endothelial Dysfunction in Heart Failure: What Is Its Role? J. Clin. Med. 2024, 13, 2534. [Google Scholar] [CrossRef] [PubMed]
- Allbritton-King, J.D.; García-Cardeña, G. Endothelial Cell Dysfunction in Cardiac Disease: Driver or Consequence? Front. Cell Dev. Biol. 2023, 11, 1278166. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019, 139, E840–E878. [Google Scholar] [CrossRef] [PubMed]
- Thind, G.S.; Loehrke, M.; Wilt, J.L. Acute Cardiorenal Syndrome: Mechanisms and Clinical Implications. Cleve Clin. J. Med. 2018, 85, 231–239. [Google Scholar] [CrossRef]
- Duan, S.; Li, Y.; Yang, P. Predictive Value of Blood Urea Nitrogen in Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2023, 10, 1189884. [Google Scholar] [CrossRef]
- Hill, J.A.; Pauly, D.F.; Olitsky, D.R.; Russell, S.; O’Connor, C.M.; Patterson, B.; Elkayam, U.; Khan, S.; Stevenson, L.W.; Brooks, K.; et al. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness: The ESCAPE Trial. JAMA 2005, 294, 1625–1633. [Google Scholar] [CrossRef]
- Mullens, W.; Verbrugge, F.H.; Nijst, P.; Tang, W.H.W. Renal Sodium Avidity in Heart Failure: From Pathophysiology to Treatment Strategies. Eur. Heart J. 2017, 38, 1872–1882. [Google Scholar] [CrossRef]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef]
- Colin-Ramirez, E.; Arcand, J.; Saldarriaga, C.; Ezekowitz, J.A. The Current State of Evidence for Sodium and Fluid Restriction in Heart Failure. Prog. Cardiovasc. Dis. 2024, 82, 43–54. [Google Scholar] [CrossRef]
- Nolan, P.; McEvoy, J.W. Salt restriction for treatment of hypertension—Current state and future directions. Curr. Opin. Cardiol. 2024, 39, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P. Salt versus no salt restriction in heart failure a review. Eur. J. Clin. Investig. 2024, 54, e14265. [Google Scholar] [CrossRef] [PubMed]
- Skouri, H.; Girerd, N.; Monzo, L.; Petrie, M.C.; Böhm, M.; Adamo, M.; Mullens, W.; Savarese, G.; Yilmaz, M.B.; Amir, O.; et al. Clinical management and therapeutic optimization of patients with heart failure with reduced ejection fraction and low blood pressure. A clinical consensus statement of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2025, 27, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Wang, X.; Mone, P. Updated ACC/AHA/HFSA 2022 guidelines on heart failure: What is new? From epidemiology to clinical management. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, e23–e24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ezekowitz, J.A.; O’Meara, E.; McDonald, M.A.; Abrams, H.; Chan, M.; Ducharme, A.; Giannetti, N.; Grzeslo, A.; Hamilton, P.G.; Heckman, G.A.; et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can. J. Cardiol. 2017, 33, 1342–1433. [Google Scholar] [CrossRef] [PubMed]
- Sodium Reduction. Available online: https://www.who.int/news-room/fact-sheets/detail/sodium-reduction (accessed on 8 August 2025).
- Cody, R.J.; Covit, A.B.; Schaer, G.L.; Laragh, J.H.; Sealey, J.E.; Feldschuh, J. Sodium and Water Balance in Chronic Congestive Heart Failure. J. Clin. Investig. 1986, 77, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Kostis, J.B.; Rosen, R.C.; Cosgrove, N.M.; Shindler, D.M.; Wilson, A.C. Nonpharmacologic Therapy Improves Functional and Emotional Status in Congestive Heart Failure. Chest 1994, 106, 996–1001. [Google Scholar] [CrossRef]
- Colín Ramírez, E.; Castillo Martínez, L.; Orea Tejeda, A.; Rebollar González, V.; Narváez David, R.; Asensio Lafuente, E. Effects of a Nutritional Intervention on Body Composition, Clinical Status, and Quality of Life in Patients with Heart Failure. Nutrition 2004, 20, 890–895. [Google Scholar] [CrossRef]
- Alvelos, M.; Ferreira, A.; Bettencourt, P.; Serrão, P.; Pestana, M.; Cerqueira-Gomes, M.; Soares-da-Silva, P. The Effect of Dietary Sodium Restriction on Neurohumoral Activity and Renal Dopaminergic Response in Patients with Heart Failure. Eur. J. Heart Fail. 2004, 6, 593–599. [Google Scholar] [CrossRef]
- Damgaard, M.; Norsk, P.; Gustafsson, F.; Kanters, J.K.; Christensen, N.J.; Bie, P.; Friberg, L.; Gadsbøll, N. Hemodynamic and Neuroendocrine Responses to Changes in Sodium Intake in Compensated Heart Failure. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 290, R1294–R1301. [Google Scholar] [CrossRef]
- Nakasato, M.; Strunk, C.M.C.; Guimarães, G.; Rezende, M.V.C.; Bocchi, E.A. A Dieta Com Baixo Teor de Sódio é de Fato Indicada Para Todos Os Pacientes Com Insuficiência Cardíaca Estável? Arq. Bras. Cardiol. 2010, 94, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Philipson, H.; Ekman, I.; Swedberg, K.; Schaufelberger, M. A Pilot Study of Salt and Water Restriction in Patients with Chronic Heart Failure. Scand. Cardiovasc. J. 2010, 44, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Colin, R.E.; Castillo, M.L.; Orea, T.A.; Montaño, H.P.; Dorantes, G.J. Impacto de Una Dieta Con Restricción de Sodio Y Líquidos Sobre el Estado Clínico de Pacientes con Insuficiencia Cardiaca. Rev. Chil. Nutr. 2010, 37, 427–437. [Google Scholar] [CrossRef]
- Philipson, H.; Ekman, I.; Forslund, H.B.; Swedberg, K.; Schaufelberger, M. Salt and Fluid Restriction Is Effective in Patients with Chronic Heart Failure. Eur. J. Heart Fail. 2013, 15, 1304–1310. [Google Scholar] [CrossRef]
- Aliti, G.B.; Rabelo, E.R.; Clausell, N.; Rohde, L.E.; Biolo, A.; Beck-da-Silva, L. Aggressive Fluid and Sodium Restriction in Acute Decompensated Heart Failure. JAMA Intern. Med. 2013, 173, 1058. [Google Scholar] [CrossRef]
- Colin-Ramirez, E.; McAlister, F.A.; Zheng, Y.; Sharma, S.; Armstrong, P.W.; Ezekowitz, J.A. The Long-Term Effects of Dietary Sodium Restriction on Clinical Outcomes in Patients with Heart Failure. The SODIUM-HF (Study of Dietary Intervention Under 100 Mmol in Heart Failure): A Pilot Study. Am. Heart J. 2015, 169, 274–281.e1. [Google Scholar] [CrossRef]
- Machado d’Almeida, K.S.; Rabelo-Silva, E.R.; Souza, G.C.; Trojahn, M.M.; Santin Barilli, S.L.; Aliti, G.; Rohde, L.E.; Biolo, A.; Beck-da-Silva, L. Aggressive Fluid and Sodium Restriction in Decompensated Heart Failure with Preserved Ejection Fraction: Results from a Randomized Clinical Trial. Nutrition 2018, 54, 111–117. [Google Scholar] [CrossRef]
- Hummel, S.L.; Karmally, W.; Gillespie, B.W.; Helmke, S.; Teruya, S.; Wells, J.; Trumble, E.; Jimenez, O.; Marolt, C.; Wessler, J.D.; et al. Home-Delivered Meals Postdischarge from Heart Failure Hospitalization. Circ. Heart Fail. 2018, 11, e004886. [Google Scholar] [CrossRef]
- Fabricio, C.G.; Tanaka, D.M.; de Souza Gentil, J.R.; Ferreira Amato, C.A.; Marques, F.; Schwartzmann, P.V.; Schmidt, A.; Simões, M.V. A Normal Sodium Diet Preserves Serum Sodium Levels during Treatment of Acute Decompensated Heart Failure: A Prospective, Blind and Randomized Trial. Clin. Nutr. ESPEN 2019, 32, 145–152. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.; Papadimitriou, L.; Georgiopoulou, V.V.; Dunbar, S.B.; Skopicki, H.; Butler, J. Low- Versus Moderate-Sodium Diet in Patients with Recent Hospitalization for Heart Failure. Circ. Heart Fail. 2020, 13, e006389. [Google Scholar] [CrossRef]
- Ivey-Miranda, J.B.; Almeida-Gutierrez, E.; Herrera-Saucedo, R.; Posada-Martinez, E.L.; Chavez-Mendoza, A.; Mendoza-Zavala, G.H.; Cigarroa-Lopez, J.A.; Magaña-Serrano, J.A.; Rivera-Leaños, R.; Treviño-Mejia, A.; et al. Sodium Restriction in Patients with Chronic Heart Failure and Reduced Ejection Fraction: A Randomized Controlled Trial. Cardiol. J. 2023, 30, 411–421. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; Colin-Ramirez, E.; Ross, H.; Escobedo, J.; Macdonald, P.; Troughton, R.; Saldarriaga, C.; Alemayehu, W.; McAlister, F.A.; Arcand, J.; et al. Reduction of Dietary Sodium to Less than 100 Mmol in Heart Failure (SODIUM-HF): An International, Open-Label, Randomised, Controlled Trial. Lancet 2022, 399, 1391–1400. [Google Scholar] [CrossRef]
- Colin-Ramirez, E.; Sepehrvand, N.; Rathwell, S.; Ross, H.; Escobedo, J.; MacDonald, P.; Troughton, R.; Saldarriaga, C.; Lanas, F.; Doughty, R.; et al. Sodium Restriction in Patients with Heart Failure: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Circ. Heart Fail. 2023, 16, e009879. [Google Scholar] [CrossRef]
- Urban, S.; Fułek, M.; Błaziak, M.; Fułek, K.; Iwanek, G.; Jura, M.; Grzesiak, M.; Szymański, O.; Stańczykiewicz, B.; Ptaszkowski, K.; et al. Role of Dietary Sodium Restriction in Chronic Heart Failure: Systematic Review and Meta-Analysis. Clin. Res. Cardiol. 2024, 113, 1331–1342. [Google Scholar] [CrossRef]
- Mai, H.J.; Kanaan, G.; Erqou, S.; Salvador, V.; Joseph, J.; Wu, W.C.; Rudolph, J.; Caputo, E.L.; Rickard, T.; Rieke, K.; et al. Prescribed In-Hospital Sodium Intake for Decompensated Heart Failure: A Systematic Review and Meta-Analysis. J. Hosp. Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Soufer, A.; Goljo, E.; Colna, M.; Rao, V.S.; Jeon, S.; Raghavendra, P.; D’Ambrosi, J.; Riello, R.; Coca, S.G.; et al. Real World Use of Hypertonic Saline in Refractory Acute Decompensated Heart Failure: A U.S. Center’s Experience. JACC Heart Fail. 2020, 8, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T. We Need to Stop Telling Heart Failure Patients to Restrict Their Salt Intake. Heart Fail. 2025, 13, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Paterna, S.; Gaspare, P.; Fasullo, S.; Sarullo, F.M.; Di Pasquale, P. Normal-Sodium Diet Compared with Low-Sodium Diet in Compensated Congestive Heart Failure: Is Sodium an Old Enemy or a New Friend? Clin. Sci. 2008, 114, 221–230. [Google Scholar] [CrossRef]
- Paterna, S.; Parrinello, G.; Cannizzaro, S.; Fasullo, S.; Torres, D.; Sarullo, F.M.; Di Pasquale, P. Medium Term Effects of Different Dosage of Diuretic, Sodium, and Fluid Administration on Neurohormonal and Clinical Outcome in Patients with Recently Compensated Heart Failure. Am. J. Cardiol. 2009, 103, 93–102. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Chatterjee, S.; O’Keefe, J.H. Dietary Salt Restriction in Heart Failure: Where Is the Evidence? Prog. Cardiovasc. Dis. 2016, 58, 401–406. [Google Scholar] [CrossRef]
- Kutz, A.; Ebrahimi, F.; Aghlmandi, S.; Wagner, U.; Bromley, M.; Illigens, B.; Siepmann, T.; Schuetz, P.; Mueller, B.; Christ-Crain, M. Risk of Adverse Clinical Outcomes in Hyponatremic Adult Patients Hospitalized for Acute Medical Conditions: A Population-Based Cohort Study. J. Clin. Endocrinol. Metab. 2020, 105, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Georgiopoulou, V.V.; Kalogeropoulos, A.P.; Dunbar, S.B.; Reilly, C.M.; Sands, J.M.; Fonarow, G.C.; Jessup, M.; Gheorghiade, M.; Yancy, C.; et al. Dietary Sodium Intake in Heart Failure. Circulation 2012, 126, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Miyajima, I.; Suzuki, N.; Greenberg, B.H.; Akashi, Y.J. Nutritional Management of Heart Failure. J. Cardiol. 2023, 81, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Irlik, K.; Hendel, M.; Piaśnik, J.; Lip, G.Y.H.; Nabrdalik, K. Prognostic Impact and Prevalence of Cachexia in Patients with Heart Failure: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 2536–2543. [Google Scholar] [CrossRef]
- Lang, F.; Busch, G.L.; Ritter, M.; Völkl, H.; Waldegger, S.; Gulbins, E.; Häussinger, D. Functional Significance of Cell Volume Regulatory Mechanisms. Physiol. Rev. 1998, 78, 247–306. [Google Scholar] [CrossRef]
- Zampieri, F.G.; Rathwell, S.; Mcalister, F.A.; Ross, H.; Escobedo, J.; Saldarriaga, C.; Colin-Ramirez, E.; Troughton, R.W.; Macdonald, P.; Alemayehu, W.; et al. Heterogeneity in Treatment Effects in the Reduction of Dietary Sodium to Less Than 100 Mmol in Heart Failure (SODIUM-HF): A Secondary Post Hoc Analysis. J. Card. Fail. 2025, S1071-9164(25)00102-2. [Google Scholar] [CrossRef]
- Balafa, O.; Kalaitzidis, R.G. Salt Sensitivity and Hypertension. J. Hum. Hypertens. 2021, 35, 184–192. [Google Scholar] [CrossRef]
- Bailey, M.A.; Dhaun, N. Salt Sensitivity: Causes, Consequences, and Recent Advances. Hypertension 2024, 81, 476–489. [Google Scholar] [CrossRef]
- Wang, J.; Olendzki, B.C.; Wedick, N.M.; Persuitte, G.M.; Culver, A.L.; Li, W.; Merriam, P.A.; Carmody, J.; Fang, H.; Zhang, Z.; et al. Challenges in Sodium Intake Reduction and Meal Consumption Patterns among Participants with Metabolic Syndrome in a Dietary Trial. Nutr. J. 2013, 12, 163. [Google Scholar] [CrossRef]
- Champagne, C.M.; Lastor, K.C. Sodium Intake: Challenges for Researchers Attempting to Assess Consumption Relative to Health Risks. J. Food Compos. Anal. 2009, 22, S19–S22. [Google Scholar] [CrossRef]
- Schutte, A.E.; Neal, B. The Sodium Hidden in Medication: A Tough Pill to Swallow. Eur. Heart J. 2022, 43, 1756–1758. [Google Scholar] [CrossRef]
- WHO. Launch of the WHO Guideline on the Use of Lower-Sodium Salt Substitutes. Available online: https://www.who.int/news-room/events/detail/2025/01/27/default-calendar/launch-of-the-who-guideline-on-the-use-of-lower-sodium-salt-substitutes (accessed on 8 July 2025).
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium Intake and Hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-G.; Li, W.; Wen, X.-X.; Li, Y.; Hu, J.-H.; Zhao, L.-C. Effects of Salt Substitutes on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2014, 100, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Vernooij, R.W.M.; Zhu, H.; Nesrallah, G.; Bai, C.; Wang, Q.; Li, Y.; Xia, D.; Bała, M.M.; Warzecha, S.; et al. Impact of Sodium Intake on Blood Pressure, Mortality and Major Cardiovascular Events: An Umbrella Review of Systematic Reviews and Meta-Analyses. Crit. Rev. Food Sci. Nutr. 2024, 65, 5903–5913. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Wu, Y.; Feng, X.; Zhang, R.; Zhang, Y.; Shi, J.; Zhang, J.; Tian, M.; Huang, L.; Li, Z.; et al. Effect of Salt Substitution on Cardiovascular Events and Death. N. Engl. J. Med. 2021, 385, 1067–1077. [Google Scholar] [CrossRef]
- Stein, C.; Helal, L.; Migliavaca, C.B.; Sangalli, C.N.; Colpani, V.; Raupp da Rosa, P.; Beck-da-Silva, L.; Rohde, L.E.; Polanczyk, C.A.; Falavigna, M. Are the Recommendation of Sodium and Fluid Restriction in Heart Failure Patients Changing over the Past Years? A Systematic Review and Meta-Analysis. Clin. Nutr. ESPEN 2022, 49, 129–137. [Google Scholar] [CrossRef]
- Hsu, S.-M.; Lin, Y.-H.; Lin, Y.-C.; Liu, S.-J.; Liu, C.-J.; Hung, C.-L.; Wang, T.-J. Fluid Intake Impact on Heart Failure: Systematic Review and Meta-Analysis with Trial Sequential Analysis. J. Formos. Med. Assoc. 2025, 124, 650–659. [Google Scholar] [CrossRef]
- Lennie, T.A.; Song, E.K.; Wu, J.-R.; Chung, M.L.; Dunbar, S.B.; Pressler, S.J.; Moser, D.K. Three Gram Sodium Intake Is Associated with Longer Event-Free Survival Only in Patients with Advanced Heart Failure. J. Card. Fail. 2011, 17, 325–330. [Google Scholar] [CrossRef]
- de Jorge-Huerta, L.; Marco-Alacid, C.; Grande, C.; Velardo Andrés, C. A Narrative Review of the Diagnosis and Treatment of Sarcopenia and Malnutrition in Patients with Heart Failure. Nutrients 2024, 16, 2717. [Google Scholar] [CrossRef]
- Belqaid, K.; Irving, G.F.; Waldréus, N. Nutritional Interventions in Older, Frail Persons with Heart Failure-A Systematic Narrative Review. JAR Life 2024, 13, 99–107. [Google Scholar] [CrossRef]
- Grossniklaus, D.A.; O’Brien, M.C.; Clark, P.C.; Dunbar, S.B. Nutrient Intake in Heart Failure Patients. J. Cardiovasc. Nurs. 2008, 23, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, M.B.; Sivalingam, S.K. The New Heart Failure Diet: Less Salt Restriction, More Micronutrients. J. Gen. Intern. Med. 2010, 25, 1136–1137. [Google Scholar] [CrossRef] [PubMed]
- Verdu-Rotellar, J.-M.; Calero, E.; Duran, J.; Navas, E.; Alonso, S.; Argemí, N.; Casademunt, M.; Furió, P.; Casajuana, E.; Vinyoles, E.; et al. Impact of Malnutrition on the Quality of Life in Older Patients with Advanced Heart Failure: A Cohort Study. Rev. Clin. Esp. 2024, 224, 105–113. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.A.; Evans, C.V.; Rushkin, M.C.; Redmond, N.; Lin, J.S. Behavioral Counseling to Promote a Healthy Diet and Physical Activity for Cardiovascular Disease Prevention in Adults with Cardiovascular Risk Factors. JAMA 2020, 324, 2076. [Google Scholar] [CrossRef]
- Low, J.H.M.; Toh, D.W.K.; Ng, M.T.T.; Fam, J.; Kua, E.H.; Kim, J.E. A Systematic Review and Meta-Analysis of the Impact of Different Intensity of Dietary Counselling on Cardiometabolic Health in Middle-Aged and Older Adults. Nutrients 2021, 13, 2936. [Google Scholar] [CrossRef]
- Elias, S.; Chen, Y.; Liu, X.; Slone, S.; Turkson-Ocran, R.-A.; Ogungbe, B.; Thomas, S.; Byiringiro, S.; Koirala, B.; Asano, R.; et al. Shared Decision-Making in Cardiovascular Risk Factor Management. JAMA Netw. Open 2024, 7, e243779. [Google Scholar] [CrossRef]
- Dennison Himmelfarb, C.R.; Beckie, T.M.; Allen, L.A.; Commodore-Mensah, Y.; Davidson, P.M.; Lin, G.; Lutz, B.; Spatz, E.S. Shared Decision-Making and Cardiovascular Health: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 912–931. [Google Scholar] [CrossRef]
- Osoro, L.; Zylla, M.M.; Braunschweig, F.; Leyva, F.; Figueras, J.; Pürerfellner, H.; Merino, J.L.; Casado-Arroyo, R.; Boriani, G. Challenging the Status Quo: A Scoping Review of Value-Based Care Models in Cardiology and Electrophysiology. Europace 2024, 26, euae210. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J. Evidence-Based Practice of Nutrition Management in Chronic Heart Failure Patients Based on Knowledge-to-Action Transformation Model. Open J. Appl. Sci. 2025, 15, 400–410. [Google Scholar] [CrossRef]
- Sokos, G.; Kido, K.; Panjrath, G.; Benton, E.; Page, R.; Patel, J.; Smith, P.J.; Korous, S.; Guglin, M. Multidisciplinary Care in Heart Failure Services. J. Card. Fail. 2023, 29, 943–958. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, S.; Lim, Y.; Ohn, J.H.; Kim, S.-W.; Cho, J.H.; Park, H.S.; Lee, J.; Kim, E.S.; Kim, N.-H.; et al. Sodium Intake Estimation in Hospital Patients Using AI-Based Imaging: Prospective Pilot Study. JMIR Form. Res. 2024, 8, e48690. [Google Scholar] [CrossRef]
- Macrì, R.; Mollace, R.; Serra, M.; Scarano, F.; Ritorto, G.; Ussia, S.; Cardamone, A.; Coppoletta, A.R.; Carresi, C.; Gliozzi, M.; et al. Nutritional and Nutraceutical Support to the Failing Myocardium: A Possible Way of Potentiating the Current Treatment of Heart Failure. Int. J. Mol. Sci. 2024, 25, 12232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arcand, J.A.L.; Brazel, S.; Joliffe, C.; Choleva, M.; Berkoff, F.; Allard, J.P.; Newton, G.E. Education by a Dietitian in Patients with Heart Failure Results in Improved Adherence with a Sodium-Restricted Diet: A Randomized Trial. Am. Heart J. 2005, 150, 716. [Google Scholar] [CrossRef]
- Kuehneman, T.; Saulsbury, D.; Splett, P.; Chapman, D.B. Demonstrating the Impact of Nutrition Intervention in a Heart Failure Program. J. Am. Diet. Assoc. 2002, 102, 1790–1794. [Google Scholar] [CrossRef]
- Compher, C.; Henstenburg, J.A.; Aloupis, M.; Sun, A.; Quinn, R.; Emery, E.; Thomas, J.; Crafford, A.G.; Schwartz, D.R. The Nutritional Impact of 7 versus 21 Home-Delivered Medically Tailored Meals in Patients with Heart Failure and Malnutrition Risk: A Random Order Crossover Feeding Trial (MEDIMEALS). BMC Nutr. 2025, 11, 56. [Google Scholar] [CrossRef]
| Study ID | Population/Method of Sodium Assessment | Setting/ HF Type | Sample Size | Intervention/ (Duration) | Comparator | Outcome (MACE) |
|---|---|---|---|---|---|---|
| Cody et al., 1986 [37] | Hospitalized patients with moderate to severe chronic HF/24 h urinary sodium excretion | Inpatient/ HFrEF | 10 | Very low sodium restriction (230 mg/d)/ (14 days) | Low sodium intake (2300 mg/d) | NA |
| Kostis et al., 1994 [38] | Patients with chronic HF and NYHA II–III/Dietary recall and counseling logs | Outpatient/ HFrEF | 13 | Sodium restriction (1200 mg/d)/ (84 days) | Routine dietary advisories | Improvement in QoL and mood indices |
| Colin-Ramirez et al., 2004 [39] | Patients with HF based on reduced systolic and diastolic function in echo/3-day food records analyzed by dietitian | Outpatient/ HFrEF | 65 | Sodium restriction at 2000–2400 mg/d/ (180 days) | Routine dietary advisories | NS difference in NYHA class, significant improvement in QoL |
| Alvelos et al., 2004 [40] | Patients with mild to moderate chronic HF/24 h urinary sodium excretion | Outpatient/ HFrEF | 24 | Sodium restriction (2300 mg/d)/ (15 days) | Diet with usual salt intake | NS difference in NYHA class |
| Damgaard et al., 2006 [41] | Male patients with ADHF/24 h urinary sodium excretion | Outpatient/ HFrEF | 12 | Sodium restriction (1610 mg/d)/ (7 days) | High sodium intake (5750 mg/d) | NA |
| Nakasato et al., 2010 [42] | Patients with chronic HF and NYHA II–III/Dietitian-recorded menus with compliance interviews | Outpatient/ HFrEF | 50 | Very low sodium restriction (800 mg/d)/ (7 days) | Low sodium intake (2400 mg/d) | Improvement in QoL |
| Philipson et al., 2010 [43] | Patients with chronic HF and NYHA II–IV and signs of fluid retention/3 consecutive 24 h urine collections | Outpatient/ HFrEF or HFpEF | 30 | Sodium restriction at 2000–3000 mg/d and fluid restriction/ (84 days) | Routine dietary advisories | NS difference in QoL |
| Colin-Ramirez et al., 2010 [44] | Patients with HF based on reduced systolic and diastolic function in echo/3-day food records; subset urinary sodium validation | Outpatient/ HFrEF or HFpEF | 203 | Sodium restriction at 2000–2400 mg/d and fluid restriction/ (1 year) | Routine dietary advisories | Significant reduction in CV hospitalization |
| Philipson et al., 2013 [45] | Patients with chronic HF and NYHA II–IV and signs of fluid retention and maximal tolerated doses of GDMT/Dietary records and repeated spot urinary sodium | Outpatient/ HFrEF or HFpEF | 97 | Sodium restriction at 2000–3000 mg/d and fluid restriction/ (84 days) | Routine dietary advisories | NS difference in mortality, NYHA class or QoL |
| Aliti et al., 2013 [46] | Hospitalized patients with ADHF and LVEF < 45%/Controlled inpatient meal sodium records | Inpatient/ HFrEF | 75 | Very low sodium restriction at 800 mg/d and fluid restriction/ (7 days) | Diet with usual salt and fluid intake | 30-day readmission rate: NS |
| Colin-Ramirez et al., 2015 [47] | Patients with chronic HF and NYHA II–III and receiving GDMT/3-day food records with counseling verification | Outpatient/ HFrEF or HFpEF | 38 | Sodium restriction at 1500 mg/d/ (180 days) | Low sodium intake (2300 mg/d) | NS difference in mortality, NYHA class or QoL |
| Machado d’ Almeida et al., 2018 [48] | Hospitalized patients with ADHF and LVEF > 50%/Hospital nutrition records and sodium content of meals | Inpatient/ HFpEF | 53 | Very low sodium restriction at 800 mg/d and fluid restriction/ (7 days) | Standard hospital diet: 4000 mg/d sodium and unlimited fluid intake | 30-day mortality or readmission rate: NS |
| Hummel et al. 2018 [49] | Patients ≥ 65 y with history of hypertension, discharged from hospital with ADHF/3-day food records analyzed using standardized software | Outpatient/ HFrEF or HFpEF | 66 | Sodium-restricted DASH diet with 1500 mg/d sodium/ (30 days) | Routine dietary advisories + phone calls | 30-day mortality or readmission rate: NS |
| Fabricio et al., 2019 [50] | Patients hospitalized with ADHF/Standardized hospital diet and 24 h urinary sodium | Inpatient/ HFrEF or HFpEF | 44 | Sodium restriction at 1200 mg/d and fluid restriction/ (7 days) | Normal-sodium diet (2800 mg/d) | 30-day readmission rate: NS |
| Kalogeropoulos et al., 2020 [51] | Patients recently hospitalized for HF, on optimal GDMT, SBP ≥ 100 mm Hg, consuming > 3000 mg Na/day/24 h urinary sodium | Outpatient/ HFrEF | 27 | Sodium restriction at 1500 mg/d/ (84 days) | Normal-sodium diet (3000 mg/d) | 30-day readmission rate: NS |
| Ivey-Miranda et al., 2021 [52] | Patients with chronic, on optimal GDMT, SBP ≥ 90 mm Hg/24 h urinary sodium with dietary logs | Outpatient/ HFrEF | 70 | Sodium restriction at 2000 mg/d/ (140 days) | Normal-sodium diet (3000 mg/d) | 30-day mortality or readmission rate: NS |
| Ezekowitz et al., 2022 [53] SODIUM-HF trial | Patients with chronic HF and NYHA functional class II or III/3-day food diaries assessed by blinded dietitians | Inpatient or outpatient/ HFrEF or HFpEF | 806 | Sodium restriction at 1500 mg/d/ (1 year) | Usual care and routine dietary advisories | 30-day mortality or readmission rate: NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachteas, P.; Nasoufidou, A.; Koiliari, M.; Arampatzi, V.; Alexaki, C.; Kofos, C.; Karakasis, P.; Karagiannidis, E.; Koufakis, T.; Fragakis, N.; et al. Safety and Efficacy of Salt Restriction Across the Spectrum of Heart Failure. J. Cardiovasc. Dev. Dis. 2025, 12, 432. https://doi.org/10.3390/jcdd12110432
Stachteas P, Nasoufidou A, Koiliari M, Arampatzi V, Alexaki C, Kofos C, Karakasis P, Karagiannidis E, Koufakis T, Fragakis N, et al. Safety and Efficacy of Salt Restriction Across the Spectrum of Heart Failure. Journal of Cardiovascular Development and Disease. 2025; 12(11):432. https://doi.org/10.3390/jcdd12110432
Chicago/Turabian StyleStachteas, Panagiotis, Athina Nasoufidou, Markella Koiliari, Vasiliki Arampatzi, Chrysa Alexaki, Christos Kofos, Paschalis Karakasis, Efstratios Karagiannidis, Theocharis Koufakis, Nikolaos Fragakis, and et al. 2025. "Safety and Efficacy of Salt Restriction Across the Spectrum of Heart Failure" Journal of Cardiovascular Development and Disease 12, no. 11: 432. https://doi.org/10.3390/jcdd12110432
APA StyleStachteas, P., Nasoufidou, A., Koiliari, M., Arampatzi, V., Alexaki, C., Kofos, C., Karakasis, P., Karagiannidis, E., Koufakis, T., Fragakis, N., & Patoulias, D. (2025). Safety and Efficacy of Salt Restriction Across the Spectrum of Heart Failure. Journal of Cardiovascular Development and Disease, 12(11), 432. https://doi.org/10.3390/jcdd12110432







