The Synergistic Risk of Insulin Resistance and Renal Dysfunction in Acute Coronary Syndrome Patients After Percutaneous Coronary Intervention
Abstract
1. Background
2. Methods
2.1. Study Population
2.2. Data Collection and Definitions
2.3. Assessment of TyG Index and SYNTAX Score
2.4. Outcome Definition
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
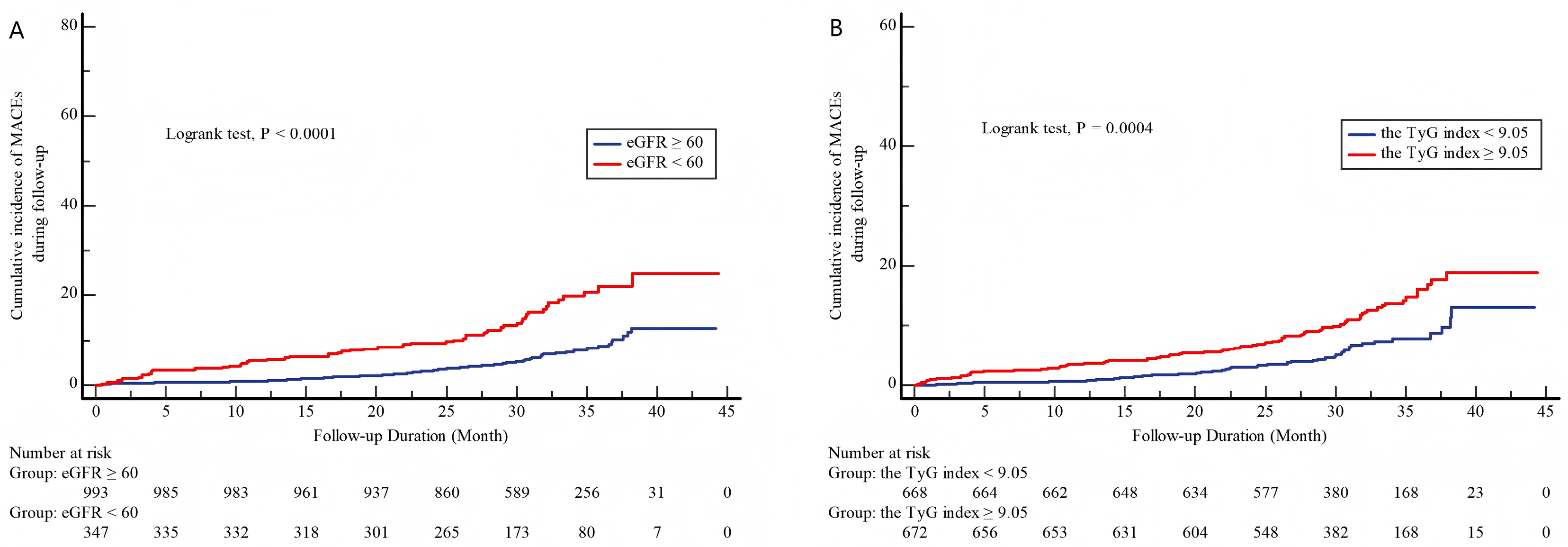
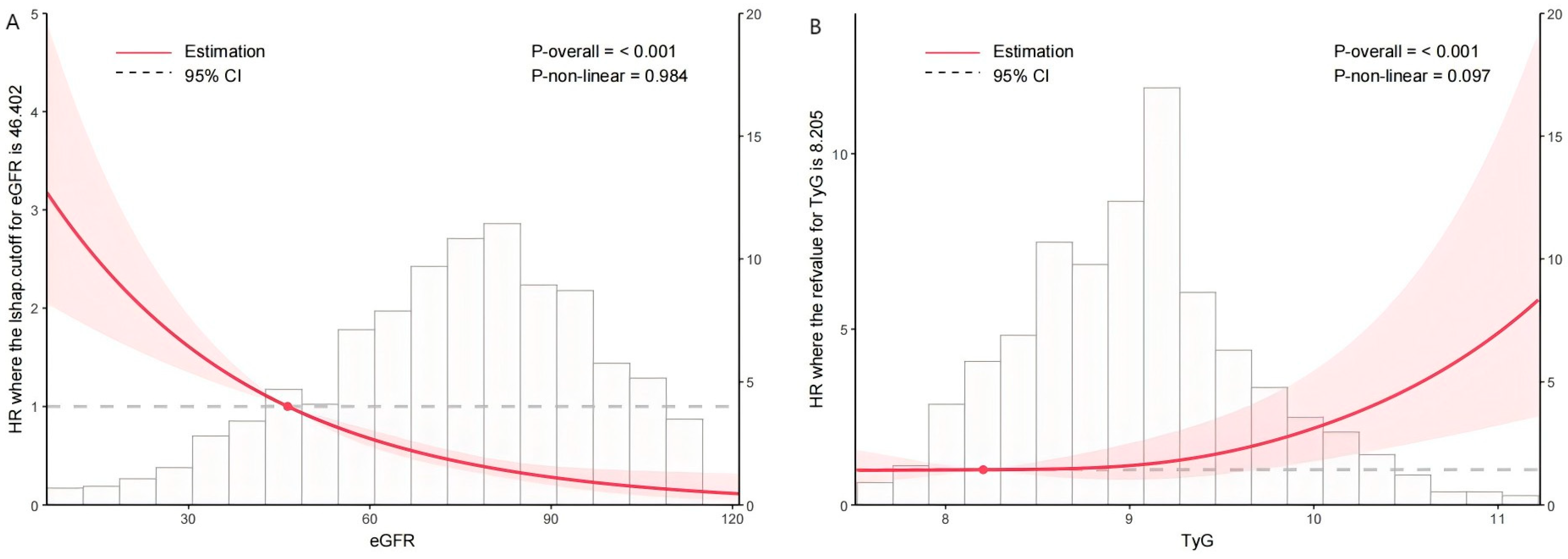
3.2. Association Between TyG Index, eGFR, and the Incidence of MACEs
3.3. Univariable and Multivariable Cox Regression Analyses
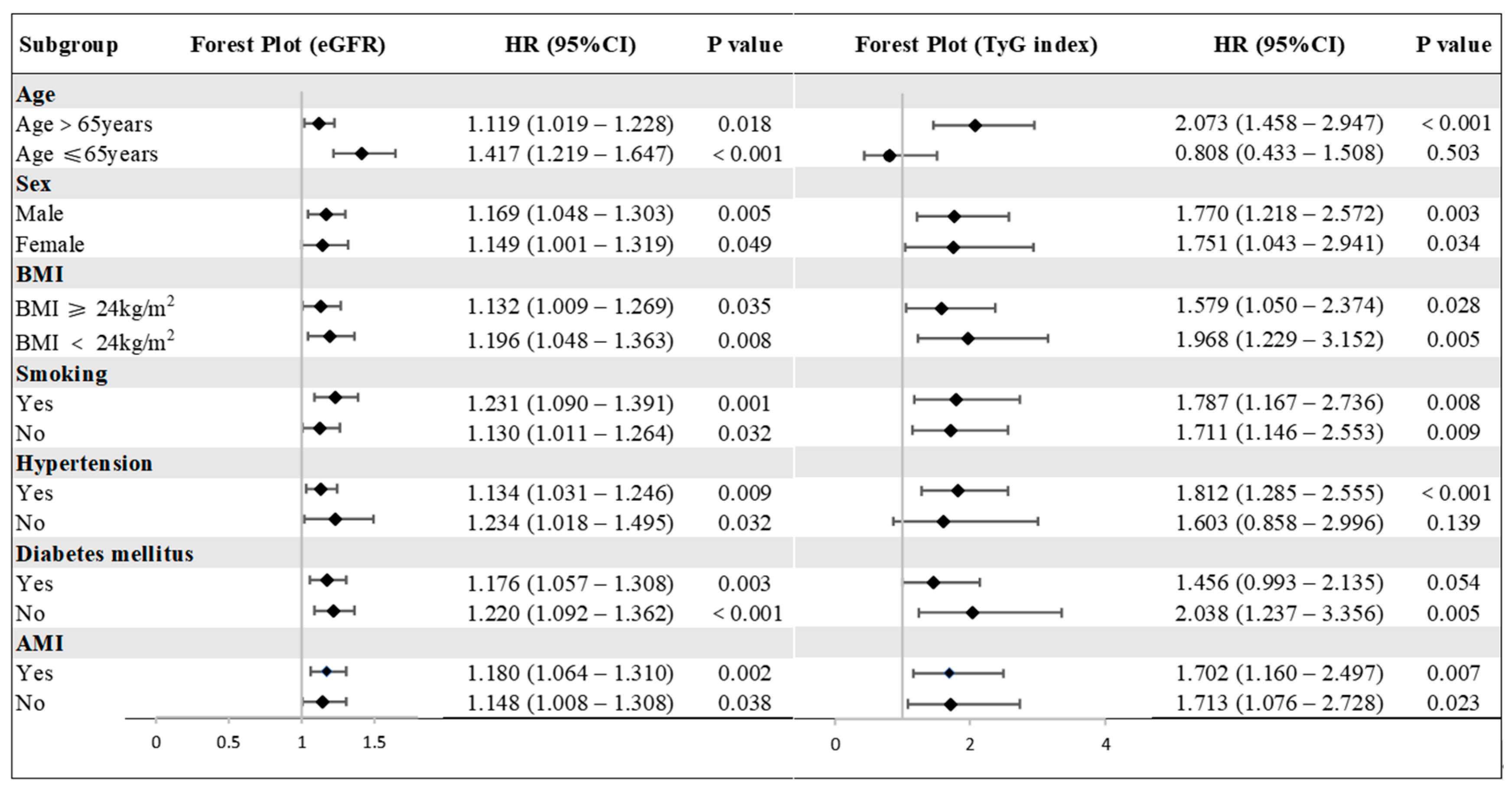
3.4. The Predictive Value of the TyG Index and eGFR for MACEs in Various Subgroups
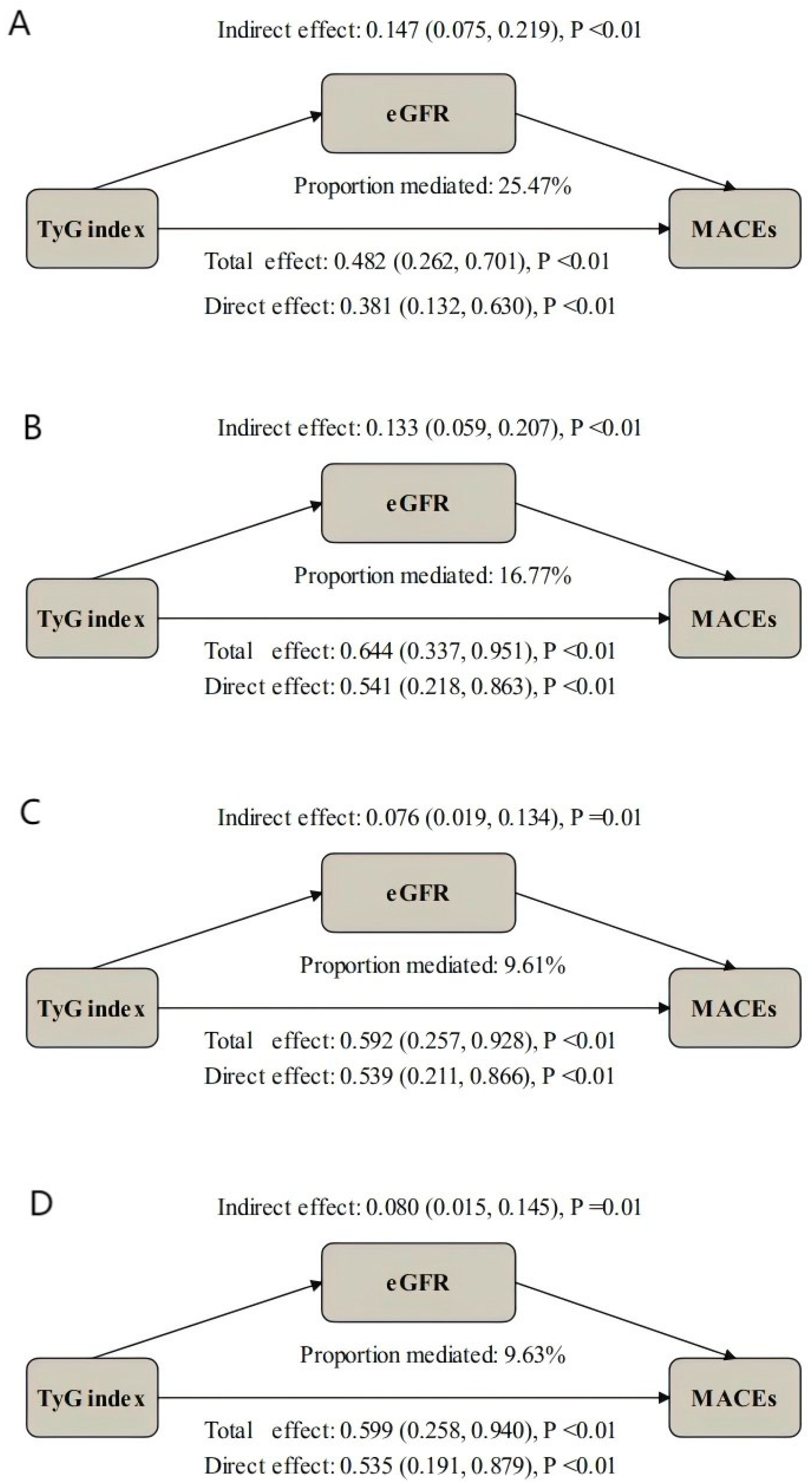
3.5. Mediation Analysis
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef]
- Gogineni, V.S.; Shah, K.B. High-Risk Percutaneous Coronary Intervention: Challenges and Considerations. CVIA 2024, 9, 949. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Iglesies-Grau, J.; Garcia-Alvarez, A.; Oliva, B.; Mendieta, G.; García-Lunar, I.; Fuster, J.J.; Devesa, A.; Pérez-Herreras, C.; Fernández-Ortiz, A.; Brugada, R.; et al. Early insulin resistance in normoglycemic low-risk individuals is associated with subclinical atherosclerosis. Cardiovasc. Diabetol. 2023, 22, 350. [Google Scholar] [CrossRef]
- Chen, Q.; Xiong, S.; Ye, T.; Gao, Y.; Wang, J.; Li, X.; Li, Y.; Cui, C.; Liu, H.; Zhang, Z.; et al. Insulin resistance, coronary artery lesion complexity and adverse cardiovascular outcomes in patients with acute coronary syndrome. Cardiovasc. Diabetol. 2024, 23, 172. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
- Son, D.H.; Lee, H.S.; Lee, Y.J.; Lee, J.H.; Han, J.H. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 596–604. [Google Scholar] [CrossRef]
- Huang, D.; Ma, R.; Zhong, X.; Jiang, Y.; Lu, J.; Li, Y.; Shi, Y. Positive association between different triglyceride glucose index-related indicators and psoriasis: Evidence from NHANES. Front. Immunol. 2023, 14, 1325557. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tan, Z.; Huang, Y.; Zhao, H.; Liu, M.; Yu, P.; Ma, J.; Zhao, Y.; Zhu, W.; Wang, J. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2022, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Won, K.B.; Park, E.J.; Han, D.; Lee, J.H.; Choi, S.Y.; Chun, E.J.; Park, S.H.; Han, H.W.; Sung, J.; Jung, H.O.; et al. Triglyceride glucose index is an independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc. Diabetol. 2020, 19, 34. [Google Scholar] [CrossRef]
- Park, K.; Ahn, C.W.; Lee, S.B.; Kang, S.; Nam, J.S.; Lee, B.K.; Kim, J.H.; Park, J.S. Elevated TyG Index Predicts Progression of Coronary Artery Calcification. Diabetes Care 2019, 42, 1569–1573. [Google Scholar] [CrossRef]
- Gao, J.W.; Hao, Q.Y.; Gao, M.; Zhang, K.; Li, X.Z.; Wang, J.F.; Vuitton, D.A.; Zhang, S.L.; Liu, P.M. Triglyceride-glucose index in the development of peripheral artery disease: Findings from the Atherosclerosis Risk in Communities (ARIC) Study. Cardiovasc. Diabetol. 2021, 20, 126. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, X.; Wang, Y.; Leng, L.; Xu, J.; Feng, L.; Jiang, S.; Wang, J.; Yang, Y.; Pan, G.; et al. The impact of triglyceride-glucose index on ischemic stroke: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2023, 22, 2. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003, 42, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Marassi, M.; Fadini, G.P. The cardio-renal-metabolic connection: A review of the evidence. Cardiovasc. Diabetol. 2023, 22, 195. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Toso, A.; Leoncini, M.; Maioli, M.; Villani, S.; Bellandi, F. Biomarkers of residual risk and all-cause mortality after acute coronary syndrome. Am. J. Prev. Cardiol. 2025, 21, 100934. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Cui, C.; Liu, L.; Zhang, T.; Fang, L.; Mo, Z.; Qi, Y.; Zheng, J.; Wang, Z.; Xu, H.; Yan, H.; et al. Triglyceride-glucose index, renal function and cardiovascular disease: A national cohort study. Cardiovasc. Diabetol. 2023, 22, 325. [Google Scholar] [CrossRef]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients With Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 151, e771–e862. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Chaker, H.; Leaming, R.; Johnson, A.; Brechtel, G.; Baron, A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Investig. 1996, 97, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Sui, W.; Chen, S.; Guo, Z.; Jing, X.; Wang, X.; Wang, Q.; Yu, X.; Xiong, W.; Ji, J.; et al. Sweetener aspartame aggravates atherosclerosis through insulin-triggered inflammation. Cell Metab. 2025, 37, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, X.; Hua, B.; Liu, Q.; Gao, H.; Chen, H.; Zhao, X.Q.; Li, W.; Li, H. High triglyceride-glucose index is associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2351–2362. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Wang, K.; Niu, J.; Liu, Y.; Ge, H. Association between the triglyceride-glucose index and in-hospital major adverse cardiovascular events in patients with acute coronary syndrome: Results from the Improving Care for Cardiovascular Disease in China (CCC)-Acute Coronary Syndrome project. Cardiovasc. Diabetol. 2024, 23, 170. [Google Scholar] [CrossRef]
- Dang, K.; Wang, X.; Hu, J.; Zhang, Y.; Cheng, L.; Qi, X.; Liu, L.; Ming, Z.; Tao, X.; Li, Y. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003-2018. Cardiovasc. Diabetol. 2024, 23, 8. [Google Scholar] [CrossRef]
- Alavi Tabatabaei, G.; Mohammadifard, N.; Rafiee, H.; Nouri, F.; Maghami Mehr, A.; Najafian, J.; Sadeghi, M.; Boshtam, M.; Roohafza, H.; Haghighatdoost, F.; et al. Association of the triglyceride glucose index with all-cause and cardiovascular mortality in a general population of Iranian adults. Cardiovasc. Diabetol. 2024, 23, 66. [Google Scholar] [CrossRef]
- Wang, L.; Cong, H.L.; Zhang, J.X.; Hu, Y.C.; Wei, A.; Zhang, Y.Y.; Yang, H.; Ren, L.B.; Qi, W.; Li, W.Y.; et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc. Diabetol. 2020, 19, 80. [Google Scholar] [CrossRef]
- da Silva, A.; Caldas, A.P.S.; Hermsdorff, H.H.M.; Bersch-Ferreira, Â.C.; Torreglosa, C.R.; Weber, B.; Bressan, J. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc. Diabetol. 2019, 18, 89. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Lu, Y.; Xiao, Y.; Zhou, X. Insulin resistance assessed by estimated glucose disposal rate and risk of incident cardiovascular diseases among individuals without diabetes: Findings from a nationwide, population based, prospective cohort study. Cardiovasc. Diabetol. 2024, 23, 194. [Google Scholar] [CrossRef]
- D’Apolito, M.; Du, X.; Pisanelli, D.; Pettoello-Mantovani, M.; Campanozzi, A.; Giacco, F.; Maffione, A.B.; Colia, A.L.; Brownlee, M.; Giardino, I. Urea-induced ROS cause endothelial dysfunction in chronic renal failure. Atherosclerosis 2015, 239, 393–400. [Google Scholar] [CrossRef]
- Yu, W.; Cheng, J.D. Uric Acid and Cardiovascular Disease: An Update From Molecular Mechanism to Clinical Perspective. Front. Pharmacol. 2020, 11, 582680. [Google Scholar] [CrossRef] [PubMed]
- Curaj, A.; Vanholder, R.; Loscalzo, J.; Quach, K.; Wu, Z.; Jankowski, V.; Jankowski, J. Cardiovascular Consequences of Uremic Metabolites: An Overview of the Involved Signaling Pathways. Circ. Res. 2024, 134, 592–613. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Meert, N.; Glorieux, G.; Goeman, J.; Van der Eycken, J.; Vanholder, R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol. Dial. Transplant. 2007, 22, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: A hypothesis proposal. Clin. J. Am. Soc. Nephrol. 2009, 4 (Suppl. S1), S49–S55. [Google Scholar] [CrossRef]
- Kaesler, N.; Babler, A.; Floege, J.; Kramann, R. Cardiac Remodeling in Chronic Kidney Disease. Toxins 2020, 12, 161. [Google Scholar] [CrossRef]
- London, G.M.; Drueke, T.B. Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int. 1997, 51, 1678–1695. [Google Scholar] [CrossRef]
- Vaziri, N.D. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat. Rev. Nephrol. 2016, 12, 37–47. [Google Scholar] [CrossRef]
- Pop, D.; Dădârlat-Pop, A.; Tomoaia, R.; Zdrenghea, D.; Caloian, B. Updates on the Renin-Angiotensin-Aldosterone System and the Cardiovascular Continuum. Biomedicines 2024, 12, 1582. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef]
- Dozio, E.; Ambrogi, F.; de Cal, M.; Vianello, E.; Ronco, C.; Corsi Romanelli, M.M. Role of the Soluble Receptor for Advanced Glycation End Products (sRAGE) as a Prognostic Factor for Mortality in Hemodialysis and Peritoneal Dialysis Patients. Mediat. Inflamm. 2018, 2018, 1347432. [Google Scholar] [CrossRef]
- Jovanovich, A.; Isakova, T.; Stubbs, J. Microbiome and Cardiovascular Disease in CKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 1598–1604. [Google Scholar] [CrossRef]
- Gupta, S.; Dominguez, M.; Golestaneh, L. Diabetic Kidney Disease: An Update. Med. Clin. N. Am. 2023, 107, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Premaratne, E.; Verma, S.; Ekinci, E.I.; Theverkalam, G.; Jerums, G.; MacIsaac, R.J. The impact of hyperfiltration on the diabetic kidney. Diabetes Metab. 2015, 41, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Cui, H.; Wang, Y.; Ju, F.; Cai, Y.; Gang, X.; Wang, G. The role of lipotoxicity in kidney disease: From molecular mechanisms to therapeutic prospects. Biomed. Pharmacother. 2023, 161, 114465. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Devarajan, P.; Haase-Fielitz, A.; Bellomo, R.; Cruz, D.N.; Wagener, G.; Krawczeski, C.D.; Koyner, J.L.; Murray, P.; Zappitelli, M.; et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: A multicenter pooled analysis of prospective studies. J. Am. Coll. Cardiol. 2011, 57, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Parikh, C.R. Current concepts and advances in biomarkers of acute kidney injury. Crit. Rev. Clin. Lab. Sci. 2021, 58, 354–368. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total Population | Non-MACEs (n = 1216) | MACEs (n = 124) | p-Value |
|---|---|---|---|---|
| Age, years | 67.02 ± 11.21 | 66.34 ± 11.11 | 73.62 ± 10.05 | <0.001 |
| Female, n (%) | 384 (28.66) | 340 (27.96) | 44 (35.48) | 0.078 |
| BMI, kg/m2 | 24.40 ± 2.97 | 24.45 ± 2.96 | 23.91 ± 2.95 | 0.050 |
| Smoking, n (%) | 715 (53.36) | 658 (54.11) | 57 (45.97) | 0.083 |
| Previous PCI, n (%) | 115 (8.58) | 101 (8.31) | 14 (11.29) | 0.258 |
| COPD, n (%) | 41 (3.06) | 31 (2.55) | 10 (8.06) | 0.001 |
| Hypertension, n (%) | 911 (67.99) | 818 (67.27) | 93 (75.00) | 0.079 |
| Diabetes mellitus, n (%) | 533 (39.78) | 470 (38.65) | 63 (50.81) | 0.008 |
| Stroke, n (%) | 63 (4.70) | 57 (4.69) | 6 (4.84) | 0.944 |
| SBP, mmHg | 132.98 ± 21.02 | 132.95 ± 20.81 | 133.22 ± 23.03 | 0.893 |
| Heart rate, bpm | 77.04 ± 14.29 | 76.73 ± 13.88 | 80.11 ± 17.65 | 0.012 |
| cTnT, pg/mL | 58.15 (12.33, 1211.90) | 50.80 (11.60, 1211.90) | 229.90 (25.75, 1919.25) | <0.001 |
| BNP, pg/mL | 124.75 (44.25, 328.49) | 118.15 (41.85, 328.49) | 328.49 (96.13, 988.13) | <0.001 |
| Uric acid, µmol/L | 367.05 (306.23, 434.00) | 365.75 (306.10, 430.58) | 384.30 (308.25, 498.10) | 0.028 |
| Serum creatinine, mg/dL | 0.87 (0.74, 1.04) | 0.87 (0.74, 1.02) | 0.97 (0.78, 1.36) | <0.001 |
| Cystatin C, mg/dL | 1.14 (0.97, 1.41) | 1.12 (0.96, 1.37) | 1.37 (1.11, 2.00) | <0.001 |
| eGFR, mL/min/1.73 m2 | 75.93 (59.35, 91.33) | 77.87 (61.38, 92.38) | 62.52 (36.52, 77.08) | <0.001 |
| eGFR < 60 mL/min/1.73 m2 | 347 (25.9) | 291 (23.93) | 56 (45.16) | <0.001 |
| FBG, mmol/L | 6.95 ± 3.21 | 6.93 ± 3.23 | 7.21 ± 3.01 | 0.356 |
| TG, mmol/L | 1.85 ± 1.37 | 1.87 ± 1.41 | 1.63 ± 0.93 | 0.063 |
| TC, mmol/L | 4.47 ± 1.27 | 4.48 ± 1.27 | 4.45 ± 1.26 | 0.847 |
| HDL-C, mmol/L | 1.16 ± 0.30 | 1.16 ± 0.30 | 1.16 ± 0.31 | 0.973 |
| LDL-C, mmol/L | 2.73 ± 0.93 | 2.74 ± 0.93 | 2.73 ± 0.93 | 0.903 |
| Hcy, µmol/L | 13.85 (11.00, 17.40) | 13.60 (10.90, 17.00) | 16.65 (12.53, 20.78) | <0.001 |
| Fib, g/L | 3.80 ± 1.31 | 3.75 ± 1.27 | 4.24 ± 1.58 | <0.001 |
| LVEF | 55.06 ± 8.68 | 55.47 ± 8.30 | 51.03 ± 11.03 | <0.001 |
| AMI, n (%) | 667 (49.78) | 591 (48.60) | 76 (61.29) | 0.007 |
| Diagnosis, n (%) | 0.016 | |||
| UA | 673 (50.22) | 625 (51.40) | 48 (38.71) | |
| NSTEMI | 283 (21.12) | 247 (20.31) | 36 (29.03) | |
| STEMI | 384 (28.66) | 344 (28.29) | 40 (32.26) | |
| Aspirin, n (%) | 1321 (98.58) | 1202 (98.85) | 119 (95.97) | 0.010 |
| P2Y12-receptor inhibitor, n (%) | 1319 (98.43) | 1199 (98.60) | 120 (96.77) | 0.119 |
| Statins, n (%) | 1312 (97.91) | 1191 (97.94) | 121 (97.58) | 0.788 |
| β-blockers, n (%) | 935 (69.78) | 850 (69.90) | 85 (68.55) | 0.755 |
| ACEI/ARB, n (%) | 607 (45.30) | 545 (44.82) | 62 (50.00) | 0.270 |
| Diuretics, n (%) | 230 (17.16) | 185 (15.21) | 45 (36.30) | <0.001 |
| Insulin, n (%) | 144 (10.75) | 121 (9.95) | 23 (18.55) | 0.003 |
| TyG index | 9.05 ± 0.69 | 9.03 ± 0.68 | 9.31 ± 0.74 | <0.001 |
| bSS | 13.00 (8.00, 20.000) | 13.00 (8.00, 19.50) | 19.00 (13.00, 27.85) | <0.001 |
| eGFR | TyG Index | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| MACEs | Unadjusted Model | 1.338 (1.125–1.432) | <0.001 | 1.620 (1.301–2.016) | <0.001 |
| Adjusted Model I | 1.205 (1.110–1.309) | <0.001 | 1.731 (1.282–2.337) | <0.001 | |
| Adjusted Model II | 1.128 (1.035–1.230) | 0.006 | 1.728 (1.278–2.336) | <0.001 | |
| Adjusted Model III | 1.127 (1.032–1.232) | 0.008 | 1.738 (1.273–2.372) | <0.001 | |
| Death | Unadjusted Model | 1.366 (1.261–1.479) | <0.001 | 1.635 (1.257–2.126) | <0.001 |
| Adjusted Model I | 1.220 (1.107–1.344) | <0.001 | 1.695 (1.184–2.426) | 0.004 | |
| Adjusted Model II | 1.140 (1.028–1.264) | 0.013 | 1.788 (1.241–2.574) | 0.002 | |
| Adjusted Model III | 1.139 (1.026–1.265) | 0.015 | 1.751 (1.203–2.546) | 0.003 | |
| Exposures | Association | |||||||
|---|---|---|---|---|---|---|---|---|
| Total Effect | Indirect Effect | Direct Effect | ||||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | PM (95% CI) | p | |
| Unadjusted Model | 1.608 (1.267, 1.950) | <0.01 | 1.162 (1.079, 1.245) | <0.01 | 1.456 (1.094, 1.819) | <0.01 | 25.47 (13.52, 53.90) | <0.01 |
| Model I | 1.895 (1.309, 2.481) | <0.01 | 1.146 (1.058, 1.234) | <0.01 | 1.714 (1.159, 2.269) | <0.01 | 16.77 (7.75, 33.64) | <0.01 |
| Model II | 1.803 (1.207, 2.400) | <0.01 | 1.081 (1.013, 1.149) | <0.01 | 1.703 (1.118, 2.288) | <0.01 | 9.61 (1.17, 23.79) | 0.02 |
| Model III | 1.823 (1.201, 2.444) | <0.01 | 1.082 (1.012, 1.153) | <0.01 | 1.721 (1.104, 2.337) | <0.01 | 9.63 (1.77, 23.40) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Jiang, M.; Liu, L.; Jia, D.; Feng, J.; Luo, Y.; Ye, T.; Xia, L.; Liu, H.; Zhang, Z.; et al. The Synergistic Risk of Insulin Resistance and Renal Dysfunction in Acute Coronary Syndrome Patients After Percutaneous Coronary Intervention. J. Cardiovasc. Dev. Dis. 2025, 12, 427. https://doi.org/10.3390/jcdd12110427
Yang G, Jiang M, Liu L, Jia D, Feng J, Luo Y, Ye T, Xia L, Liu H, Zhang Z, et al. The Synergistic Risk of Insulin Resistance and Renal Dysfunction in Acute Coronary Syndrome Patients After Percutaneous Coronary Intervention. Journal of Cardiovascular Development and Disease. 2025; 12(11):427. https://doi.org/10.3390/jcdd12110427
Chicago/Turabian StyleYang, Guoshu, Maoling Jiang, Lin Liu, Dongyue Jia, Jie Feng, Yan Luo, Tao Ye, Long Xia, Hanxiong Liu, Zhen Zhang, and et al. 2025. "The Synergistic Risk of Insulin Resistance and Renal Dysfunction in Acute Coronary Syndrome Patients After Percutaneous Coronary Intervention" Journal of Cardiovascular Development and Disease 12, no. 11: 427. https://doi.org/10.3390/jcdd12110427
APA StyleYang, G., Jiang, M., Liu, L., Jia, D., Feng, J., Luo, Y., Ye, T., Xia, L., Liu, H., Zhang, Z., Fu, J., Cai, L., Chen, Q., & Xiong, S. (2025). The Synergistic Risk of Insulin Resistance and Renal Dysfunction in Acute Coronary Syndrome Patients After Percutaneous Coronary Intervention. Journal of Cardiovascular Development and Disease, 12(11), 427. https://doi.org/10.3390/jcdd12110427





