Abnormal Blood Biomarkers and Cumulative Disability Burden in Middle-Aged and Older Adults: Evidence from Two Nationally Representative Surveys in the United States and China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. The Health and Retirement Study (HRS)
2.1.2. The China Health and Retirement Longitudinal Study (CHARLS)
2.2. Defining Abnormal Levels of Blood Biomarkers in Different Biological Systems
2.3. Assessment of Disability
2.4. Covariates
2.5. Statistical Analysis
2.6. Sensitivity Analysis
3. Results
3.1. Characteristics of the Participants
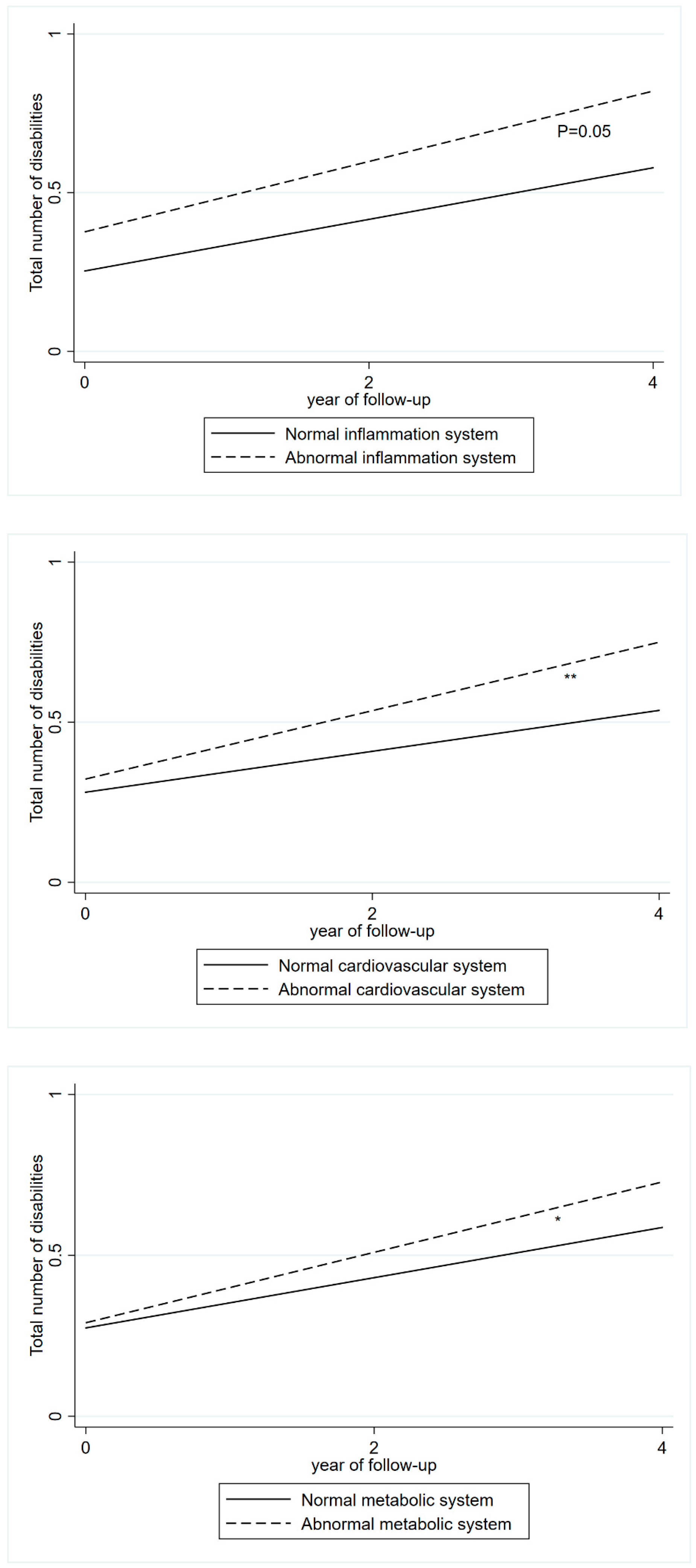
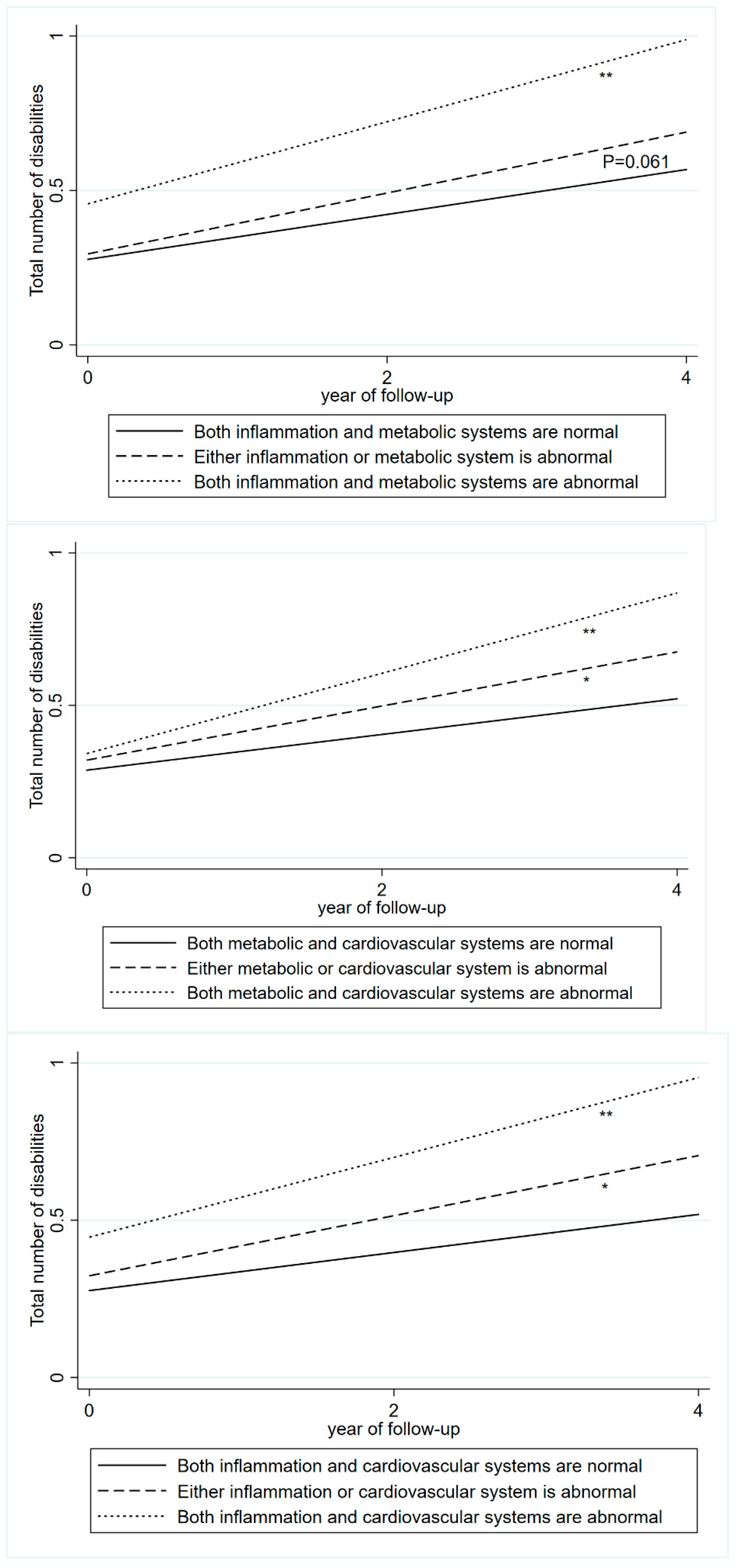
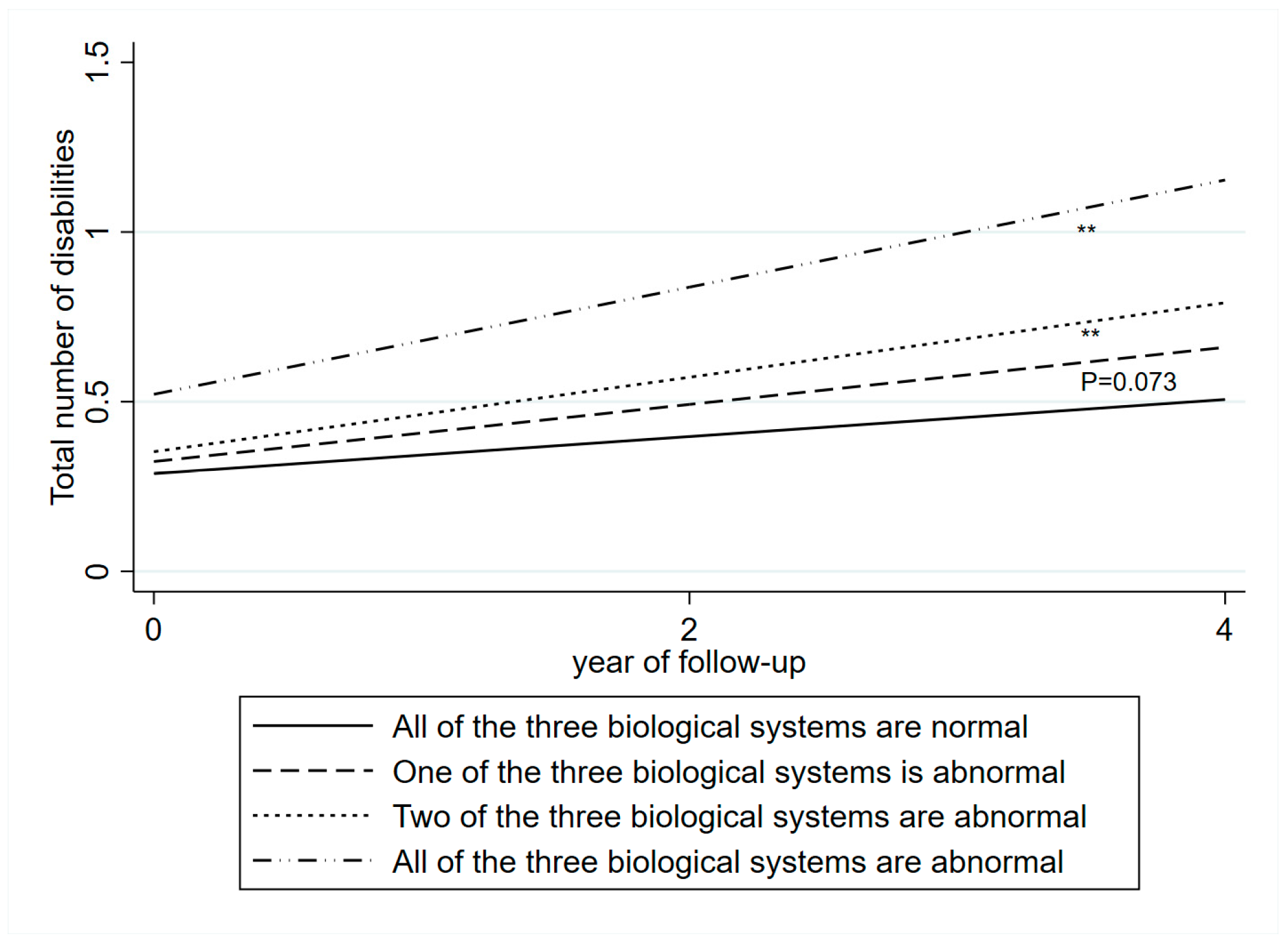
3.2. Blood Biomarkers and Disabilities
3.3. Sensitivity Analysis
4. Discussion
4.1. Comparison with Previous Studies
4.2. Methodological Considerations and Cohort Differences
4.3. The Modifying Role of Education
4.4. Possible Mechanisms
4.5. Public Health and Clinical Implications
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | Activities of daily living |
| AL | Allostatic load |
| ANOVA | Analysis of variance |
| BMI | Body mass index |
| CES-D | Center for Epidemiological Studies Depression scale |
| CHARLS | China Health and Retirement Longitudinal Study |
| China CDC | Chinese Center for Disease Control and Prevention |
| CRP | C-reactive protein |
| DBP | Diastolic blood pressure |
| DBS | Dried blood spot |
| ELISA | Enzyme-linked immunosorbent assay |
| HbA1c | Glycosylated hemoglobin |
| HDL-C | High-density lipoprotein cholesterol |
| HRS | Health and Retirement Study |
| IADL | Instrumental activities of daily living |
| SBP | Systolic blood pressure |
References
- Hosseinpoor, A.R.; Stewart Williams, J.; Jann, B.; Kowal, P.; Officer, A.; Posarac, A.; Chatterji, S. Social determinants of sex differences in disability among older adults: A multi-country decomposition analysis using the World Health Survey. Int. J. Equity Health 2012, 11, 52. [Google Scholar] [CrossRef]
- Dhamoon, M.S.; Cheung, Y.-K.; Moon, Y.P.; Wright, C.B.; Willey, J.Z.; Sacco, R.; Elkind, M.S. C-reactive protein is associated with disability independently of vascular events: The Northern Manhattan Study. Age Ageing 2017, 46, 77–83. [Google Scholar] [CrossRef]
- Hajjar, I.; Lackland, D.T.; Cupples, L.A.; Lipsitz, L.A. Association between concurrent and remote blood pressure and disability in older adults. Hypertension 2007, 50, 1026–1032. [Google Scholar] [CrossRef]
- Odden, M.C.; Rawlings, A.M.; Arnold, A.M.; Cushman, M.; Biggs, M.L.; Psaty, B.M.; Newman, A.B. Patterns of cardiovascular risk factors in old age and survival and health status at 90. J. Gerontol. Ser. A 2020, 75, 2207–2214. [Google Scholar] [CrossRef]
- Sanders, J.L.; Boudreau, R.M.; Penninx, B.W.; Simonsick, E.M.; Kritchevsky, S.B.; Satterfield, S.; Harris, T.B.; Bauer, D.C.; Newman, A.B.; Study, H.A. Association of a Modified Physiologic Index with mortality and incident disability: The Health, Aging, and Body Composition study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2012, 67, 1439–1446. [Google Scholar] [CrossRef]
- Wu, C.; Newman, A.B.; Dong, B.R.; Odden, M.C. Index of healthy aging in Chinese older adults: China health and retirement longitudinal study. J. Am. Geriatr. Soc. 2018, 66, 1303–1310. [Google Scholar] [CrossRef]
- Vu, T.-H.T.; Lloyd-Jones, D.M.; Liu, K.; Stamler, J.; Garside, D.B.; Daviglus, M.L. Optimal levels of all major cardiovascular risk factors in younger age and functional disability in older age: The Chicago Heart Association Detection Project in Industry 2016, 32-year follow-up health survey. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 355–363. [Google Scholar] [CrossRef]
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 2021, 90, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Juster, R.P.; McEwen, B.S.; Lupien, S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Robledo, L.M.; García-Chanes, R.E.; Pérez-Zepeda, M.U. Allostatic Load as a Biological Substrate to Intrinsic Capacity: A Secondary Analysis of CRELES. J. Nutr. Health Aging 2019, 23, 788–795. [Google Scholar] [CrossRef]
- Freire, A.; Barbosa, J.F.S.; Pereira, D.S.; Gomes, C.D.S.; Guerra, R.O. Allostatic load and stress biomarkers in a sample of community-dwelling older adults. Arch. Gerontol. Geriatr. 2020, 87, 104006. [Google Scholar] [CrossRef]
- Seplaki, C.L.; Goldman, N.; Glei, D.; Weinstein, M. A comparative analysis of measurement approaches for physiological dysregulation in an older population. Exp. Gerontol. 2005, 40, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Seplaki, C.L.; Goldman, N.; Weinstein, M.; Lin, Y.-H. Measurement of cumulative physiological dysregulation in an older population. Demography 2006, 43, 165–183. [Google Scholar] [CrossRef]
- Duignan, L.; Dutton, D. Comprehensiveness vs Efficiency: A Cross-Sectional Analysis of the Association Between Allostatic Load and the Frailty Index Using the CLSA. J. Am. Med. Dir. Assoc. 2025, 26, 105829. [Google Scholar] [CrossRef] [PubMed]
- Germano, M.L.; dos Santos Gomes, C.; de Souza Barbosa, J.F.; Neto, N.J.; Pereira, D.S.; Ahmed, T.; Borrero, C.L.C.; Guerra, R.O. Allostatic load and physical performance in older adults: Findings from the International Mobility in Aging Study (IMIAS). Arch. Gerontol. Geriatr. 2023, 109, 104961. [Google Scholar] [CrossRef]
- Gruenewald, T.L.; Seeman, T.E.; Ryff, C.D.; Karlamangla, A.S.; Singer, B.H. Combinations of biomarkers predictive of later life mortality. Proc. Natl. Acad. Sci. USA 2006, 103, 14158–14163. [Google Scholar] [CrossRef] [PubMed]
- Gruenewald, T.L.; Seeman, T.E.; Karlamangla, A.S.; Sarkisian, C.A. Allostatic load and frailty in older adults. J. Am. Geriatr. Soc. 2009, 57, 1525–1531. [Google Scholar] [CrossRef]
- Arkesteijn, M.; Bennett, R.; Davies, J.L.; Sumner, R.C. Does allostatic load in 50–89-year-olds predict the development of frailty? Evidence from a national longitudinal study over 12 years. Stress Health 2025, 41, e3517. [Google Scholar] [CrossRef]
- López-Cepero, A.; McClain, A.C.; Rosal, M.C.; Tucker, K.L.; Mattei, J. Examination of the Allostatic Load Construct and Its Longitudinal Association with Health Outcomes in the Boston Puerto Rican Health Study. Psychosom. Med. 2022, 84, 104–115. [Google Scholar] [CrossRef]
- Ding, Y.Y.; Kuha, J.; Murphy, M. Multidimensional predictors of physical frailty in older people: Identifying how and for whom they exert their effects. Biogerontology 2017, 18, 237–252. [Google Scholar] [CrossRef]
- Mayeux, R. Biomarkers potential uses and limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef]
- Crimmins, E.; Faul, J.; Kim, J.K.; Guyer, H.; Langa, K.; Ofstedal, M.B.; Sonnega, A.; Wallace, R.; Weir, D. Documentation of Biomarkers in the 2006 and 2008 Health and Retirement Study; Survey Research Center University of Michigan: Ann Arbor, MI, USA, 2013. [Google Scholar]
- Zhao, Y.; Hu, Y.; Smith, J.P.; Strauss, J.; Yang, G. Cohort profile: The China health and retirement longitudinal study (CHARLS). Int. J. Epidemiol. 2014, 43, 61–68. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O.; Criqui, M., 3rd; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.J.C. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Crimmins, E.; Faul, J.; Kim, J.K.; Weir, D.J.A.A. Documentation of Biomarkers in the 2010 and 2012 Health and Retirement Study; Survey Research Center, University of Michigan: Ann Arbor, MI, USA, 2015. [Google Scholar]
- CHARLS Blood Sample Users’ Guide. Available online: https://charls.pku.edu.cn/wenjian/xuejianshujuyonghushiyongshouce.pdf (accessed on 11 February 2025).
- Crimmins, E.; Kim, J.K.; McCreath, H.; Faul, J.; Weir, D.; Seeman, T. Validation of blood-based assays using dried blood spots for use in large population studies. Biodemogr Soc. Biol. 2014, 60, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Spector, W.D.; Fleishman, J.A. Combining activities of daily living with instrumental activities of daily living to measure functional disability. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 1998, 53, S46–S57. [Google Scholar] [CrossRef] [PubMed]
- Karim, J.; Weisz, R.; Bibi, Z.; ur Rehman, S. Validation of the eight-item center for epidemiologic studies depression scale (CES-D) among older adults. Curr. Psychol. 2015, 34, 681–692. [Google Scholar] [CrossRef]
- Andresen, E.M.; Malmgren, J.A.; Carter, W.B.; Patrick, D.L. Screening for Depression in Well Older Adults: Evaluation of a Short Form of the CES-D. Am. J. Prev. Med. 1994, 10, 77–84. [Google Scholar] [CrossRef]
- Zajacova, A.; Lawrence, E.M. The Relationship Between Education and Health: Reducing Disparities Through a Contextual Approach. Annu. Rev. Public. Health 2018, 39, 273–289. [Google Scholar] [CrossRef]
- Zhao, W.; Si, Y.; Li, X.; Zhao, Y.; Jia, S.; Dong, B. Association of allostatic load with functional disability in the China Health and Retirement Longitudinal Study. J. Nutr. Health Aging 2024, 28, 100367. [Google Scholar] [CrossRef]
- Li, C.; Howard, S.P.; Rogers, C.R.; Andrzejak, S.; Gilbert, K.L.; Watts, K.J.; Bevel, M.S.; Moody, M.D.; Langston, M.E.; Doty, J.V.; et al. Allostatic Load, Educational Attainment, and Risk of Cancer Mortality Among US Men. JAMA Netw. Open 2024, 7, e2449855. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, E.M.; Zhang, Y.S.; Kim, J.K.; Frochen, S.; Kang, H.; Shim, H.; Ailshire, J.; Potter, A.; Cofferen, J.; Faul, J. Dried blood spots: Effects of less than optimal collection, shipping time, heat, and humidity. Am. J. Hum. Biol. 2020, 32, e23390. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, P.; Zhou, T.; Lu, J.; Spatz, E.S.; Nasir, K.; Jiang, L.; Krumholz, H.M. Comparison of Prevalence, Awareness, Treatment, and Control of Cardiovascular Risk Factors in China and the United States. J. Am. Heart Assoc. 2018, 7, e007462. [Google Scholar] [CrossRef] [PubMed]
- Read, S.; Grundy, E. Allostatic Load—A Challenge to Measure Multisystem Physiological Dysregulation. Available online: https://eprints.ncrm.ac.uk/id/eprint/2879/1/NCRM_workingpaper_0412.pdf (accessed on 12 March 2025).
- Landi, F.; Russo, A.; Cesari, M.; Pahor, M.; Bernabei, R.; Onder, G. HDL-cholesterol and physical performance: Results from the ageing and longevity study in the sirente geographic area (ilSIRENTE Study). Age Ageing 2007, 36, 514–520. [Google Scholar] [CrossRef]

| HRS | CHARLS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal Levels of Blood Biomarkers in the Biological Systems (n = 1624) | Abnormal Levels of Blood Biomarkers in One of the Biological Systems (n = 3835) | Abnormal Levels of Blood Biomarkers in Two of the Biological Systems (n = 2931) | Abnormal Levels of Blood Biomarkers in all of the Biological Systems a (n = 860) | p | Normal Levels of Blood Biomarkers in the Biological Systems (n = 2088) | Abnormal Levels of Blood Biomarkers in One of the Biological Systems (n = 2842) | Abnormal Levels of Blood Biomarkers in Two of the Biological Systems (n = 1549) | Abnormal Levels of Blood Biomarkers in All of the Biological Systems (n = 365) | p | |
| Age (years) | 62.3 ± 9.4 | 65.7 ± 10.1 | 65.8 ± 10 | 64.6 ± 9.7 | <0.001 | 61.1 ± 7.4 | 62.3 ± 8 | 62.6 ± 8.1 | 63.8 ± 8.2 | <0.001 |
| Female sex | 901 (55.5) | 2042 (53.3) | 1851 (63.2) | 663 (77.1) | <0.001 | 903 (43.3) | 1449 (51) | 985 (63.6) | 244 (66.9) | <0.001 |
| Education | <0.001 | 0.323 | ||||||||
| ≤9 years | 112 (6.9) | 403 (10.6) | 376 (12.9) | 122 (14.3) | 1891 (90.6) | 2598 (91.5) | 1428 (92.3) | 331 (90.7) | ||
| >9 years | 1501 (93.1) | 3414 (89.4) | 2542 (87.1) | 734 (85.8) | 196 (9.4) | 243 (8.6) | 119 (7.7) | 34 (9.3) | ||
| Marital status | <0.001 | <0.001 | ||||||||
| Married | 1111 (68.5) | 2319 (60.5) | 1644 (56.1) | 437 (50.8) | 1819 (87.1) | 2347 (82.6) | 1252 (80.8) | 290 (79.5) | ||
| Unmarried | 512 (31.6) | 1514 (39.5) | 1287 (43.9) | 423 (49.2) | 269 (12.9) | 495 (17.4) | 297 (19.2) | 75 (20.6) | ||
| Smoking | <0.001 | <0.001 | ||||||||
| Current smokers | 207 (12.8) | 514 (13.4) | 481 (16.4) | 177 (20.6) | 772 (37) | 883 (31.3) | 359 (23.4) | 73 (20.2) | ||
| Non-current smokers | 1417 (87.3) | 3320 (86.6) | 2450 (83.6) | 682 (79.4) | 1312 (63) | 1938 (68.7) | 1177 (76.6) | 289 (79.8) | ||
| Alcohol consumption | <0.001 | <0.001 | ||||||||
| Regular alcohol drinkers | 276 (17) | 559 (14.6) | 270 (9.2) | 41 (4.8) | 353 (18.2) | 371 (13.8) | 119 (8.1) | 28 (7.9) | ||
| Irregular alcohol drinkers | 1347 (83) | 3271 (85.4) | 2656 (90.8) | 817 (95.2) | 1589 (81.8) | 2316 (86.2) | 1360 (92) | 327 (92.1) | ||
| BMI | <0.001 | <0.001 | ||||||||
| Underweight (<18.5) | 29 (1.8) | 30 (0.8) | 15 (0.6) | 1 (0.2) | 227 (11) | 174 (6.5) | 48 (3.4) | 5 (1.5) | ||
| Normal weight (18.5–24.99) | 565 (35.9) | 782 (21.9) | 325 (13.3) | 53 (8.6) | 1544 (75.1) | 1724 (64.7) | 683 (48.8) | 129 (38.7) | ||
| Overweight (25–29.99) | 607 (38.6) | 1405 (39.3) | 857 (35) | 169 (27.5) | 272 (13.2) | 670 (25.1) | 561 (40) | 141 (42.3) | ||
| Obese (≥30) | 371 (23.6) | 1354 (37.9) | 1250 (51.1) | 391 (63.7) | 14 (0.7) | 97 (3.6) | 109 (7.8) | 58 (17.4) | ||
| Health status b | <0.001 | <0.001 | ||||||||
| Healthy | 654 (40.5) | 507 (13.3) | 187 (6.4) | 25 (2.9) | 783 (39) | 735 (27) | 266 (17.8) | 36 (10.3) | ||
| Unhealthy | 960 (59.5) | 3300 (86.7) | 2725 (93.6) | 825 (97.1) | 1223 (61) | 1989 (73) | 1229 (82.2) | 313 (89.7) | ||
| Depressive symptom | 307 (19) | 921 (24.1) | 957 (32.9) | 330 (38.8) | <0.001 | 734 (37.5) | 1053 (39.7) | 588 (41.2) | 145 (43.4) | 0.069 |
| ADL disability | 138 (8.5) | 538 (14) | 596 (20.3) | 254 (29.5) | <0.001 | 332 (15.9) | 539 (19) | 319 (20.6) | 91 (24.9) | <0.001 |
| IADL disability | 92 (5.7) | 383 (10) | 426 (14.5) | 178 (20.7) | <0.001 | 399 (19.1) | 665 (23.4) | 415 (26.8) | 111 (30.4) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, R.; Pei, J.-J.; Wolthon, A.; Li, Y.; Wang, H.-X. Abnormal Blood Biomarkers and Cumulative Disability Burden in Middle-Aged and Older Adults: Evidence from Two Nationally Representative Surveys in the United States and China. J. Cardiovasc. Dev. Dis. 2025, 12, 429. https://doi.org/10.3390/jcdd12110429
Tu R, Pei J-J, Wolthon A, Li Y, Wang H-X. Abnormal Blood Biomarkers and Cumulative Disability Burden in Middle-Aged and Older Adults: Evidence from Two Nationally Representative Surveys in the United States and China. Journal of Cardiovascular Development and Disease. 2025; 12(11):429. https://doi.org/10.3390/jcdd12110429
Chicago/Turabian StyleTu, Raoping, Jin-Jing Pei, Alexander Wolthon, Yueping Li, and Hui-Xin Wang. 2025. "Abnormal Blood Biomarkers and Cumulative Disability Burden in Middle-Aged and Older Adults: Evidence from Two Nationally Representative Surveys in the United States and China" Journal of Cardiovascular Development and Disease 12, no. 11: 429. https://doi.org/10.3390/jcdd12110429
APA StyleTu, R., Pei, J.-J., Wolthon, A., Li, Y., & Wang, H.-X. (2025). Abnormal Blood Biomarkers and Cumulative Disability Burden in Middle-Aged and Older Adults: Evidence from Two Nationally Representative Surveys in the United States and China. Journal of Cardiovascular Development and Disease, 12(11), 429. https://doi.org/10.3390/jcdd12110429






