Pericardial Closure Preserves Early Right Ventricular Function After Cardiac Surgery: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design & Patient Population
2.2. Data Collection
2.3. Surgical Technique
2.4. Echocardiography
2.5. Endpoints
2.6. Ethics
2.7. Statistical Analysis
3. Results
3.1. Baseline (Preoperative) Characteristics
3.2. Changes Within Each Group
3.3. Postoperative Inter-Group Comparison
3.4. Correlation Between Open vs. Closed Pericardium and the Incidence of Postoperative Atrial Fibrillation
4. Discussion
4.1. Clinical Perspective
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| (2D-)STE | (Two-dimensional) Speckle-tracking Echocardiography |
| 3D | Three-dimensional |
| 4AC | Apical Four Chamber View |
| 5AC | Apical Five Chamber View |
| A (TV) | Late Diastolic Tricuspid Inflow Velocity during Atrial Contraction |
| A′ | Late Diastolic Tricuspid Annular velocity during Atrial Contraction |
| AF | Atrial Fibrillation |
| APLAX | Apical Long Axis |
| AUC | Area Under the Curve |
| AVR | Aortic Valve Replacement |
| CABG | Coronary Artery Bypass Grafting |
| CMR | Cardiovascular Magnetic Resonance |
| COPD | Chronic Obstructive Pulmonary Disease |
| CPB | Cardiopulmonary Bypass |
| DeT TV | E-wave Deceleration Time |
| E (TV) | Early Diastolic Tricuspid Inflow Velocity |
| E′ | Early Diastolic Tricuspid Annular Velocity |
| ECG | Electrocardiography |
| ET | Ejection Time |
| ET RVOT | Ejection Time over the Right Ventricular Outflow Tract |
| IVCT | Isovolumetric Contraction Time |
| IVRT | Isovolumetric Relaxation Time |
| LV | Left Ventricle |
| LV GLS | Left Ventricular Global Longitudinal Strain |
| LVEDD | Left Ventricular End-Diastolic Diameter |
| LVEDVI | Left Ventricular End-Diastolic Volume Index |
| LVEF | Left-Ventricular Ejection Fraction |
| LVOTd | Left Ventricular Outflow Tract Diameter |
| LVSVI | Left Ventricular Stroke Volume Index |
| MPI | Myocardial Performance Index |
| PAPsys | Pulmonary Artery Systolic Pressure |
| PLAX | Parasternal Long Axis |
| PSAX | Parasternal Short Axis |
| PVR | Pulmonary Vascular Resistance |
| PW-TDI | PW-Tissue Doppler Imaging |
| RA | Right Atrium |
| RAP | Right Atrial Pressure |
| ROC | Receiver Operating Characteristic |
| RV | Right Ventricle |
| RV ET | Right Ventricular Ejection Time |
| RV Str | Right Ventricular StrainRate |
| RV Strain | Right Ventricular Strain |
| RVEDD1 | Right Ventricular Basal Diameter at End-Diastole |
| RVEDD3 | Right Ventricular Longitudinal diameter at End-Diastole |
| RVEF | Right Ventricular Ejection Fraction |
| RVF | Right Ventricular Function |
| RV-FAC | Right Ventricular Fractional Area Change |
| SD | Standard Deviation |
| SVI | Stroke Volume Index |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| TASV, S′ | Tricuspid Annular Systolic Velocity |
| TEE | Transesophageal echocardiography |
| Transpulmonale T | Transpulmonary Time |
| TRVmax | Maximum Flow Velocity of Systolic Tricuspid Regurgitation |
| TTE | Transthoracic Echocardiography |
| VCI | Vena Cava Inferior |
| VTI | Velocity-Time Integral |
References
- Purmah, Y.; Lei, L.Y.; Dykstra, S.; Mikami, Y.; Cornhill, A.; Satriano, A.; Flewitt, J.; Rivest, S.; Sandonato, R.; Seib, M.; et al. Right Ventricular Ejection Fraction for the Prediction of Major Adverse Cardiovascular and Heart Failure-Related Events. Circ. Cardiovasc. Imaging 2021, 14, e011337. [Google Scholar] [CrossRef]
- Denault, A.; Haddad, F.; Lamarche, Y.; Bouabdallaoui, N.; Deschamps, A.; Desjardins, G. Postoperative right ventricular dysfunction-Integrating right heart profiles beyond long-axis function. J. Thorac. Cardiovasc. Surg. 2020, 159, e315–e317. [Google Scholar]
- Towheed, A.; Sabbagh, E.; Gupta, R.; Assiri, S.; Chowdhury, M.A.; Moukarbel, G.V.; Khuder, S.A.; Schwann, T.A.; Bonnell, M.R.; Cooper, C.J.; et al. Right Ventricular Dysfunction and Short-Term Outcomes Following Left-Sided Valvular Surgery: An Echocardiographic Study. J. Am. Heart Assoc. 2021, 10, e016283. [Google Scholar]
- Donauer, M.; Schneider, J.; Jander, N.; Beyersdorf, F.; Keyl, C. Perioperative Changes of Right Ventricular Function in Cardiac Surgical Patients Assessed by Myocardial Deformation Analysis and 3-Dimensional Echocardiography. J. Cardiothorac. Vasc. Anesth. 2020, 34, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.; Mohamed, A.; Arends, S.; Ishak, B.; Tsarenko, O.; Balaj, I.; Kamler, M.; Brenner, T.; Shehada, S.E. Echocardiographic right ventricular evaluation in cardiac surgery patients undergoing mitral valve reconstruction: A single center prospective observational study. J. Thorac. Dis. 2024, 16, 2259–2273. [Google Scholar] [CrossRef]
- Bitcon, C.J.; Tousignant, C. The effect of pericardial incision on right ventricular systolic function: A prospective observational study. Can. J. Anaesth. 2017, 64, 1194–1201. [Google Scholar] [CrossRef]
- Bootsma, I.T.; Scheeren, T.W.L.; de Lange, F.; Jainandunsing, J.S.; Boerma, E.C. The Reduction in Right Ventricular Longitudinal Contraction Parameters Is Not Accompanied by a Reduction in General Right Ventricular Performance During Aortic Valve Replacement: An Explorative Study. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2140–2147. [Google Scholar] [CrossRef]
- Gaudino, M.; Pragliola, C.; Anselmi, A.; Pieroni, M.; De Paulis, S.; Leone, A.; De Caterina, A.R.; Massetti, M. Randomized trial of HTK versus warm blood cardioplegia for right ventricular protection in mitral surgery. Scand. Cardiovasc. J. 2013, 47, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Merlo, A.; Cirelli, C.; Vizzardi, E.; Fiorendi, L.; Roncali, F.; Marino, M.; Merlo, M.; Senni, M.; Sciatti, E. Right Ventricular Dysfunction before and after Cardiac Surgery: Prognostic Implications. J. Clin. Med. 2024, 13, 1609. [Google Scholar] [CrossRef]
- Unsworth, B.; Casula, R.P.; Yadav, H.; Baruah, R.; Hughes, A.D.; Mayet, J.; Francis, D.P. Contrasting effect of different cardiothoracic operations on echocardiographic right ventricular long axis velocities, and implications for interpretation of post-operative values. Int. J. Cardiol. 2013, 165, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, B.; Casula, R.P.; Kyriacou, A.A.; Yadav, H.; Chukwuemeka, A.; Cherian, A.; de Lisle Stanbridge, R.; Athanasiou, T.; Mayet, J.; Francis, D.P. The right ventricular annular velocity reduction caused by coronary artery bypass graft surgery occurs at the moment of pericardial incision. Am. Heart J. 2010, 159, 314–322. [Google Scholar] [CrossRef]
- Mauermann, E.; Vandenheuvel, M.; François, K.; Bouchez, S.; Wouters, P. Right Ventricular Systolic Assessment by Transesophageal Versus Transthoracic Echocardiography: Displacement, Velocity, and Myocardial Deformation. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2152–2161. [Google Scholar] [CrossRef]
- Geyer, H.; Caracciolo, G.; Abe, H.; Wilansky, S.; Carerj, S.; Gentile, F.; Nesser, H.J.; Khandheria, B.; Narula, J.; Sengupta, P.P. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J. Am. Soc. Echocardiogr. 2010, 23, 351–369. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of, Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 412. [Google Scholar]
- Negishi, K.; Negishi, T.; Kurosawa, K.; Hristova, K.; Popescu, B.A.; Vinereanu, D.; Yuda, S.; Marwick, T.H. Practical guidance in echocardiographic assessment of global longitudinal strain. JACC Cardiovasc. Imaging 2015, 8, 489–492. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Raina, A.; Vaidya, A.; Gertz, Z.M.; Susan, C.; Forfia, P.R. Marked changes in right ventricular contractile pattern after cardiothoracic surgery: Implications for post-surgical assessment of right ventricular function. J. Heart Lung Transplant. 2013, 32, 777–783. [Google Scholar] [CrossRef]
- Tamborini, G.; Muratori, M.; Brusoni, D.; Celeste, F.; Maffessanti, F.; Caiani, E.G.; Alamanni, F.; Pepi, M. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur. J. Echocardiogr. 2009, 10, 630–634. [Google Scholar] [CrossRef]
- Korshin, A.; Grønlykke, L.; Nilsson, J.C.; Møller-Sørensen, H.; Ihlemann, N.; Kjøller, S.M.; Damgaard, S.; Lehnert, P.; Hassager, C.; Kjaergaard, J.; et al. Tricuspid annular plane systolic excursion is significantly reduced during uncomplicated coronary artery bypass surgery: A prospective observational study. J. Thorac. Cardiovasc. Surg. 2019, 158, 480–489. [Google Scholar] [CrossRef]
- Singh, A.; Huang, X.; Dai, L.; Wyler, D.; Alfirevic, A.; Blackstone, E.H.; Pettersson, G.B.; Duncan, A.E. Right ventricular function is reduced during cardiac surgery independent of procedural characteristics, reoperative status, or pericardiotomy. J. Thorac. Cardiovasc. Surg. 2020, 159, 1430–1438.e4. [Google Scholar] [CrossRef]
- Kovács, A.; Lakatos, B.; Tokodi, M.; Merkely, B. Right ventricular mechanical pattern in health and disease: Beyond longitudinal shortening. Heart Fail. Rev. 2019, 24, 511–520. [Google Scholar] [CrossRef]
- Vitarelli, A.; Mangieri, E.; Terzano, C.; Gaudio, C.; Salsano, F.; Rosato, E.; Capotosto, L.; D’Orazio, S.; Azzano, A.; Truscelli, G.; et al. Three-Dimensional Echocardiography and 2D-3D Speckle-Tracking Imaging in Chronic Pulmonary Hypertension: Diagnostic Accuracy in Detecting Hemodynamic Signs of Right Ventricular (RV) Failure. J. Am. Heart Assoc. 2015, 4, e001584. [Google Scholar] [CrossRef]
- Lee, J.Z.; Low, S.W.; Pasha, A.K.; Howe, C.L.; Lee, K.S.; Suryanarayana, P.G. Comparison of tricuspid annular plane systolic excursion with fractional area change for the evaluation of right ventricular systolic function: A meta-analysis. Open Heart 2018, 5, e000667. [Google Scholar] [CrossRef]
- Keller, M.; Heller, T.; Lang, T.; Patzelt, J.; Schreieck, J.; Schlensak, C.; Rosenberger, P.; Magunia, H. Acute changes of global and longitudinal right ventricular function: An exploratory analysis in patients undergoing open-chest mitral valve surgery, percutaneous mitral valve repair and off-pump coronary artery bypass grafting. Cardiovasc. Ultrasound 2020, 18, 32. [Google Scholar] [CrossRef]
- Zanobini, M.; Loardi, C.; Poggio, P.; Tamborini, G.; Veglia, F.; Di Minno, A.; Myasoedova, V.; Mammana, L.F.; Biondi, R.; Pepi, M.; et al. The impact of pericardial approach and myocardial protection onto postoperative right ventricle function reduction. J. Cardiothorac. Surg. 2018, 13, 55. [Google Scholar] [CrossRef]
- Rao, V.; Komeda, M.; Weisel, R.D.; Cohen, G.; Borger, M.A.; David, T.E. Should the pericardium be closed routinely after heart operations? Ann. Thorac. Surg. 1999, 67, 484–488. [Google Scholar] [CrossRef]
- Santamore, W.P.; Dell’Italia, L.J. Ventricular interdependence: Significant left ventricular contributions to right ventricular systolic function. Prog. Cardiovasc. Dis. 1998, 40, 289–308. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Reddy, Y.N. The role of the pericardium in heart failure: Implications for pathophysiology and treatment. JACC Heart Fail. 2019, 7, 574–585. [Google Scholar] [CrossRef]
- David, J.S.; Tousignant, C.P.; Bowry, R. Tricuspid annular velocity in patients undergoing cardiac operation using transesophageal echocardiography. J. Am. Soc. Echocardiogr. 2006, 19, 329–334. [Google Scholar] [CrossRef]
| Open (n = 32) | Closed (n = 65) | p-Values | |
|---|---|---|---|
| preoperative | |||
| LV GLS (%) | −19.2 ± 3.6 | −17.4 ± 3.7 | <0.001 |
| LVEDD (mm) | 47.7 ± 7.0 | 48.4 ± 8.3 | 0.479 |
| LVEDVI (mL/m2) | 48.6 ± 11.2 | 55.7 ± 24.3 | 0.007 |
| LVEF (%) | 58.1 ± 8.4 | 55.5 ± 8.9 | 0.018 |
| SVI (mL/m2) | 37.6 ± 12.4 | 37.1 ± 11.5 | 0.728 |
| IVSd (mm) | 13.6 ± 3.0 | 13.3 ± 3.1 | 0.683 |
| RVEDD 1 (mm) | 31.8 ± 7.0 | 33.0 ± 6.5 | 0.126 |
| RVEDD 3 (mm) | 80.5 ± 7.4 | 82.7 ± 10.1 | 0.052 |
| TAPSE (mm) | 21.6 ± 3.3 | 22.2 ± 4.4 | 0.558 |
| TASV (cm/s) | 11.7 ± 2.8 | 12.0 ± 3.6 | 0.709 |
| Time to peak RV S′ (ms) | 181.3 ± 43.7 | 187.6 ± 42.9 | 0.540 |
| RVFAC (%) | 41.5 ± 11.6 | 43.5 ± 10.0 | 0.382 |
| Ratio TAPSE/RVFAC | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.506 |
| RV Str | −1.5 ± 0.4 | −1.6 ± 0.5 | 0.643 |
| RV Strain (%) | −21.1 ± 6.0 | −22.5 ± 5.8 | 0.320 |
| PAPsys (mmHg) | 19.9 ± 15.4 | 20.3 ± 11.0 | 0.880 |
| RAP (mmHg) | 18 ± 56.2 | 34 ± 52.3 | 0.480 |
| VTI RVOT (cm) | 16.5 ± 3.4 | 17.1 ± 3.7 | 0.582 |
| Vmax TR (m/s) | 1.9 ± 0.9 | 1.9 ± 0.6 | 0.502 |
| PVR (wood units) | 1.3 ± 0.9 | 1.3 ± 0.7 | 0.966 |
| TAPSE/sPAP | 1.0 ± 1.0 | 0.8 ± 0.6 | 0.233 |
| E TV (cm/s) | 60.8 ± 18.5 | 57.3 ± 11.7 | 0.074 |
| A TV (cm/s) | 50.6 ± 13.3 | 49.0 ± 10.9 | 0.334 |
| E′ (cm/s) | 8.4 ± 3.1 | 9.2 ± 4.0 | 0.091 |
| A′ (cm/s) | 11.7 ± 4.7 | 12.7 ± 5.6 | 0.184 |
| E/A | 1.2 ± 0.4 | 1.2 ± 0.3 | 0.672 |
| E/E′ | 8.0 ± 3.8 | 6.9 ± 3.2 | 0.228 |
| DeT TV (ms) | 314.7 ± 116.2 | 339.7 ± 146.2 | 0.448 |
| IVCT (ms) | 74.5 ± 20.1 | 72.0 ± 19.4 | 0.741 |
| IVRT (ms) | 83.7 ± 30.3 | 83.3 ± 43.4 | 0.968 |
| Ejection Time (ms) | 278.1 ± 54.8 | 270.7 ± 49.3 | 0.530 |
| Tei Index (TDI) | 0.6 ± 0.3 | 0.6 ± 0.5 | 0.651 |
| ET spectral RVOT (ms) | 267.9 ± 58.6 | 369.8 ± 51.3 | 0.888 |
| Transpulmonale T (ms) | 295.0 ± 84.5 | 295.4 ± 81.3 | 0.982 |
| Tei-Index (Spectral) | 0.0 ± 0.6 | 0.0 ± 0.5 | 0.748 |
| Postoperative | |||
| GLS LV (%) | −15.1 ± 3.7 | −16.9 ± 3.7 | <0.001 |
| LVEDD (mm) | 48.7 ± 5.7 | 48.4 ± 7.5 | 0.697 |
| LVEDVI (mL/m2) | 47.4 ± 15.0 | 59.9 ± 69.8 | 0.093 |
| LVEF (%) | 53.3 ± 11.5 | 52.2 ± 9.3 | 0.435 |
| SVI (mL/m2) | 32.0 ± 10.6 | 31.1 ± 11.8 | 0.532 |
| RVEDD 1 (mm) | 33.5 ± 7.5 | 35.5 ± 6.4 | 0.170 |
| RVEDD 3 (mm) | 81.2 ± 9.3 | 82.0 ± 10.7 | 0.529 |
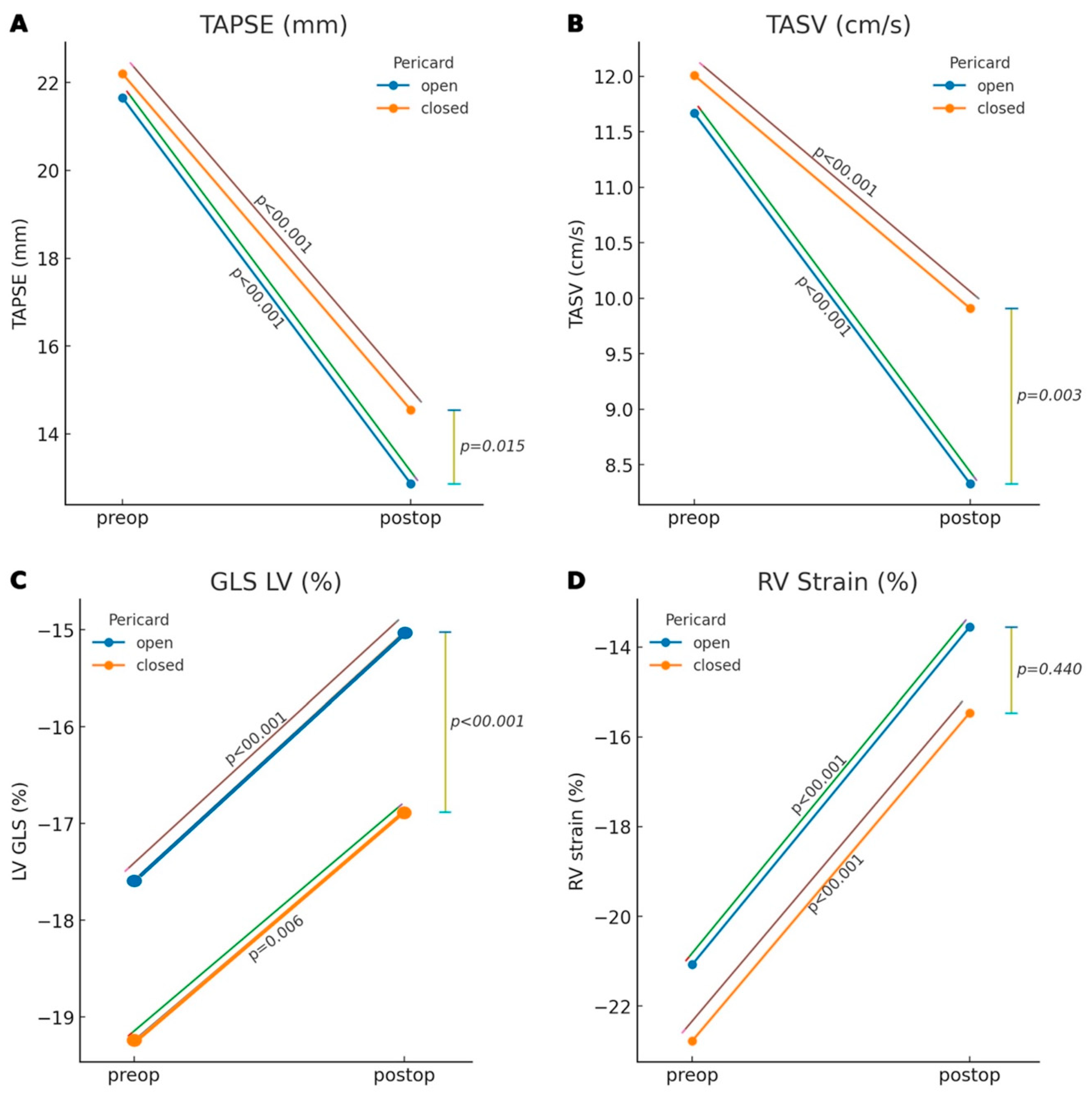
| TAPSE (mm) | 12.9 ± 3.1 | 14.6 ± 3.1 | 0.015 |
| TASV (cm/s) | 7.9 ± 2.5 | 9.8 ± 2.7 | 0.003 |
| Time to peak RV S′ (ms) | 147.1 ± 33.8 | 158.7 ± 31.8 | 0.187 |
| RVFAC (%) | 41.7 ± 15.8 | 42.2 ± 10.6 | 0.882 |
| Ratio TAPSE/RVFAC | 0.3 ± 0.2 | 0.4 ± 0.1 | 0.579 |
| RV Str | −1.3 ± 0.8 | −1.5 ± 1.5 | 0.311 |
| RV Strain (%) | −14.2 ± 6.3 | −15.3 ± 6.9 | 0.440 |
| PAPsys (mmHg) | 26.9 ± 21.4 | 23.4 ± 11.6 | 0.362 |
| RAP (mmHg) | 16 ± 50.0 | 30 ± 46.2 | 0.183 |
| VTI RVOT (cm) | 16.7 ± 5.5 | 14.5 ± 4.7 | 0.126 |
| Vmax TR (m/s) | 2.1 ± 0.9 | 2.0 ± 0.7 | 0.162 |
| PVR (wood units) | 1.2 ± 0.9 | 1.4 ± 0.9 | 0.443 |
| TAPSE/sPAP | 44.7 ± 42.2 | 39.1 ± 23.3 | 0.450 |
| E TV (cm/s) | 75.1 ± 20.0 | 63.3 ± 21.4 | <0.001 |
| A TV (cm/s) | 52.6 ± 16.2 | 49.9 ± 18.3 | 0.282 |
| E′ (cm/s) | 5.7 ± 2.9 | 6.7 ± 2.7 | 0.006 |
| A′ (cm/s) | 5.7 ± 3.5 | 8.2 ± 3.3 | <0.001 |
| E/A | 1.5 ± 0.4 | 1.3 ± 0.4 | 0.163 |
| E/E′ | 18.4 ± 23.6 | 11.5 ± 9.2 | 0.089 |
| DeT TV (ms) | 303.5 ± 196.6 | 222.6 ± 80.6 | 0.015 |
| IVCT (ms) | 62.8 ± 15.0 | 66.0 ± 18.0 | 0.427 |
| IVRT (ms) | 78.1 ± 31.6 | 82.8 ± 34.6 | 0.545 |
| Ejection Time (ms) | 226.6 ± 65.4 | 205.6 ± 39.5 | 0.070 |
| Tei Index (TDI) | 0.7 ± 0.3 | 0.8 ± 0.3 | 0.036 |
| ET spectral RVOT (ms) | 248.0 ± 43.4 | 230.4 ± 41.2 | 0.145 |
| Transpulmonale T (ms) | 265.7 ± 61.8 | 253.3 ± 61.0 | 0.407 |
| Tei Index Spektral | −0.1 ± 0.5 | −0.1 ± 0.5 | 0.926 |
| Open (n = 32) | Closed (n = 65) | |||||
|---|---|---|---|---|---|---|
| Preoperative | Postoperative | p Values | Preoperative | Postoperative | p Values | |
| LV GLS (%) | −19.246 ± 3.654 | −16.879 ± 3.896 | 0.006 | −17.589 ± 3.647 | −15.023 ± 3.584 | <0.001 |
| LVEDD (mm) | 46.773 ± 6.102 | 48.682 ± 5.875 | 1.000 | 48.263 ± 8.134 | 48.351 ± 7.499 | 1.000 |
| LVEDVI (mL/m2) | 48.910 ± 11.262 | 48.034 ± 14.949 | 1.000 | 56.878 ± 24.914 | 60.267 ± 70.746 | 1.000 |
| LVEF (%) | 58.379 ± 8.432 | 53.448 ± 11.776 | 0.164 | 55.492 ± 8.997 | 52.576 ± 9.058 | 0.283 |
| SVI (mL/m2) | 37.719 ± 12.775 | 32.032 ± 10.687 | 0.127 | 36.967 ± 11.463 | 31.323 ± 11.992 | 0.019 |
| RVEDD 1 (mm) | 31.750 ± 7.030 | 33.469 ± 7.577 | 0.707 | 33.319 ± 6.573 | 35.532 ± 6.467 | 0.175 |
| RVEDD 3 (mm) | 80.469 ± 7.457 | 81.156 ± 9.395 | 1.000 | 82.516 ± 10.300 | 81.968 ± 10.770 | 1.000 |
| TAPSE (mm) | 21.650 ± 3.300 | 12.869 ± 3.147 | <0.001 | 22.197 ± 4.411 | 14.552 ± 3.103 | <0.001 |
| TASV (cm/s) | 11.667 ± 2.817 | 8.329 ± 2.328 | <0.001 | 12.009 ± 3.521 | 9.909 ± 2.708 | <0.001 |
| Time to peak RV S′ (ms) | 168.059 ± 39.213 | 145.412 ± 33.577 | 0.072 | 185.310 ± 44.337 | 158.881 ± 33.207 | 0.001 |
| RVFAC (%) | 41.542 ± 11.634 | 41.750 ± 15.815 | 0.940 | 43.570 ± 9.885 | 41.159 ± 10.626 | 0.477 |
| Ratio TAPSE/RVFAC | 0.568 ± 0.211 | 0.347 ± 0.157 | <0.001 | 0.541 ± 0.183 | 0.362 ± 0.113 | <0.001 |
| RV Str | −1.535 ± 0.383 | −1.204 ± 0.849 | 0.267 | −1.618 ± 0.512 | −1.556 ± 1.508 | 0.745 |
| RV Strain (%) | −21.074 ± 5.988 | −13.552 ± 6.720 | <0.001 | −22.777 ± 5.755 | −15.460 ± 6.779 | <0.001 |
| PAPsys (mmHg) | 20.588 ± 16.567 | 28.832 ± 22.333 | 0.071 | 19.863 ± 10.628 | 22.802 ± 11.607 | 0.342 |
| RAP (mmHg) | 3.952 ± 2.012 | 5.333 ± 3.864 | 0.193 | 5.122 ± 3.120 | 6.122 ± 3.603 | 0.150 |
| VTI RVOT (cm) | 17.207 ± 3.761 | 16.450 ± 6.134 | 0.597 | 17.885 ± 3.596 | 14.565 ± 4.775 | <0.001 |
| Vmax TR (m/s) | 1.866 ± 0.947 | 2.243 ± 0.955 | 0.567 | 1.891 ± 0.641 | 1.992 ± 0.720 | 1.000 |
| PVR (wood units) | 1.238 ± 0.612 | 1.387 ± 0.858 | 0.532 | 1.162 ± 0.448 | 1.361 ± 0.766 | 0.161 |
| TAPSE/sPAP | 1.044 ± 1.020 | 44.701 ± 42.223 | <0.001 | 0.817 ± 0.633 | 39.060 ± 23.320 | <0.001 |
| E TV (cm/s) | 50.455 ± 13.412 | 51.364 ± 16.552 | 1.000 | 48.690 ± 11.507 | 48.214 ± 13.377 | 1.000 |
| A TV (cm/s) | 50.455 ± 13.412 | 51.364 ± 16.552 | 0.807 | 48.690 ± 11.507 | 48.214 ± 16.377 | 0.860 |
| E′ (cm/s) | 8.535 ± 3.191 | 6.217 ± 2.621 | 0.014 | 9.362 ± 3.582 | 6.840 ± 2.788 | <0.001 |
| A′ (cm/s) | 11.917 ± 5.147 | 6.304 ± 3.080 | <0.001 | 13.055 ± 4.937 | 8.489 ± 3.053 | <0.001 |
| E/A | 1.194 ± 0.288 | 1.513 ± 0.400 | 0.006 | 1.207 ± 0.268 | 1.327 ± 0.451 | 0.140 |
| E/E′ (cm/s) | 8.300 ± 4.007 | 12.218 ± 4.867 | 0.048 | 6.755 ± 3.126 | 11.478 ± 9.846 | <0.001 |
| DeT TV (ms) | 318.636 ± 119.110 | 301.136 ± 181.893 | 0.630 | 354.214 ± 152.561 | 223.190 ± 84.594 | <0.001 |
| IVCT (ms) | 72.458 ± 19.583 | 63.875 ± 12.585 | 0.099 | 72.321 ± 19.583 | 66.396 ± 18.137 | 0.091 |
| IVRT (ms) | 80.167 ± 27.886 | 79.625 ± 24.182 | 0.962 | 81.811 ± 45.349 | 83.849 ± 34.759 | 0.793 |
| Ejection Time (ms) | 276.542 ± 58.755 | 230.083 ± 61.679 | <0.001 | 271.472 ± 47.013 | 205.585 ± 39.922 | <0.001 |
| Tei Index (TDI) | 0.594 ± 0.271 | 0.686 ± 0.293 | 1.000 | 0.612 ± 0.487 | 0.776 ± 0.359 | 0.322 |
| ET spectral RVOT (ms) | 271.538 ± 67.002 | 242.615 ± 47.072 | 0.080 | 279.950 ± 45.034 | 232.025 ± 42.225 | <0.001 |
| Transpulmonale T (ms) | 290.045 ± 88.245 | 263.591 ± 60.970 | 0.207 | 288.957 ± 88.241 | 251.957 ± 60.582 | 0.012 |
| Tei Index (Spectral) | 0.081 ± 0.499 | 0.027 ± 0.410 | 1.000 | 0.046 ± 0.356 | −0.101 ± 0.470 | 0.818 |
| Outcome | AMD (Closed–Open) | 95% CI | p | p (Holm) |
|---|---|---|---|---|
| TAPSE (mm) | 1.531 | 0.130 to 2.931 | 0.033 | 0.033 |
| TASV (cm/s) | 1.694 | 0.437 to 2.951 | 0.009 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breuer, H.; Sales, M.C.; Ramnath, N.W.M.; Shieba, Y.; Kolashov, A.; Moza, A.; Tewarie, L.; Zayat, R.; Hatam, N. Pericardial Closure Preserves Early Right Ventricular Function After Cardiac Surgery: A Retrospective Cohort Study. J. Cardiovasc. Dev. Dis. 2025, 12, 431. https://doi.org/10.3390/jcdd12110431
Breuer H, Sales MC, Ramnath NWM, Shieba Y, Kolashov A, Moza A, Tewarie L, Zayat R, Hatam N. Pericardial Closure Preserves Early Right Ventricular Function After Cardiac Surgery: A Retrospective Cohort Study. Journal of Cardiovascular Development and Disease. 2025; 12(11):431. https://doi.org/10.3390/jcdd12110431
Chicago/Turabian StyleBreuer, Hannah, Marjolijn C. Sales, Natasja W. M. Ramnath, Yusuf Shieba, Alish Kolashov, Ajay Moza, Lachmandath Tewarie, Rashad Zayat, and Nima Hatam. 2025. "Pericardial Closure Preserves Early Right Ventricular Function After Cardiac Surgery: A Retrospective Cohort Study" Journal of Cardiovascular Development and Disease 12, no. 11: 431. https://doi.org/10.3390/jcdd12110431
APA StyleBreuer, H., Sales, M. C., Ramnath, N. W. M., Shieba, Y., Kolashov, A., Moza, A., Tewarie, L., Zayat, R., & Hatam, N. (2025). Pericardial Closure Preserves Early Right Ventricular Function After Cardiac Surgery: A Retrospective Cohort Study. Journal of Cardiovascular Development and Disease, 12(11), 431. https://doi.org/10.3390/jcdd12110431






