Ventricular Topology in Congenital Heart Defects Associated with Heterotaxy: Can We Find Patterns Reflecting the Syndrome-Specific Tendency for Visceral Symmetry?
Abstract
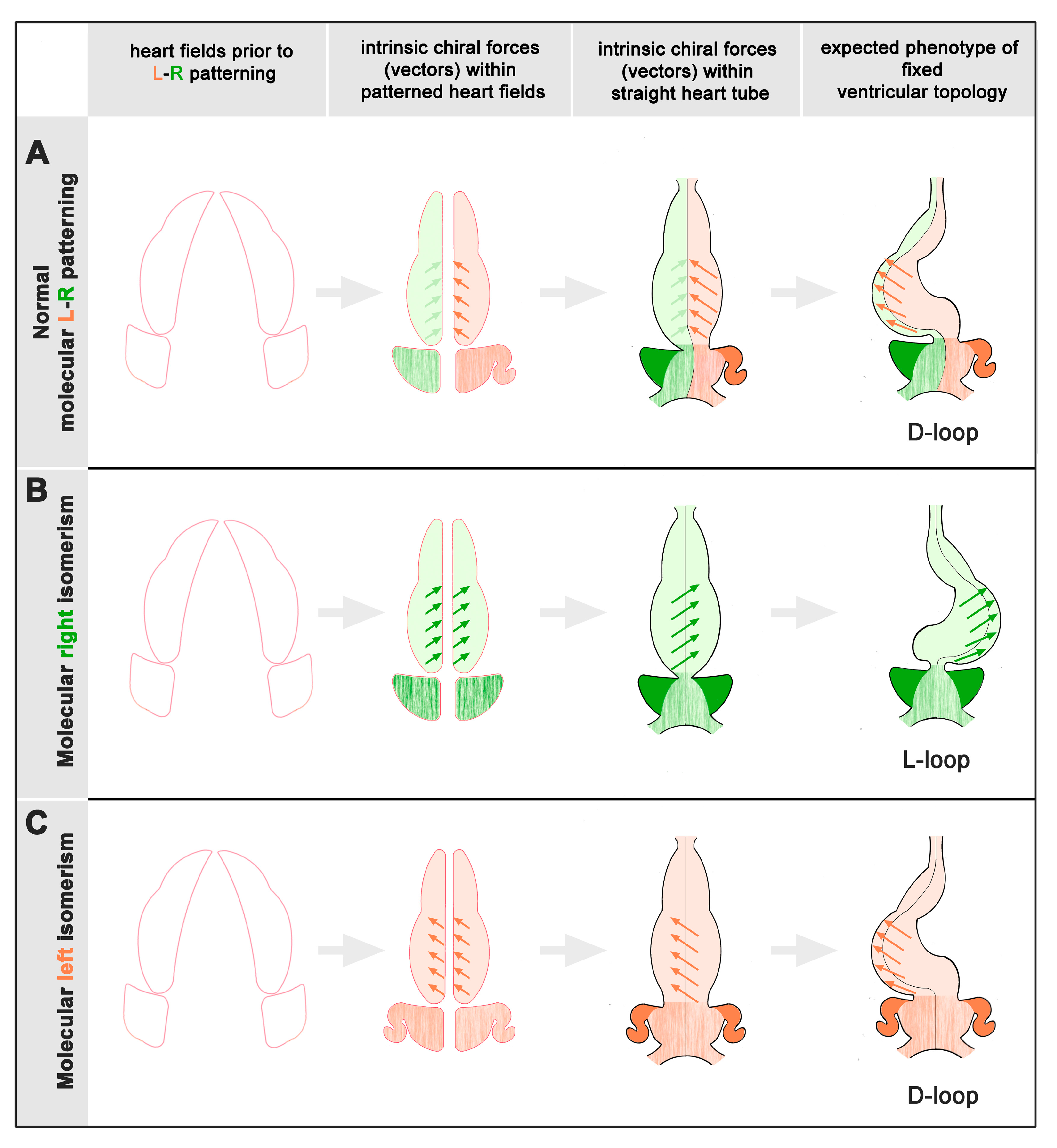
1. Introduction
2. Methods
2.1. Patient Population
- All patients that met our diagnostic criteria for LAI or RAI.
- Patients who did not have good imaging data facilitating correct diagnosis of LAI or RAI.
- Patients who did not meet our inclusion diagnostic criteria for LAI or RAI during detailed data review process despite the fact that they were erroneously labeled or suspected as such.
2.2. Variables
- Demographic data for the patients including sex and date of birth (DOB)
- Mortality
- Duration of follow-up (calculated from date of first echo, date of birth until the date of death or date of last follow-up)
- –
- Ventricular topology; D-hand or L-hand
- –
- Orientation of the cardiac apex; left-sided, right-sided, or midline
- –
- Aortic arch patterning; unilateral left, unilateral right, or bilateral (double aortic arch)
- –
- Superior caval vein patterning; unilateral left, unilateral right, or bilateral
- –
- Inferior caval vein patterning; normal or interrupted
- –
- Anomalies of pulmonary venous drainage; partial or total
- –
- Atrioventricular septal defect; balanced or unbalanced
- –
- Common atrial cavity
- –
- Functionally univentricular heart (FUVH) including the morphological identity of its dominant ventricle
- –
- Hypoplastic left heart syndrome
- –
- Transposition of the great arteries
- –
- Double outlet right ventricle
- –
- Anatomy of pulmonary valve; stenosis, atresia, or normal
- –
- Aortic arch abnormalities; coarctation, interruption, or hypoplasia
- –
- Generation of atrial activity (determined by the p-wave axis) divided into 4 groups:
- Left inferior axis (0 to +90)
- Left superior axis (0 to −90)
- Right inferior axis (+90 to 180)
- Right superior axis (−90 to 180)
- –
- We further analyzed generation of atrial activity by combining the above 4 axis to another 4 groups
- Left axis (combining left inferior axis and left superior axis)
- Right axis (combining right inferior axis and right superior axis)
- Inferior axis (combining left inferior axis and right inferior axis)
- Superior axis (combining left superior axis and right superior axis)
- –
- Presence of documented atrial arrhythmias (SVT, atrial fibrillation, atrial flutter, atrial tachycardia)
- –
- Conduction abnormalities (complete heart block, junctional rhythm whether inter mittent or permanent).
- Complete heart block (congenital)
- Complete heart block (post-op)
- Complete heart block (acquired)
- –
- Ventricular arrhythmias (ventricular fibrillation, ventricular tachycardia)
- –
- Permanent pacemaker insertion
- (1)
- The proportions of ventricular D- and L-hand topology in the whole study population.
- (2)
- The proportions of ventricular D- and L-hand topology in cases of (a) LAI, and (b) RAI.
- (3)
- Statistically significant differences in mortality between LAI and RAI patients.
- (4)
- Statistically significant associations between the type of ventricular topology (D-hand, L-hand) and mortality (a) in the whole study population and (b, c) in the LAI and RAI subsets.
- (5)
- Statistically significant associations between the type of ventricular topology (D-hand, L-hand) and orientation of the cardiac apex, aortic arch patterning, and patterning of SVC and IVC (a) in the whole study population and (b, c) in the two study subsets (LAI, RAI).
- (6)
- Statistically significant associations between the type of ventricular topology (D-hand, L-hand) and specific CHDs (AVSD, functionally univentricular heart, common atrial cavity, TGA, DORV, PV defects, anomalies in pulmonary and systemic venous drainage) (a) in the whole study population and (b, c) in the two study sub-populations (LAI, RAI).
- (7)
- Statistically significant associations between the type of ventricular topology (D-hand, L-hand) and congenital disorders of excitation and conduction (a) in the whole study population and (b, c) in the two study subsets (LAI, RAI).
2.3. Study Design and Data Collection
- (8)
- Usual arrangement (situs solitus) is when the aorta and inferior caval vein lie apart, on opposite sides of the spine, with the aorta on the left.
- (9)
- Mirror-imagery (situs inversus) is the mirror-imaged arrangement with the aorta on the right and the inferior caval vein on the left.
- (10)
- RAI is when the aorta and inferior caval vein are on the same side of the spine, with the vein slightly anterior.
- (11)
- LAI is when the aorta is in the midline and the azygos vein is located in a posterolateral position.
- Morphologic identities of the ventricles were identified based on their distinct morphological features.
- Ventricular topology was determined using the "handedness" method, independent of bodily orientation. Right-hand topology corresponds to D-looped ventricles, and left-hand topology corresponds to L-looped ventricles.
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Size of Study Population and Subsets (Figure 1 and Supplementary Table S2)
3.2. Sex Distribution
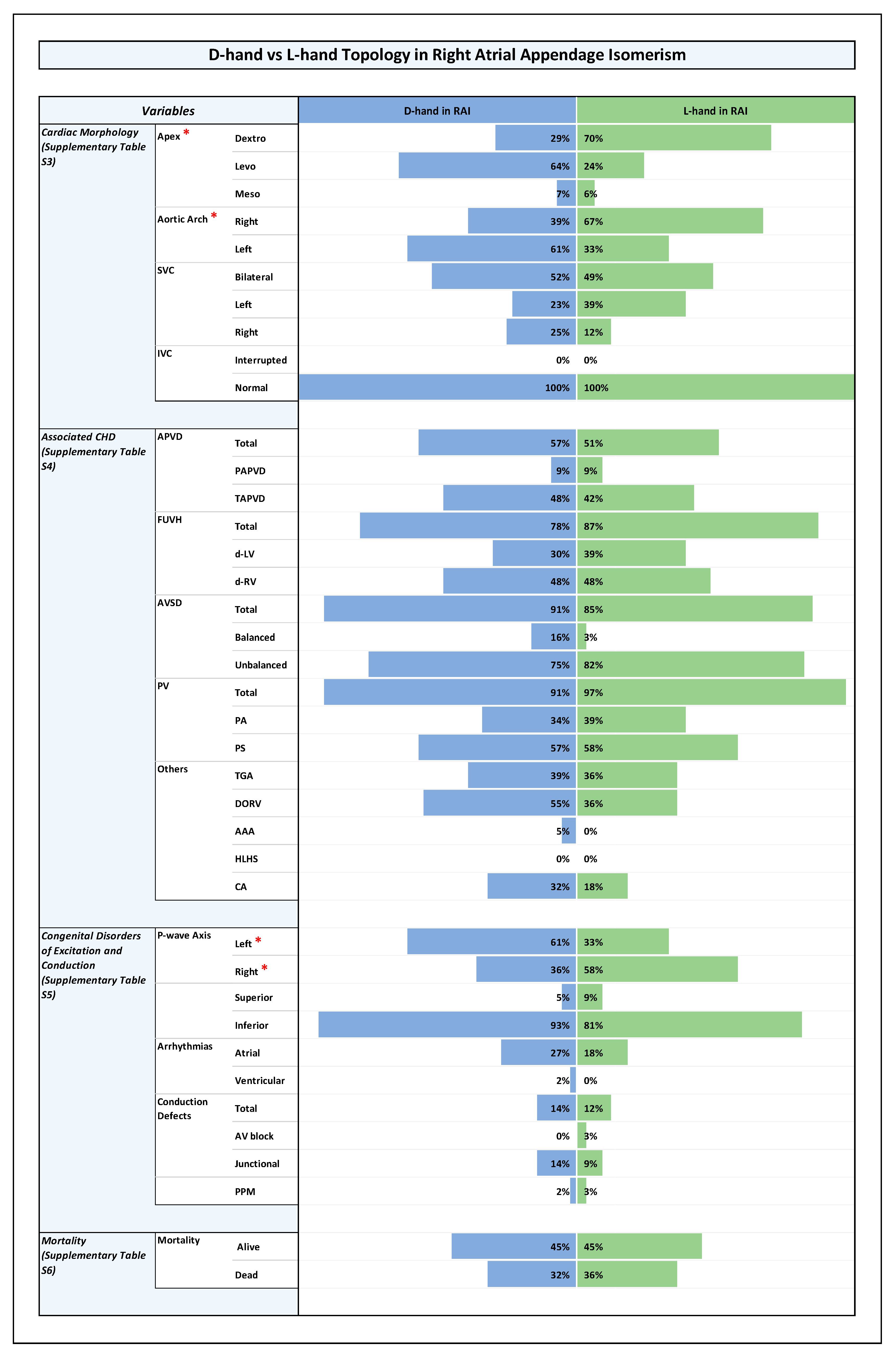
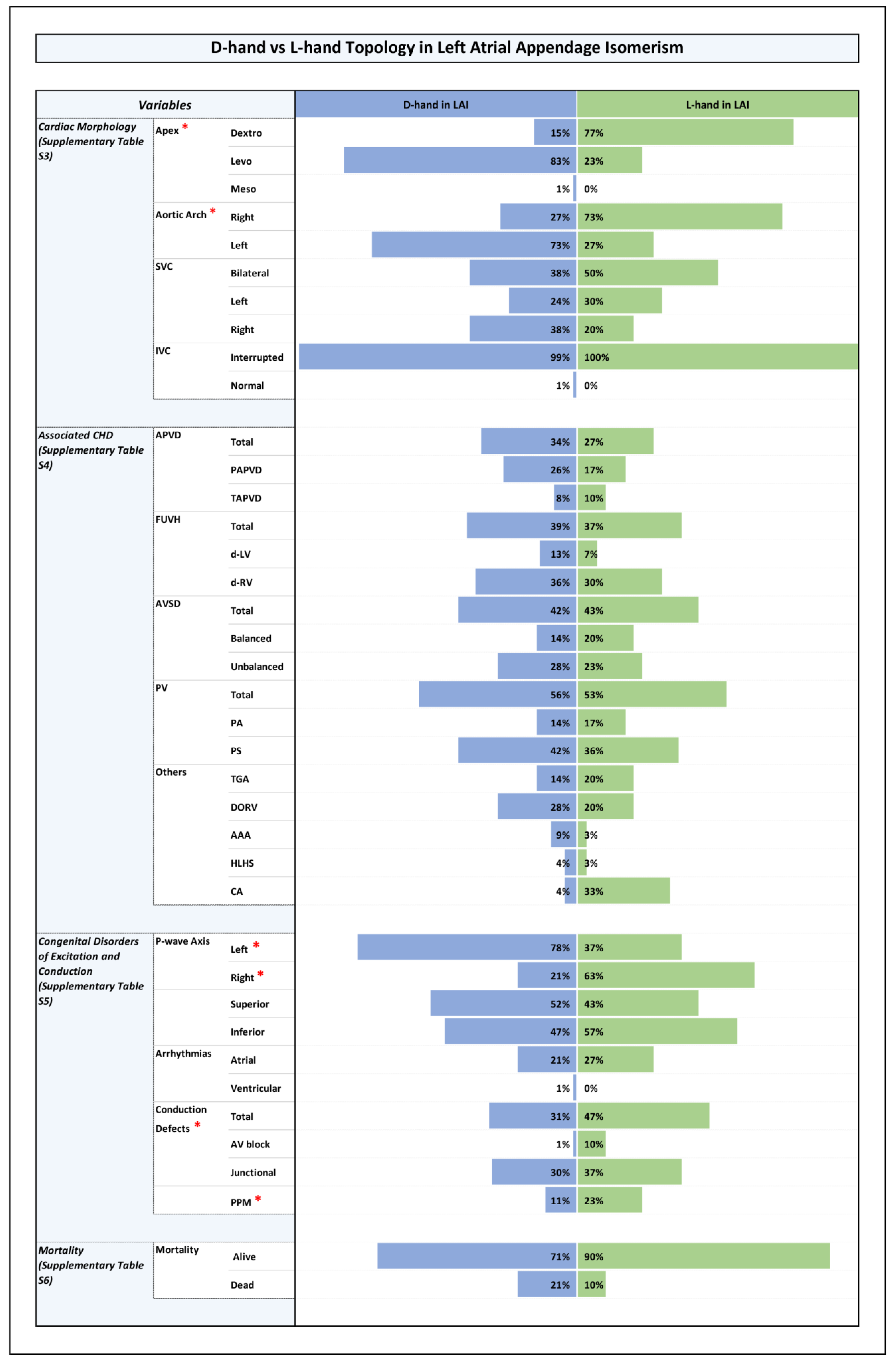
3.3. Distribution of Ventricular Topologies (Figure 1 and Supplementary Table S2)
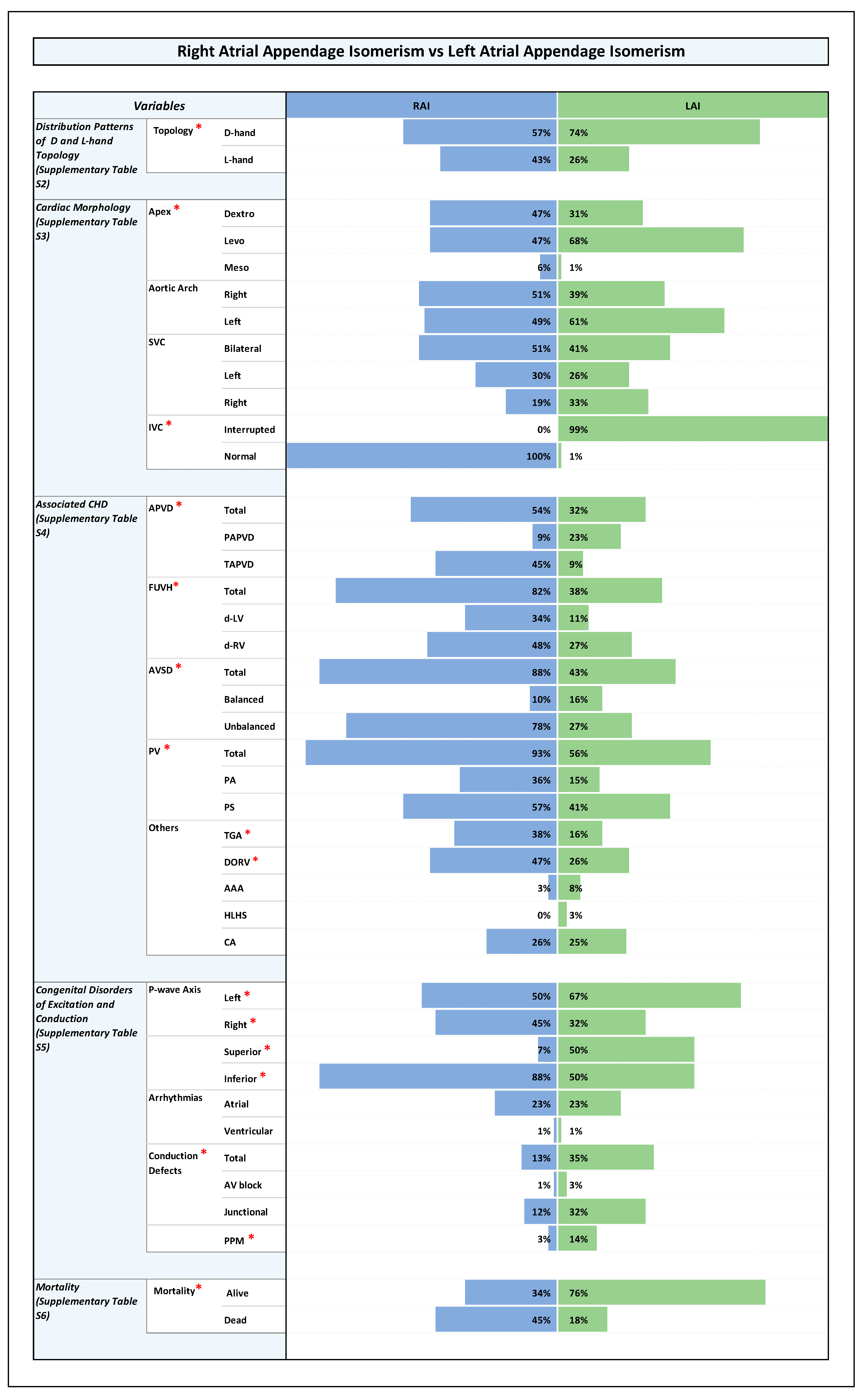
3.4. Orientation of the Cardiac Apex and Patterning of Aortic Arch, SVC, and IVC (Figure 1, Figure 2, Figure 3 and Figure 4 and Supplementary Table S3)
3.4.1. Orientation of the Cardiac Apex
3.4.2. Aortic Arch Patterning (Bilateral, Unilateral Right or Unilateral Left)
3.4.3. Patterning of SVC (Bilateral, Unilateral Right or Unilateral Left)
3.4.4. Patterning of IVC (Normal or Interrupted)
3.5. Associated CHDs (Figure 1, Figure 2, Figure 3 and Figure 4 and Supplementary Table S4)
- –
- Aortic arch abnormalities (coarctation, interruption, or hypoplasia)
- –
- Anomalies in pulmonary venous drainage (partially anomalous, totally anomalous)
- –
- Atrioventricular septal defect (balanced or unbalanced)
- –
- Functionally univentricular hearts with a dominant ventricle of left or right morphology
- –
- Hypoplastic left heart syndrome
- –
- Common atrial cavity
- –
- Transposition of the great arteries
- –
- Double outlet right ventricle
- –
- Anomalies of pulmonary valve (pulmonary stenosis, pulmonary atresia)
- 1.
- Abnormal pulmonary venous drainage (PAPVD), mainly TAPVD.Please note that in the setting of RAI, by definition, the pulmonary veins are connected anomalously, since even if returning to the heart, they are returning to an atrial chamber with a morphologically right atrial appendage.
- 2.
- AVSD, mainly unbalanced AVSD.
- 3.
- Functionally univentricular heart, mostly with a dominant ventricle of right morphology.Please note that in our patient populations data, we have not encountered any case of what some people erroneously call “single ventricle of indeterminate morphology”.
- 4.
- Pulmonary stenosis or atresia.
- 5.
- TGA and DORV.
3.6. Congenital Disorders of Excitation and Conduction (Figure 1, Figure 2, Figure 3 and Figure 4 and Supplementary Table S5)
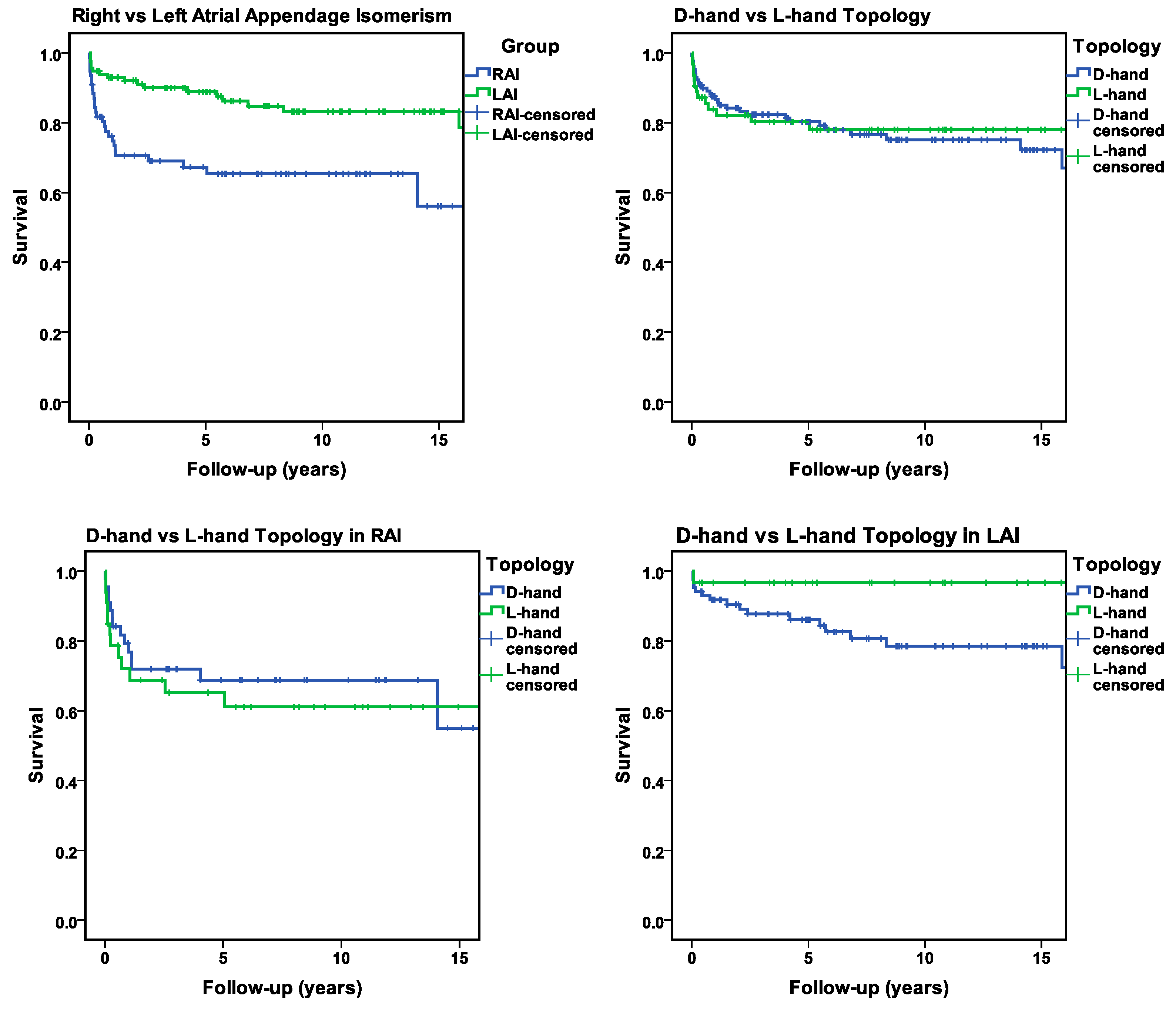
- (1)
- RAI vs. LAI;
- (2)
- Ventricular D-hand topology vs. L-hand topology;
- (3)
- Ventricular D-hand topology in RAI vs. L-hand topology in RAI;
- (4)
- Ventricular D-hand topology in LAI vs. L-hand topology in LAI.
3.7. Mortality and Long-Term Survival
4. Discussion
4.1. Statistical Distribution Patterns of Ventricular D-Hand and L-Hand Topology
4.2. Cardiovascular Anomalies in the Setting of Heterotaxy, Their Distribution Among RAI and LAI Subsets, and Associations with Ventricular D-Hand and L-Hand Topology
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | Aortic arch abnormalities |
| APVD | Anomalous pulmonary venous drainage |
| AV | Atrioventricular |
| AVSD | Atrioventricular septal defect |
| CA | Common atrial cavity |
| CHB-c | Complete heart block (congenital) |
| CHB-ac | Complete heart block (acquired) |
| CHB-po | Complete heart block (post-op) |
| CHD | Congenital heart disease/defects |
| Dextro | Dextro-position of cardiac apex |
| D-hand | D-hand topology |
| d-LV | Dominant left ventricle |
| DORV | Double outlet right ventricle |
| d-RV | Dominant right ventricle |
| ECG | Electrocardiogram |
| FUV | Functionally univentricular heart |
| HLHS | Hypoplastic left heart syndrome |
| IVC | Inferior vena cava |
| IRB | Institutional Review Board |
| JIR | Junctional intermittent rhythm |
| JPR | Junctional persistent rhythm |
| KACC | King Abdulaziz Cardiac Center |
| KAMC | King Abdulaziz Medical City |
| LAI | Left atrial appendage isomerism |
| Levo | Levo-position of cardiac apex |
| LIA | Left inferior axis (0 to +90) |
| LSA | Left superior axis (0 to −90) |
| L-hand | L-hand topology |
| LR | Left right |
| Meso | Meso-position of cardiac apex |
| MRN | Medical record number |
| ODS | Online data supplements |
| P | p-value |
| PA | Pulmonary atresia |
| PAPVD | Partially anomalous pulmonary venous drainage |
| PPM | Permanent pacemaker |
| PS | Pulmonary stenosis |
| PV | Pulmonary valve |
| RAI | Right atrial appendage isomerism |
| RIA | Right inferior axis (+90 to 180) |
| RSA | Right superior axis (−90 to 180) |
| SA | Sinoatrial |
| SVC | Superior vena cava |
| TAPVD | Totally anomalous pulmonary venous drainage |
| TGA | Transposition of the great arteries |
| V | Ventricle |
References
- Ramsdell, A.F. Left–right asymmetry and congenital cardiac defects: Getting to the heart of the matter in vertebrate left–right axis determination. Dev. Biol. 2005, 288, 1–20. [Google Scholar] [CrossRef]
- Nakamura, T.; Hamada, H. Left-right patterning: Conserved and divergent mechanisms. Development 2012, 139, 3257–3262. [Google Scholar] [CrossRef]
- Männer, J. The functional significance of cardiac looping: Comparative embryology, anatomy, and physiology of the looped design of vertebrate hearts. J. Cardiovasc. Dev. Dis. 2024, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Ivemark, B.I. Implications of agenesis of the spleen on the pathogenesis of cono-truncus anomalies in childhood. Acta Paediatr. 1955, 44 (Suppl. 104), 7–113. [Google Scholar] [CrossRef]
- Moller, J.H.; Nakib, A.; Anderson, R.C.; Edwards, J.E. Congenital cardiac disease associated with polysplenia. A developmental complex of bilateral „left-sidedness“. Circulation 1967, 36, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Stanger, P.; Benassi, R.C.; Korns, M.E.; Jue, K.L.; Edwards, J.E. Diagrammatic portrayal of variations in cardiac structure. Reference to transposition, dextrocardia and the concept of four normal hearts. Circulation 1968, 7 (Suppl. 4), 1–16. [Google Scholar]
- Bartram, U.; Wirbelauer, J.; Speer, C.P. Heterotaxy syndrome–asplenia and polysplenia as indicators of visceral malposition and complex congenital heart disease. Neonatology 2005, 88, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Schidlow, D.; Emani, S.M.; Teele, S.A. Heterotaxy syndrome. In Nadas’ Pediatric Cardiology; Walsh, E.P., Mayer, J.E., Teele, S.A., Brown, D.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 418–432. [Google Scholar]
- Jacobs, J.P.; Mavroudis, C.; Jacobs, M.L.; Maruszewski, B.; Tchervenkov, C.I.; Lacour-Gayet, F.G.; Clarke, D.R.; Gaynor, J.W.; Spray, T.L.; Kurosawa, H.; et al. Nomenclature and databases—The past, the present, and the future: A primer for the congenital heart surgeon. Pediatr. Cardiol. 2007, 28, 105–115. [Google Scholar] [CrossRef]
- Loomba, R.S.; Hlavacek, A.M.; Spicer, D.E.; Anderson, R.H. Isomerism or heterotaxy: Which term leads to better understanding? Cardiol. Young 2015, 25, 1037–1043. [Google Scholar] [CrossRef]
- Devine, W.A. What if Ivemark had suggested the term "Syndrome of Visceral Symmetry with Asplenia" instead of "Asplenia, a Teratologic Syndrome of Visceral Heterotaxy“? Cardiol. Young 1992, 2, 108–113. [Google Scholar] [CrossRef]
- Uemura, H.; Ho, S.Y.; Devine, W.A.; Kilpatrick, L.L.; Anderson, R.H. Atrial appendages and venoatrial connections in hearts with patients with visceral heterotaxy. Ann. Thorac. Surg. 1995, 60, 561–569. [Google Scholar] [CrossRef]
- De Tommasi, S.; Daliento, L.; Ho, S.Y.; Macartney, F.J.; Anderson, R.H. Analysis of atrioventricular junction, ventricular mass, and ventriculoarterial junction in 43 specimens with atrial isomerism. Br. Heart J. 1981, 45, 236–247. [Google Scholar] [CrossRef]
- Ho, S.Y.; Seo, J.W.; Brown, N.A.; Cook, A.C.; Fagg, N.L.; Anderson, R.H. Morphology of the sinus node in human and mouse hearts with isomerism of the atrial appendages. Br. Heart J. 1995, 74, 437–442. [Google Scholar] [CrossRef]
- Smith, A.; Ho, S.Y.; Anderson, R.H.; Connell, M.G.; Arnold, R.; Wilkinson, J.L.; Cook, A.C. The diverse cardiac morphology seen in hearts with isomerism of the atrial appendages with reference to the disposition of the specialised conduction system. Cardiol. Young 2006, 16, 437–454. [Google Scholar] [CrossRef]
- Anderson, R.H.; Brown, N.A.; Meno, C.; Spicer, D.E. The importance of being isomeric. Clin. Anat. 2015, 28, 477–486. [Google Scholar] [CrossRef]
- Loomba, R.S.; Ahmed, M.M.; Spicer, D.E.; Backer, C.L.; Anderson, R.H. Manifestations of bodily isomerism. Cardiovasc. Pathol. 2016, 25, 173–180. [Google Scholar] [CrossRef]
- Anderson, R.H.; Spicer, D.E.; Loomba, R.S. Is an appreciation of isomerism the key to unlocking the mysteries of cardiac findings in heterotaxy? J. Cardiovasc. Dev. Dis. 2018, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Ticho, B.S.; Goldstein, A.M.; Van Praagh, R. Extracardiac anomalies in the heterotaxy syndromes with focus on anomalies of midline-associated structures. Am. J. Cardiol. 2000, 85, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.M.; Ticho, B.S.; Fishman, M.C. Patterning the heart’s left-right axis: From zebrafish to man. Dev. Genet. 1998, 22, 278–287. [Google Scholar] [CrossRef]
- Lander, A.; King, T.; Brown, N.A. Left–right development: Mammalian phenotypes and conceptual models. Semin. Cell Dev. Biol. 1998, 9, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Webb, S.; Brown, N.A. Defective lateralisation in children with congenitally malformed hearts. Cardiol. Young 1998, 8, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Campione, M.; Ros, M.A.; Icardo, J.M.; Piedra, E.; Christoffels, V.M.; Schweickert, A.; Blum, M.; Franco, D.; Moorman, A.F. Pitx2 expression defines a left cardiac lineage of cells: Evidence for atrial and ventricular molecular isomerism in the iv/iv mice. Dev. Biol. 2001, 231, 252–264. [Google Scholar] [CrossRef]
- Männer, J. The anatomy of cardiac looping: A step towards the understanding of the morphogenesis of several forms of congenital malformations. Clin. Anat. 2009, 22, 21–35. [Google Scholar] [CrossRef]
- Moorman, A.F.M.; Soufan, A.T.; Hagoort, J.; De Boer, P.A.J.; Christoffels, V.M. Development of the building plan of the heart. Ann. N. Y. Acad. Sci. 2004, 1015, 171–181. [Google Scholar] [CrossRef]
- Palmer, A.R. Symmetry breaking and the evolution of development. Science 2004, 306, 828–833. [Google Scholar] [CrossRef]
- Inaki, M.; Liu, J.; Matsuno, K. Cell chirality: Its origin and roles in left–right asymmetric development. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150403. [Google Scholar] [CrossRef]
- Ray, P.; Chin, A.S.; Worley, K.E.; Fan, J.; Kaur, G.; Wu, M.; Wan, L.Q. Intrinsic cellular chirality regulates left–right symmetry breaking during cardiac looping. Proc. Nat. Acad. Sci. USA 2018, 115, E11568–E11577. [Google Scholar] [CrossRef]
- Rahman, T.; Zhang, H.; Fan, J.; Wan, L.Q. Cell chirality in cardiovascular development and disease. APL Bioeng. 2020, 4, 031503. [Google Scholar] [CrossRef] [PubMed]
- Ekman, G. Experimentelle Beiträge zur Herzentwicklung der Amphibien. Roux’s Arch. Dev. Biol. 1925, 106, 320–352. [Google Scholar] [CrossRef] [PubMed]
- Copenhaver, W.M. Experiments on the development of the heart of Amblystoma punctatum. J. Exp. Zool. 1926, 43, 321–371. [Google Scholar] [CrossRef]
- Goerttler, K. Die Bedeutung der ventrolateralen Mesodermbezirke für die Herzanlage der Amphibienkeime. Anat. Anz. 1928, 66 (Suppl. 2), 132–139. [Google Scholar]
- DeHaan, R. Cardia bifida and the development of pacemaker function in the early chick heart. Dev. Biol. 1959, 1, 586–602. [Google Scholar] [CrossRef]
- Zwirner, R.; Kuhlo, B. Die prospektive Potenz der rechten und der linken Herzanlage (ein experimenteller Beitrag zur Asymmetrie des Herzens). Roux’s Arch. Dev. Biol. 1964, 155, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Ginard, B.; Paz Garcia, M. The morphologic expression of each cardiac primordium in the chick embryo. J. Embryol. Exp. Morphol. 1972, 28, 141–152. [Google Scholar] [CrossRef]
- Weiss, P.A. Entwicklungsphysiologie der Tiere; Steinkopff: Dresden, Germany; Leipzig, Germany, 1930. [Google Scholar]
- Taber, L.A. Biophysical mechanisms of cardiac looping. Int. J. Dev. Biol. 2006, 50, 323–332. [Google Scholar] [CrossRef]
- Taber, L.A.; Voronov, D.A.; Ramasubramanian, A. The role of mechanical forces in the torsional component of cardiac looping. Ann. N. Y. Acad. Sci. 2010, 1188, 103–110. [Google Scholar] [CrossRef]
- Shi, Y.; Yao, J.; Young, J.M.; Fee, J.A.; Perucchio, R.; Taber, L.A. Bending and twisting the embryonic heart: A computational model for c-looping based on realistic geometry. Front. Physiol. 2014, 5, 297. [Google Scholar] [CrossRef]
- Bayraktar, M.; Männer, J. Cardiac looping may be driven by compressive loads resulting from unequal growth of the heart and pericardial cavity. Observations on a physical simulation model. Front. Physiol. 2014, 5, 112. [Google Scholar] [CrossRef]
- Desgrange, A.; Le Garrec, J.F.; Meilhac, S.M. Left-right asymmetry in heart development and disease: Forming the right loop. Development 2018, 145, dev162776. [Google Scholar] [CrossRef] [PubMed]
- Desgrange, A.; Le Garrec, J.F.; Bernheim, S.; Bønnelykke, T.H.; Meilhac, S.M. Transient nodal signaling in left precursors coordinates opposed asymmetries shaping the heart loop. Dev. Cell 2020, 55, 413–431. [Google Scholar] [CrossRef]
- Huhta, J.C.; Smallhorn, J.F.; MacCartney, F.J. Two dimensional echocardiographic diagnosis of situs. Br. Heart J. 1982, 48, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, R.S.; Alharbi, S.H.; Tuwaijri, R.M.; Alzomaili, B.T.; Althubaiti, A.; Yelbuz, T.M. Transposition of the great arteries: A laterality defect in the group of heterotaxy syndromes or an outflow tract malformation? Ann. Pediatr. Cardiol. 2018, 11, 237–249. [Google Scholar] [CrossRef]
- Crucean, A.C.; Spicer, D.E.; Anderson, R.H. The Significance of Ventricular Topology in the Analysis of Congenitally Malformed Hearts. J. Cardiovasc. Dev. Dis. 2022, 9, 155. [Google Scholar] [CrossRef]
- Yoo, S.J.; Saprungruang, A.; Lam, C.Z.; Anderson, R.H. Disharmonious Ventricular Relationship and Topology for the Given Atrioventricular Connections. Contemporary Diagnostic Approach Using 3D Modeling and Printing. Congenit. Heart Dis. 2022, 17, 495–504. [Google Scholar] [CrossRef]
- Costa, S.; Carriço, A.; Valente, F. Prenatal diagnosis of left isomerism with normal heart. Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef]
- Kiram, V.S.; Choudhary, S.; Shaik, A.; Gadabanahalli, K.; Raj, V.; Bhat, V. Spectrum of cardiac anomalies associated with heterotaxy: A single-center study of a large series based on computed tomography. Pediatr. Cardiol. 2020, 41, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Oreto, L.; Mandraffino, G.; Ciliberti, P.; Santangelo, T.P.; Romeo, P.; Celona, A.; Gitto, P.; Galetti, L.; Iorio, F.S.; Di Pino, A.; et al. Classifying anomalies in right and left isomerism: Concordant and discordant patterns. Congenit. Heart Dis. 2023, 18, 97–111. [Google Scholar] [CrossRef]
- Carvalho, J.S.; Rigby, M.L.; Shinebourne, E.A.; Anderson, R.H. Cross sectional echocardiography for recognition of ventricular topology in atrioventricular septal defect. Heart 1989, 61, 285–288. [Google Scholar] [CrossRef]
- Vairo, U.; Marino, B.; Parretti di Iulio, D.; Guccione, P.; Carotti, A.; Formigari, R.; Grazioli, S.; Bolla, G.; Squitieri, C.; Pasquini, L. Ventricular-infundibular morphology in visceral heterotaxia with left isomerism. An echocardiographic-angiocardiographic study. G. Ital. Cardiol. 1991, 21, 969–974. [Google Scholar]
- Francalanci, P.; Marino, B.; Boldrini, R.; Abella, R.; Iorio, F.; Bosman, C. Morphology of the atrioventricular valve in asplenia syndrome: A peculiar type of atrioventricular canal defect. Cardiovasc. Pathol. 1996, 5, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Cook, A.; Anderson, R.H.; Allan, L.D.; Fagg, N. Isomerism of the atrial appendages in the fetus. Pediatr. Pathol. 1991, 11, 589–608. [Google Scholar] [CrossRef]
- Tremblay, C.; Loomba, R.S.; Frommelt, P.C.; Perrin, D.; Spicer, D.E.; Backer, C.; Anderson, R.H. Segregating bodily isomerism or heterotaxy: Potential echocardiographic correlations of morphological findings. Cardiol. Young 2017, 27, 1470–1480. [Google Scholar] [CrossRef]
- Stanger, P.; Abraham, M.R.; Edwards, J.E. Cardiac malpositions. An overview based on sixty-five necropsy specimens. Circulation 1977, 56, 159–172. [Google Scholar] [CrossRef]
- Yildirim, S.V.; Tokel, K.; Varan, B.; Aslamaci, S.; Ekici, E. Clinical investigations over 13 years to establish the nature of the cardiac defects in patients having abnormalities of lateralization. Cardiol. Young 2007, 17, 275–282. [Google Scholar] [CrossRef]
- Berg, C.; Geipel, A.; Kamil, D.; Knüppel, M.; Breuer, J.; Krapp, M.; Baschat, A.; Germer, U.; Hansmann, M.; Gembruch, U. The syndrome of left atrial isomerism. Sonographic findings and outcome in prenatally diagnosed cases. J. Ultrasound Med. 2005, 24, 921–931. [Google Scholar] [CrossRef]
- Buca, D.I.P.; Khalil, A.; Rizzo, G.; Familiari, A.; Di Giovanni, S.; Liberati, M.; Murgano, D.; Ricciardulli, A.; Fanfani, F.; Scambia, G.; et al. Outcome of prenatally diagnosed fetal heterotaxy: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Männer, J. Spontaneous Left Cardiac Isomerism in chick embryos: Case report, review of the literature, and possible significance for the understanding of ventricular non-compaction cardiomyopathy in the setting of human heterotaxy syndromes. J. Cardiovas. Dev. Dis. 2019, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Xia, H.; Deng, S. Genetic basis of human left-right asymmetry disorders. Expert Rev. Mol. Med. 2014, 16, e19. [Google Scholar] [CrossRef]
- Icardo, J.M.; Sanchez de Vega, M.J. Spectrum of heart malformations in mice with situs solitus, situs inversus, and associated visceral heterotaxy. Circulation 1991, 84, 2547–2558. [Google Scholar] [CrossRef] [PubMed]
- Weininger, W.J.; Lopes Floro, K.; Bennett, M.B.; Withington, S.L.; Preis, J.I.; Barbera, J.P.; Mohun, T.J.; Dunwoodie, S.L. Cited2 is required both for heart morphogenesis and establishment of the left-right axis in mouse development. Development 2005, 132, 1337–1348. [Google Scholar] [CrossRef]
- Bamforth, S.D.; Braganca, J.; Farthing, C.R.; Schneider, J.E.; Broadbent, C.; Michell, A.C.; Clarke, K.; Neubauer, S.; Norris, D.; Brown, N.; et al. Cited2 controls left-right patterning and heart development through a Nodal-Pitx2c pathway. Nat. Genet. 2004, 11, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Meno, C.; Shimono, A.; Saijoh, Y.; Yashiro, K.; Mochida, K.; Ohishi, S.; Noji, S.; Kondho, H.; Hamada, H. Lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal. Cell 1998, 94, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Capdevila, J.; Tamura, K.; Ruiz-Lozano, P.; Rodriguez-Esteban, C.; Yonei-Tamura, S.; Magallon, J.; Chandraratna, R.A.; Chien, K.; Blumberg, B.; et al. Multiple left-right asymmetry defects in Shh (-/-) mice unveil a convergence of the Shh and retinoic acid pathways in the control of Lefty-1. Proc. Nat. Acad. Sci. USA 1999, 96, 11356–11381. [Google Scholar] [CrossRef]
- Hildreth, V.; Webb, S.; Chaudry, B.; Peat, J.D.; Phillips, H.M.; Brown, N.; Anderson, R.H.; Henderson, D.J. Left cardiac isomerism in the sonic hedgehog null mouse. J. Anat. 2009, 214, 894–904. [Google Scholar] [CrossRef]
- Lin, C.R.; Kioussi, C.; O’Cannell, S.; Briata, P.; Szeto, D.; Liu, F.; Izpisua-Belmonte, J.C.; Rosenfeld, M.G. Pitx2 regulated lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature 1999, 401, 279–282. [Google Scholar] [CrossRef]
- Green, J.M.; Chiaramida, A.J. 12-Lead EKG Confidence: A Step-by-Step Guide; Springer Publishing Company: Berlin/Heidelberg, Germany, 2014; pp. 115–117. [Google Scholar]
- Surawicz, B.; Knilans, T. Chou’s Electrocardiography in Clinical Practice: Adult and Pediatric; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008; pp. 8–9. [Google Scholar]
- Congdon, E.D. Transformation of the aortic arch system during development of the human embryo. Contrib. Embryol. 1922, 68, 49–110. [Google Scholar]
- Rana, M.S.; Sizarov, A.; Christoffels, V.M.; Moorman, A.F.M. Development of the human aortic arch system captured in an interactive three-dimensional reference model. Am. J. Med. Genet. A 2014, 164, 1372–1383. [Google Scholar] [CrossRef]
- Anderson, R.H.; Bamforth, S.D. Morphogenesis of the mammalian aortic arch arteries. Front. Cell Dev. Biol. 2022, 10, 892900. [Google Scholar] [CrossRef]
- Yashihiro, K.; Shiratori, H.; Hamada, H. Haemodynamics determined by a genetic program govern asymmetric development of the aortic arch. Nature 2007, 450, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Hastreiter, A.R.; D’Cruz, I.A.; Cantez, T. Right-sided aortic arch. Part I: Occurrence of right aortic arch in various types of congenital heart disease. Br. Heart J. 1966, 28, 722–725. [Google Scholar] [CrossRef]
- Rose, V.; Izukawa, T.; Moes, C.A.F. Syndromes of asplenia and polysplenia. A review of cardiac and non-cardiac malformations in 60 cases with special reference to diagnosis and prognosis. Br. Heart J. 1975, 37, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Peoples, W.M.; Moller, J.H.; Edwards, J.E. Polysplenia: A review of 146 cases. Pediatr. Cardiol. 1983, 4, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, W.J.; Teslovich, N.C.; Dur, O.; Keller, B.B.; Pekkan, K. Computational hemodynamics optimization predicts dominant aortic arch selection is driven by embryonic outflow tract orientation in the chick embryo. Biomech. Model. Mechanobiol. 2012, 11, 1057–1073. [Google Scholar] [CrossRef] [PubMed]

| RAI | LAI | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Case No. | D-Hand | L-Hand | Undetermined | Case No. | D-Hand | L-Hand | Undetermined |
| Stanger et al. [55] | 23 * | 34.8% (8) | 65.2% (15) | 17 * | 70.6% (12) | 29.4% (5) | ||
| Carvalho et al. [50] | 13 | 54% (7) | 46% (6) | 12 | 50% (6) | 50% (6) | ||
| Vairo et al. [51] | 28 | 53% (15) | 47% (13) | |||||
| Francalanci et al. [52] | 33 * | 60.6% (20) | 39.4% (13) | |||||
| Ho et al. [53] | 10 * | 60% (6) | 40% (4) | 20 * | 65% (13) | 10% (2) | 25% (5) | |
| Uemura et al. [12] | 125 * | 54% (68) | 42% (52) | 4% (5) | 58 * | 79% (46) | 16% (9) | 5% (3) |
| Smith et al. [15] | 10 * | 20% (2) | 20% (2) | 60% (6) | 25 * | 60% (15) | 32% (8) | 8% (2) |
| Yildirim et al. [56] | 43 | 53.5% (23) | 18.6% (8) | 27.9% (12) | 88 | 59.1% (52) | 22.7% (20) | 18.2% (16) |
| Loomba et al. [17] | 37 * | 50% | 42% | 8% | 12 * | 47% | 53% | |
| Tremblay et al. [54] | 131 * | 61% (80) | 36% (47) | 3% (4) | 56 * | 66% (37) | 34% (19) | |
| Kiram et al. [48] | 184 | 57.6% (106) | 41.9% (77) | 0.5% (1) | 118 | 66.1% (78) | 33.1% (39) | 0.8% (1) |
| Oreto et al. [49] | 43 | 60.5% (26) | 39.5% (17) | 35 | 57% (20) | 43% (15) | ||
| Pooled data | 652 | 55.4% (361) | 39.9% (260) | 4.7% (31) | 469 | 63.8% (299) | 30.5% (143) | 5.7% (27) |
| RAI | LAI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Case No. | Left Arch | Right Arch | Double Arch | Undetermined | Case No. | Left Arch | Right Arch | Double Arch | Undetermined |
| Ho et al. [53] | 10 * | 50% (5) | 50% (5) | 20 * | 75% (15) | 25% (5) | ||||
| Francalanci et al. [52] | 33 * | 60.6% (20) | 39.4% (13) | |||||||
| Smith et al. [15] | 10 * | 90% (9) | 10% (1) | 25 * | 88% (22) | 12% (3) | ||||
| Loomba et al. [17] | 37 * | 70% (26) | 30% (11) | 12 * | 95% (11) | 5% (1) | ||||
| Tremblay et al. [54] | 131 * | 63% (77) | 37% (46) | 8 | 57 * | 75% (41) | 25% (15) | 1 | ||
| Kiran et al. [48] | 184 | 46.7% (86) | 53.3% (98) | 118 | 60% (71) | 40% (47) | ||||
| Oreto et al. [49] | 43 | 70% (30) | 30% (13) | 35 | 51% (18) | 49% (17) | ||||
| Pooled data | 448 | 56.5% (253) | 41.7% (187) | 1.8% (8) | 267 | 66.6% (178) | 33% (88) | 0.4% (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, T.; Alsaiad, A.M.A.; Jijeh, A.M.Z.; Männer, J.; Yelbuz, T.M. Ventricular Topology in Congenital Heart Defects Associated with Heterotaxy: Can We Find Patterns Reflecting the Syndrome-Specific Tendency for Visceral Symmetry? J. Cardiovasc. Dev. Dis. 2025, 12, 430. https://doi.org/10.3390/jcdd12110430
Othman T, Alsaiad AMA, Jijeh AMZ, Männer J, Yelbuz TM. Ventricular Topology in Congenital Heart Defects Associated with Heterotaxy: Can We Find Patterns Reflecting the Syndrome-Specific Tendency for Visceral Symmetry? Journal of Cardiovascular Development and Disease. 2025; 12(11):430. https://doi.org/10.3390/jcdd12110430
Chicago/Turabian StyleOthman, Takhfif, Abdulsalam Mohammad Adnan Alsaiad, Abdulraouf M. Z. Jijeh, Jörg Männer, and Talat Mesud Yelbuz. 2025. "Ventricular Topology in Congenital Heart Defects Associated with Heterotaxy: Can We Find Patterns Reflecting the Syndrome-Specific Tendency for Visceral Symmetry?" Journal of Cardiovascular Development and Disease 12, no. 11: 430. https://doi.org/10.3390/jcdd12110430
APA StyleOthman, T., Alsaiad, A. M. A., Jijeh, A. M. Z., Männer, J., & Yelbuz, T. M. (2025). Ventricular Topology in Congenital Heart Defects Associated with Heterotaxy: Can We Find Patterns Reflecting the Syndrome-Specific Tendency for Visceral Symmetry? Journal of Cardiovascular Development and Disease, 12(11), 430. https://doi.org/10.3390/jcdd12110430








