Antithrombotic Therapy in Transcatheter Aortic Valve Implantation: Focus on Gender Differences
Abstract
1. Introduction
2. Rationale for Antithrombotic Therapy
3. Bleeding Risk Assessment
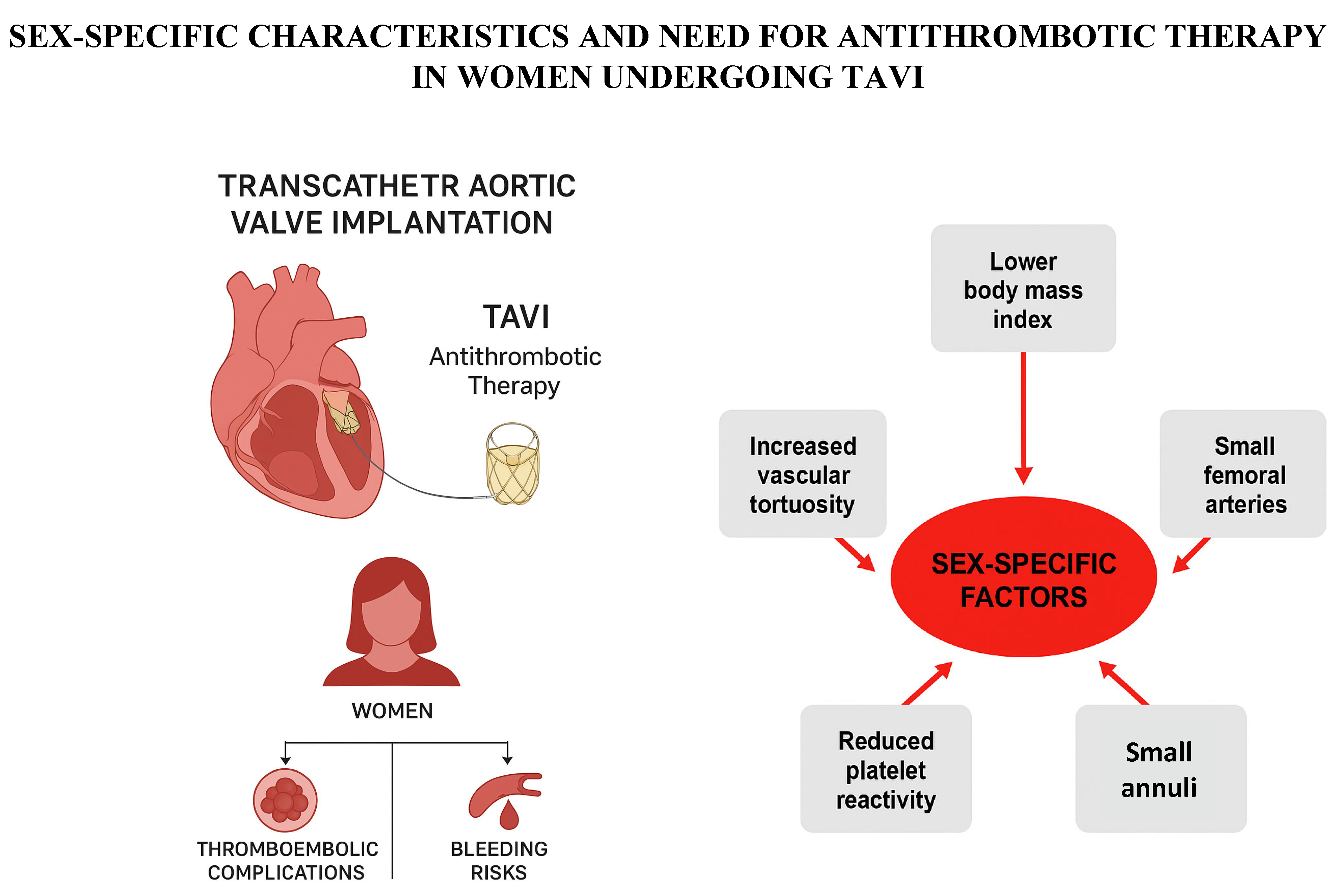
4. Sex-Specific Outcomes in Women Undergoing TAVI
5. Current Guideline Recommendations
6. Emerging Evidence from Randomized Clinical Trials
6.1. Antiplatelet Therapy
6.2. Anticoagulant Therapy
6.2.1. Patients Without a Prior Indication for OAC
6.2.2. Patients with a Prior Indication for OAC
6.3. Antiplatelet, Anticoagulation, or Antithrombotic?
7. Hormonal Influence and Sex-Related Variability
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, R.D.; Ellis, U.M.; Barry, A.R. Antithrombotic therapy in patients after transcatheter aortic valve implantation: A network meta-analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2024, 10, 454–464. [Google Scholar] [CrossRef]
- Chakravarty, T.; Leong, D.; de la Rosa, A.; Bhardwaj, N.; Makkar, R.R. Low-Intensity vs. High-Intensity Antithrombotic Therapy After Transcatheter Aortic Valve Replacement: Meta-Analysis of Randomized Controlled Trials. Struct. Heart 2023, 7, 100133. [Google Scholar] [CrossRef]
- Denegri, A.; Romano, M.; Petronio, A.S.; Angelillis, M.; Giannini, C.; Fiorina, C.; Branca, L.; Barbanti, M.; Costa, G.; Brambilla, N.; et al. Gender Differences after Transcatheter Aortic Valve Replacement (TAVR): Insights from the Italian Clinical Service Project. J. Cardiovasc. Dev. Dis. 2021, 8, 114. [Google Scholar] [CrossRef]
- Rotman, O.M.; Bianchi, M.; Ghosh, R.P.; Kovarovic, B.; Bluestein, D. Principles of TAVR valve design, modelling, and testing. Expert. Rev. Med. Devices 2018, 15, 771–791. [Google Scholar] [CrossRef]
- Guedeney, P.; Chieffo, A.; Snyder, C.; Mehilli, J.; Petronio, A.S.; Claessen, B.E.; Sartori, S.; Lefèvre, T.; Presbitero, P.; Capranzano, P.; et al. WIN-TAVI Investigators. Impact of Baseline Atrial Fibrillation on Outcomes Among Women Who Underwent Contemporary Transcatheter Aortic Valve Implantation (from the Win-TAVI Registry). Am. J. Cardiol. 2018, 122, 1909–1916. [Google Scholar] [CrossRef]
- Faroux, L.; Munoz-Garcia, E.; Serra, V.; Alperi, A.; Nombela-Franco, L.; Fischer, Q.; Veiga, G.; Donaint, P.; Asmarats, L.; Vilalta, V.; et al. Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2020, 13, e008620. [Google Scholar] [CrossRef]
- Jochheim, D.; Zadrozny, M.; Ricard, I.; Sadry, T.M.; Theiss, H.; Baquet, M.; Schwarz, F.; Bauer, A.; Khandoga, A.; Sadoni, S.; et al. Predictors of cerebrovascular events at mid-term after transcatheter aortic valve implantation—Results from EVERY-TAVI registry. Int. J. Cardiol. 2017, 244, 106–111. [Google Scholar] [CrossRef]
- Puri, R.; Auffret, V.; Rodés-Cabau, J. Bioprosthetic Valve Thrombosis. J. Am. Coll. Cardiol. 2017, 69, 2193–2211. [Google Scholar] [CrossRef]
- Sharma, A.; Lavie, C.J.; Elmariah, S.; Borer, J.S.; Sharma, S.K.; Vemulapalli, S.; Yerokun, B.A.; Li, Z.; Matsouaka, R.A.; Marmur, J.D. Relationship of Body Mass Index with Outcomes After Transcatheter Aortic Valve Replacement: Results From the National Cardiovascular Data-STS/ACC TVT Registry. Mayo Clin. Proc. 2020, 95, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Nuis, R.J.; Sinning, J.M.; Rodés-Cabau, J.; Gotzmann, M.; van Garsse, L.; Kefer, J.; Bosmans, J.; Yong, G.; Dager, A.E.; Revilla-Orodea, A.; et al. Prevalence, factors associated with, and prognostic effects of preoperative anemia on short- and long-term mortality in patients undergoing transcatheter aortic valve implantation. Circ. Cardiovasc. Interv. 2013, 6, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Allende, R.; Webb, J.G.; Munoz-Garcia, A.J.; de Jaegere, P.; Tamburino, C.; Dager, A.E.; Cheema, A.; Serra, V.; Amat-Santos, I.; Velianou, J.L.; et al. Advanced chronic kidney disease in patients undergoing transcatheter aortic valve implantation: Insights on clinical outcomes and prognostic markers from a large cohort of patients. Eur. Heart J. 2014, 35, 2685–2696. [Google Scholar] [CrossRef]
- Fuchs, A.; De Backer, O.; Brooks, M.; de Knegt, M.C.; Bieliauskas, G.; Yamamoto, M.; Yanagisawa, R.; Hayashida, K.; Søndergaard, L.; Kofoed, K.F. Subclinical leaflet thickening and stent frame geometry in self-expanding transcatheter heart valves. EuroIntervention 2017, 13, e1067–e1075. [Google Scholar] [CrossRef]
- Kopanidis, A.; Pantos, I.; Alexopoulos, N.; Theodorakakos, A.; Efstathopoulos, E.; Katritsis, D. Aortic Flow Patterns After Simulated Implantation of Transcatheter Aortic Valves. Hell. J. Cardiol. 2015, 56, 418–428. [Google Scholar] [PubMed]
- Vahidkhah, K.; Barakat, M.; Abbasi, M.; Javani, S.; Azadani, P.N.; Tandar, A.; Dvir, D.; Azadani, A.N. Valve thrombosis following transcatheter aortic valve replacement: Significance of blood stasis on the leaflets. Eur. J. Cardiothorac. Surg. 2017, 51, 927–935. [Google Scholar] [CrossRef]
- Marechaux, S.; Corseaux, D.; Vincentelli, A.; Richardson, M.; Ung, A.; Susen, S.; Zawadzki, C.; Beregi, J.P.; Ezekowitz, M.D.; Jude, B.; et al. Identification of tissue factor in experimental aortic valve sclerosis. Cardiovasc. Pathol. 2009, 18, 67–76. [Google Scholar] [CrossRef]
- Breyne, J.; Juthier, F.; Corseaux, D.; Marechaux, S.; Zawadzki, C.; Jeanpierre, E.; Ung, A.; Ennezat, P.V.; Susen, S.; Van Belle, E.; et al. Atherosclerotic-like process in aortic stenosis: Activation of the tissue factor-thrombin pathway and potential role through osteopontin alteration. Atherosclerosis 2010, 213, 369–376. [Google Scholar] [CrossRef]
- Overtchouk, P.; Guedeney, P.; Rouanet, S.; Verhoye, J.P.; Lefevre, T.; Van Belle, E.; Eltchaninoff, H.; Gilard, M.; Leprince, P.; Iung, B.; et al. Long-Term Mortality and Early Valve Dysfunction According to Anticoagulation Use: The FRANCE TAVI Registry. J. Am. Coll. Cardiol. 2019, 73, 13–21. [Google Scholar] [CrossRef]
- Jose, J.; Sulimov, D.S.; El-Mawardy, M.; Sato, T.; Allali, A.; Holy, E.W.; Becker, B.; Landt, M.; Kebernik, J.; Schwarz, B.; et al. Clinical Bioprosthetic Heart Valve Thrombosis After Transcatheter Aortic Valve Replacement: Incidence, Characteristics, and Treatment Outcomes. JACC Cardiovasc. Interv. 2017, 10, 686–697. [Google Scholar] [CrossRef]
- Yanagisawa, R.; Hayashida, K.; Yamada, Y.; Tanaka, M.; Yashima, F.; Inohara, T.; Arai, T.; Kawakami, T.; Maekawa, Y.; Tsuruta, H.; et al. Incidence, Predictors, and Mid-Term Outcomes of Possible Leaflet Thrombosis After TAVR. JACC Cardiovasc. Imaging 2016, 8, S1936-878X(16)30897-X. [Google Scholar] [CrossRef]
- Del Trigo, M.; Muñoz-Garcia, A.J.; Wijeysundera, H.C.; Nombela-Franco, L.; Cheema, A.N.; Gutierrez, E.; Serra, V.; Kefer, J.; Amat-Santos, I.J.; Benitez, L.M.; et al. Incidence, Timing, and Predictors of Valve Hemodynamic Deterioration After Transcatheter Aortic Valve Replacement: Multicenter Registry. J. Am. Coll. Cardiol. 2016, 67, 644–655. [Google Scholar] [CrossRef]
- Ducci, A.; Tzamtzis, S.; Mullen, M.J.; Burriesci, G. Hemodynamics in the Valsalva sinuses after transcatheter aortic valve implantation (TAVI). J. Heart Valve Dis. 2013, 22, 688–696. [Google Scholar] [PubMed]
- Van Mieghem, N.M.; El Faquir, N.; Rahhab, Z.; Rodríguez-Olivares, R.; Wilschut, J.; Ouhlous, M.; Galema, T.W.; Geleijnse, M.L.; Kappetein, A.P.; Schipper, M.E.; et al. Incidence and predictors of debris embolizing to the brain during transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 2015, 8, 718–724. [Google Scholar] [CrossRef] [PubMed]
- VARC-3 WRITING COMMITTEE; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H.; Chakravarty, T.; Kofoed, K.F.; De Backer, O.; Asch, F.M.; Ruiz, C.E.; Olsen, N.T.; Trento, A.; et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef]
- Dangas, G.; Nicolas, J. Anticoagulation and Subclinical Valve Thrombosis After TAVR. JACC Cardiovasc. Interv. 2022, 15, 1805–1807. [Google Scholar] [CrossRef]
- Mangieri, A.; Montalto, C.; Poletti, E.; Sticchi, A.; Crimi, G.; Giannini, F.; Latib, A.; Capodanno, D.; Colombo, A. Thrombotic Versus Bleeding Risk After Transcatheter Aortic Valve Replacement: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2088–2101. [Google Scholar] [CrossRef]
- Batur, P.; Stewart, W.J.; Isaacson, J.H. Increased prevalence of aortic stenosis in patients with arteriovenous malformations of the gastrointestinal tract in Heyde syndrome. Arch. Intern. Med. 2003, 163, 1821–1824. [Google Scholar] [CrossRef]
- Vincentelli, A.; Susen, S.; Le Tourneau, T.; Six, I.; Fabre, O.; Juthier, F.; Bauters, A.; Decoene, C.; Goudemand, J.; Prat, A.; et al. Acquired von Willebrand syndrome in aortic stenosis. N. Engl. J. Med. 2003, 349, 343–349. [Google Scholar] [CrossRef]
- O’Connor, S.A.; Morice, M.C.; Gilard, M.; Leon, M.B.; Webb, J.G.; Dvir, D.; Rodés-Cabau, J.; Tamburino, C.; Capodanno, D.; D’Ascenzo, F.; et al. Revisiting Sex Equality with Transcatheter Aortic Valve Replacement Outcomes: A Collaborative, Patient-Level Meta-Analysis of 11,310 Patients. J. Am. Coll. Cardiol. 2015, 66, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Gracia, E.; Callahan, S.; Bilfinger, T.; Tannous, H.; Pyo, R.; Kort, S.; Skopicki, H.; Weinstein, J.; Patel, N.; et al. Gender Disparities in Management and Outcomes Following Transcatheter Aortic Valve Implantation with Newer Generation Transcatheter Valves. Am. J. Cardiol. 2019, 123, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Vlastra, W.; Chandrasekhar, J.; García Del Blanco, B.; Tchétché, D.; de Brito, F.S., Jr.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A.; Ribichini, F.; et al. Sex Differences in Transfemoral Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 74, 2758–2767. [Google Scholar] [CrossRef]
- Piccolo, R.; Pilgrim, T.; Franzone, A.; Valgimigli, M.; Haynes, A.; Asami, M.; Lanz, J.; Räber, L.; Praz, F.; Langhammer, B.; et al. Frequency, Timing, and Impact of Access-Site and Non-Access-Site Bleeding on Mortality Among Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 1436–1446. [Google Scholar] [CrossRef]
- Naoum, C.; Blanke, P.; Dvir, D.; Pibarot, P.; Humphries, K.; Webb, J.; Leipsic, J. Clinical Outcomes and Imaging Findings in Women Undergoing TAVR. JACC Cardiovasc. Imaging 2016, 9, 483–493. [Google Scholar] [CrossRef]
- Patti, G.; De Caterina, R.; Abbate, R.; Andreotti, F.; Biasucci, L.M.; Calabrò, P.; Cioni, G.; Davì, G.; Di Sciascio, G.; Golia, E.; et al. Platelet function and long-term antiplatelet therapy in women: Is there a gender-specificity? A ‘state-of-the-art’ paper. Eur. Heart J. 2014, 35, 2213–2223b. [Google Scholar] [CrossRef]
- Tchetche, D.; Pibarot, P.; Bax, J.J.; Bonaros, N.; Windecker, S.; Dumonteil, N.; Nietlispach, F.; Messika-Zeitoun, D.; Pocock, S.J.; Berthoumieu, P.; et al. Transcatheter vs. surgical aortic valve replacement in women: The RHEIA trial. Eur. Heart J. 2025, 46, 2079–2088. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Zaman, A.; Prendergast, B.; Hildick-Smith, D.; Blackman, D.; Anderson, R.; Spence, M.S.; Mylotte, D.; Smith, D.; Wilding, B.; Chapman, C.; et al. An Update on Anti-thrombotic Therapy Following Transcatheter Aortic Valve Implantation: Expert Cardiologist Opinion from a UK and Ireland Delphi Group. Interv. Cardiol. 2023, 18, e13. [Google Scholar] [CrossRef]
- Ten Berg, J.; Sibbing, D.; Rocca, B.; Van Belle, E.; Chevalier, B.; Collet, J.P.; Dudek, D.; Gilard, M.; Gorog, D.A.; Grapsa, J.; et al. Management of antithrombotic therapy in patients undergoing transcatheter aortic valve implantation: A consensus document of the ESC Working Group on Thrombosis and the European Association of Percutaneous Cardiovascular Interventions (EAPCI), in collaboration with the ESC Council on Valvular Heart Disease. Eur. Heart J. 2021, 42, 2265–2269. [Google Scholar]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [PubMed]
- Rodés-Cabau, J.; Masson, J.B.; Welsh, R.C.; Garcia Del Blanco, B.; Pelletier, M.; Webb, J.G.; Al-Qoofi, F.; Généreux, P.; Maluenda, G.; Thoenes, M.; et al. Aspirin Versus Aspirin Plus Clopidogrel as Antithrombotic Treatment Following Transcatheter Aortic Valve Replacement with a Balloon-Expandable Valve: The ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) Randomized Clinical Trial. JACC Cardiovasc. Interv. 2017, 10, 1357–1365. [Google Scholar] [PubMed]
- Brouwer, J.; Nijenhuis, V.J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.F.; Frambach, P.; De Bruyne, B.; van Houwelingen, G.K.; Van Der Heyden, J.A.S.; et al. Aspirin with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2020, 383, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Kobari, Y.; Inohara, T.; Tsuruta, H.; Yashima, F.; Shimizu, H.; Fukuda, K.; Naganuma, T.; Mizutani, K.; Yamawaki, M.; Tada, N.; et al. No Antithrombotic Therapy After Transcatheter Aortic Valve Replacement: Insight From the OCEAN-TAVI Registry. JACC Cardiovasc. Interv. 2023, 16, 79–91. [Google Scholar] [CrossRef]
- Madukauwa-David, I.D.; Sadri, V.; Kamioka, N.; Midha, P.A.; Raghav, V.; Oshinski, J.N.; Sharma, R.; Babaliaros, V.; Yoganathan, A.P. Transcatheter aortic valve deployment influences neo-sinus thrombosis risk: An in vitro flow study. Catheter. Cardiovasc. Interv. 2020, 95, 1009–1016. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Salizzoni, S.; Saglietto, A.; Cortese, M.; Latib, A.; Franzone, A.; Barbanti, M.; Nietlispach, F.; Holy, E.W.; Burriesci, G.; et al. Incidence, predictors and cerebrovascular consequences of leaflet thrombosis after transcatheter aortic valve implantation: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2019, 56, 488–494. [Google Scholar] [CrossRef]
- Hansson, N.C.; Grove, E.L.; Andersen, H.R.; Leipsic, J.; Mathiassen, O.N.; Jensen, J.M.; Jensen, K.T.; Blanke, P.; Leetmaa, T.; Tang, M.; et al. Transcatheter Aortic Valve Thrombosis: Incidence, Predisposing Factors, and Clinical Implications. J. Am. Coll. Cardiol. 2016, 68, 2059–2069. [Google Scholar] [CrossRef]
- Rosseel, L.; De Backer, O.; Søndergaard, L. Clinical Valve Thrombosis and Subclinical Leaflet Thrombosis Following Transcatheter Aortic Valve Replacement: Is There a Need for a Patient-Tailored Antithrombotic Therapy? Front. Cardiovasc. Med. 2019, 6, 44. [Google Scholar] [CrossRef]
- Bogyi, M.; Schernthaner, R.E.; Loewe, C.; Gager, G.M.; Dizdarevic, A.M.; Kronberger, C.; Postula, M.; Legutko, J.; Velagapudi, P.; Hengstenberg, C.; et al. Subclinical Leaflet Thrombosis After Transcatheter Aortic Valve Replacement: A Meta-Analysis. JACC Cardiovasc. Interv. 2021, 14, 2643–2656. [Google Scholar] [PubMed]
- Chakravarty, T.; Søndergaard, L.; Friedman, J.; De Backer, O.; Berman, D.; Kofoed, K.F.; Jilaihawi, H.; Shiota, T.; Abramowitz, Y.; Jørgensen, T.H.; et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: An observational study. Lancet 2017, 389, 2383–2392. [Google Scholar] [CrossRef]
- Rashid, H.N.; Gooley, R.P.; Nerlekar, N.; Ihdayhid, A.R.; McCormick, L.M.; Nasis, A.; Cameron, J.D.; Brown, A.J. Bioprosthetic aortic valve leaflet thrombosis detected by multidetector computed tomography is associated with adverse cerebrovascular events: A meta-analysis of observational studies. EuroIntervention 2018, 13, e1748–e1755. [Google Scholar] [CrossRef]
- Pache, G.; Schoechlin, S.; Blanke, P.; Dorfs, S.; Jander, N.; Arepalli, C.D.; Gick, M.; Buettner, H.J.; Leipsic, J.; Langer, M.; et al. Early hypo-attenuated leaflet thickening in balloon-expandable transcatheter aortic heart valves. Eur. Heart J. 2016, 37, 2263–2271. [Google Scholar] [CrossRef]
- Giustino, G.; Tijssen, J.; Windecker, S.; Dangas, G. Rivaroxaban after transcatheter aortic valve replacement: The GALILEO trial. Cardiovasc. Res. 2020, 116, e39–e41. [Google Scholar] [CrossRef]
- Wöhrle, J.; Gilard, M.; Didier, R.; Kini, A.; Tavenier, A.H.; Tijssen, J.G.P.; Sartori, S.; Snyder, C.; Nicolas, J.; Seeger, J.; et al. GALILEO Investigator. Outcomes After Transcatheter Aortic Valve Implantation in Men Versus Women. Am. J. Cardiol. 2022, 180, 108–115. [Google Scholar] [CrossRef]
- Collet, J.P.; Van Belle, E.; Thiele, H.; Berti, S.; Lhermusier, T.; Manigold, T.; Neumann, F.J.; Gilard, M.; Attias, D.; Beygui, F.; et al. Apixaban vs. standard of care after transcatheter aortic valve implantation: The ATLANTIS trial. Eur. Heart J. 2022, 43, 2783–2797. [Google Scholar] [CrossRef]
- De Backer, O.; Dangas, G.D.; Jilaihawi, H.; Leipsic, J.A.; Terkelsen, C.J.; Makkar, R.; Kini, A.S.; Veien, K.T.; Abdel-Wahab, M.; Kim, W.K.; et al. GALILEO-4D Investigators. Reduced Leaflet Motion after Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 130–139. [Google Scholar] [CrossRef]
- Montalescot, G.; Redheuil, A.; Vincent, F.; Desch, S.; De Benedictis, M.; Eltchaninoff, H.; Trenk, D.; Serfaty, J.M.; Charpentier, E.; Bouazizi, K.; et al. ATLANTIS Investigators of the ACTION Group. Apixaban and Valve Thrombosis After Transcatheter Aortic Valve Replacement: The ATLANTIS-4D-CT Randomized Clinical Trial Substudy. JACC Cardiovasc. Interv. 2022, 15, 1794–1804. [Google Scholar] [CrossRef]
- Nijenhuis, V.J.; Brouwer, J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.F.; Frambach, P.; De Bruyne, B.; van Houwelingen, G.K.; Van Der Heyden, J.A.S.; et al. Anticoagulation with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2020, 382, 1696–1707. [Google Scholar] [CrossRef]
- van Bergeijk, K.H.; van Ginkel, D.J.; Brouwer, J.; Nijenhuis, V.J.; van der Werf, H.W.; van den Heuvel, A.F.M.; Voors, A.A.; Wykrzykowska, J.J.; Ten Berg, J.M. Sex Differences in Outcomes After Transcatheter Aortic Valve Replacement: A POPular TAVI Subanalysis. JACC Cardiovasc. Interv. 2023, 16, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; Unverdorben, M.; Hengstenberg, C.; Möllmann, H.; Mehran, R.; López-Otero, D.; Nombela-Franco, L.; Moreno, R.; Nordbeck, P.; Thiele, H.; et al. ENVISAGE-TAVI AF Investigators. Edoxaban versus Vitamin K Antagonist for Atrial Fibrillation after TAVR. N. Engl. J. Med. 2021, 385, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Khan, M.S.; Mufarrih, S.H.; Kazimuddin, M.; Waheed, M.A.; Tripathi, A.; Bavishi, C.; Hyder, O.N.; Aronow, H.D.; Saad, M.; et al. Meta-Analysis Assessing Efficacy and Safety of Vitamin K Antagonists Versus Direct Oral Anticoagulants for Atrial Fibrillation After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2023, 201, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.H.; De Backer, O.; Olesen, J.B.; Gerds, T.A.; Havers-Borgersen, E.; Gislason, G.H.; Torp-Pedersen, C.; Søndergaard, L.; Køber, L.; Fosbøl, E.L. Vitamin K antagonists vs. direct oral anticoagulants after transcatheter aortic valve implantation in atrial fibrillation. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 11–19. [Google Scholar] [CrossRef]
- Jochheim, D.; Barbanti, M.; Capretti, G.; Stefanini, G.G.; Hapfelmeier, A.; Zadrozny, M.; Baquet, M.; Fischer, J.; Theiss, H.; Todaro, D.; et al. Oral Anticoagulant Type and Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 1566–1576. [Google Scholar] [CrossRef]
- Kawashima, H.; Watanabe, Y.; Hioki, H.; Kozuma, K.; Kataoka, A.; Nakashima, M.; Nagura, F.; Nara, Y.; Yashima, F.; Tada, N.; et al. OCEAN-TAVI Investigator. Direct Oral Anticoagulants Versus Vitamin K Antagonists in Patients with Atrial Fibrillation After TAVR. JACC Cardiovasc. Interv. 2020, 13, 2587–2597. [Google Scholar] [CrossRef]
- Mangner, N.; Crusius, L.; Haussig, S.; Woitek, F.J.; Kiefer, P.; Stachel, G.; Leontyev, S.; Schlotter, F.; Spindler, A.; Höllriegel, R.; et al. Continued Versus Interrupted Oral Anticoagulation During Transfemoral Transcatheter Aortic Valve Implantation and Impact of Postoperative Anticoagulant Management on Outcome in Patients with Atrial Fibrillation. Am. J. Cardiol. 2019, 123, 1134–1141. [Google Scholar] [CrossRef]
- Seeger, J.; Gonska, B.; Rodewald, C.; Rottbauer, W.; Wöhrle, J. Apixaban in Patients with Atrial Fibrillation After Transfemoral Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 66–74. [Google Scholar] [CrossRef]
- Tanawuttiwat, T.; Stebbins, A.; Marquis-Gravel, G.; Vemulapalli, S.; Kosinski, A.S.; Cheng, A. Use of Direct Oral Anticoagulant and Outcomes in Patients with Atrial Fibrillation after Transcatheter Aortic Valve Replacement: Insights From the STS/ACC TVT Registry. J. Am. Heart Assoc. 2022, 11, e023561. [Google Scholar] [CrossRef]
- Teede, H.J.; McGrath, B.P.; Smolich, J.J.; Malan, E.; Kotsopoulos, D.; Liang, Y.L.; Peverill, R.E. Postmenopausal hormone replacement therapy increases coagulation activity and fibrinolysis. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1404–1409. [Google Scholar] [CrossRef]
- Thijs, A.; van Baal, W.M.; van der Mooren, M.J.; Kenemans, P.; Dräger, A.M.; Huijgens, P.C.; Stehouwer, C.D. Effects of hormone replacement therapy on blood platelets. Eur. J. Clin. Investig. 2002, 32, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Al-Farra, H.M.; Al-Fahoum, S.K.; Tabbaa, M.A. Effect of hormone replacement therapy on hemostatic variables in post-menopausal women. Saudi Med. J. 2005, 26, 1930–1935. [Google Scholar]
- Vigen, C.; Hodis, H.N.; Chandler, W.L.; Lobo, R.A.; Mack, W.J. Postmenopausal oral estrogen therapy affects hemostatic factors, but does not account for reduction in the progression of subclinical atherosclerosis. J. Thromb. Haemost. 2007, 5, 1201–1208. [Google Scholar] [CrossRef]
- Kalmanti, L.; Lindhoff-Last, E. Bleeding Issues in Women Under Oral Anticoagulation. Hamostaseologie 2022, 42, 337–347. [Google Scholar] [CrossRef]
- Palareti, G.; Legnani, C.; Antonucci, E.; Cosmi, B.; Falanga, A.; Poli, D.; Mastroiacovo, D.; Pengo, V.; Ageno, W.; Testa, S. Do women with venous thromboembolism bleed more than men during anticoagulation? Data from the real-life, prospective START-Register. Ther. Adv. Drug Saf. 2021, 12, 20420986211062965. [Google Scholar] [CrossRef]
| Study | Population | Treatment Comparison | Findings |
|---|---|---|---|
| WIN-TAVI | 1000 women | DAPT vs. SAPT vs. OAC | At 1 year, most patients are on SAPT or no therapy. No male comparator. High bleeding risk in elderly females. Following multivariable stepwise Cox regression, DAPT use results as a borderline independent predictor of the 1-year primary VARC-2 efficacy endpoint (HR: 0.70; 95% CI: 0.49 to 1.01; p = 0.059). |
| FRANCE-TAVI | >10,000 (mixed) | SAPT/OAC/DAPT | Female sex is associated with a higher risk of major bleeding and vascular complications. Male sex independently predicted mortality (HR: 1.63; 95% confidence interval [CI]: 1.44 to 1.84; p < 0.001). Anticoagulation at discharge results is independently associated with lower rates of bioprosthetic valve dysfunction (OR: 0.54; 95% CI: 0.35 to 0.82; p = 0.005) but independently correlated with all-cause mortality (HR: 1.18 95% CI: 1.04 to 1.29; p = 0.013). |
| Eurointervention Registry | ~1300 (mixed) | Per center protocol (heterogeneous) | Similar NACE in women (HR 1.16) and men (HR 1.08); edoxaban ↑ major bleeding in both but increase attenuated in women (HR 1.11 vs. 1.75 in men; interaction p = 0.170) |
| PARTNER 2 and 3 subanalysis | >1000 | DAPT vs. SAPT (historical context) | Women had a higher risk of vascular complications (17.3% vs. 10.0%; 95% CI 4.63–9.95; p < 0.001) and major bleeding (10.5% vs. 7.7%; 95% CI 0.57–5.04; p = 0.012). Not primarily focused on therapy but supports anatomical susceptibility. |
| Study | Year | Population/Women (%) | Treatment Comparison | Follow-Up | Findings |
|---|---|---|---|---|---|
| POPULAR TAVI | 2020 | 978/46.7% women | Aspirin (±OAC) vs. DAPT | 12 months | Total bleeding and ischemia similar; major/life-threatening bleeding higher in women (12.5 % vs. 7.4%; p = 0.011), especially with aspirin pre- and post-TAVI. In the SAPT vs. DAPT comparison, SAPT results in fewer bleeding events (15.1% vs. 26.6%; RR 0.57; 95% CI 0.42–0.77; p = 0.001). In OAC alone vs. OAC + antiplatelet, OAC alone results in fewer bleeding events (21.7% vs. 34.6%; RR 0.63; 95% CI 0.43–0.90; p = 0.011). Both strategies have non-inferiority for the composite ischemic endpoint. |
| ARTE | 2017 | 222/36.9% women | 3 mo. DAPT vs. ASA | 3 months | No sex-stratified analysis available (overall trend: higher combined endpoint with DAPT, p = 0.065) |
| ENVISAGE TAVI AF | 2021 | 1426/~47.5% women | Edoxaban vs. VKA (±antiplatelet) | 540 days | Similar NACE in women (HR 1.16) and men (HR 1.08); edoxaban ↑ major bleeding in both; the relative increase is smaller in women than in men (HR 1.11 in women vs. 1.75 in men; interaction p = 0.170) |
| GALILEO | 2020 | ~1644/49.5% women | Rivaroxaban + aspirin vs. DAPT | 17 months | Women had a lower risk of MACE (HR 0.69), all-cause mortality (HR 0.54), and non-cardiovascular mortality (HR 0.33). |
| ATLANTIS | 2022 | ~1510/53% women | Apixaban vs. standard care (antiplatelet or VKA) | 12 months | No sex-stratified analysis available |
| ADAPT TAVR | 2022 | 229 women (% not reported) | Edoxaban vs. DAPT | 6 months | No sex-stratified data available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Gregorio, M.; Denegri, A.; Gurgoglione, F.L.; Benatti, G.; Tadonio, I.; Solinas, E.; Carino, D.; Agostinelli, A.; Vignali, L.; Niccoli, G. Antithrombotic Therapy in Transcatheter Aortic Valve Implantation: Focus on Gender Differences. J. Cardiovasc. Dev. Dis. 2025, 12, 433. https://doi.org/10.3390/jcdd12110433
De Gregorio M, Denegri A, Gurgoglione FL, Benatti G, Tadonio I, Solinas E, Carino D, Agostinelli A, Vignali L, Niccoli G. Antithrombotic Therapy in Transcatheter Aortic Valve Implantation: Focus on Gender Differences. Journal of Cardiovascular Development and Disease. 2025; 12(11):433. https://doi.org/10.3390/jcdd12110433
Chicago/Turabian StyleDe Gregorio, Mattia, Andrea Denegri, Filippo Luca Gurgoglione, Giorgio Benatti, Iacopo Tadonio, Emilia Solinas, Davide Carino, Andrea Agostinelli, Luigi Vignali, and Giampaolo Niccoli. 2025. "Antithrombotic Therapy in Transcatheter Aortic Valve Implantation: Focus on Gender Differences" Journal of Cardiovascular Development and Disease 12, no. 11: 433. https://doi.org/10.3390/jcdd12110433
APA StyleDe Gregorio, M., Denegri, A., Gurgoglione, F. L., Benatti, G., Tadonio, I., Solinas, E., Carino, D., Agostinelli, A., Vignali, L., & Niccoli, G. (2025). Antithrombotic Therapy in Transcatheter Aortic Valve Implantation: Focus on Gender Differences. Journal of Cardiovascular Development and Disease, 12(11), 433. https://doi.org/10.3390/jcdd12110433









