Prediction of Postoperative Mortality After Fontan Procedure: A Clinical Prediction Model Study Using Deep Learning Artificial Intelligence Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Variables and Data Preprocessing
2.3. Hemodynamic Data
2.4. Deep Learning Model Development
2.5. Handling Class Imbalance
2.6. Model Interpretability and Feature Importance
2.7. Model Validation
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Model Performance
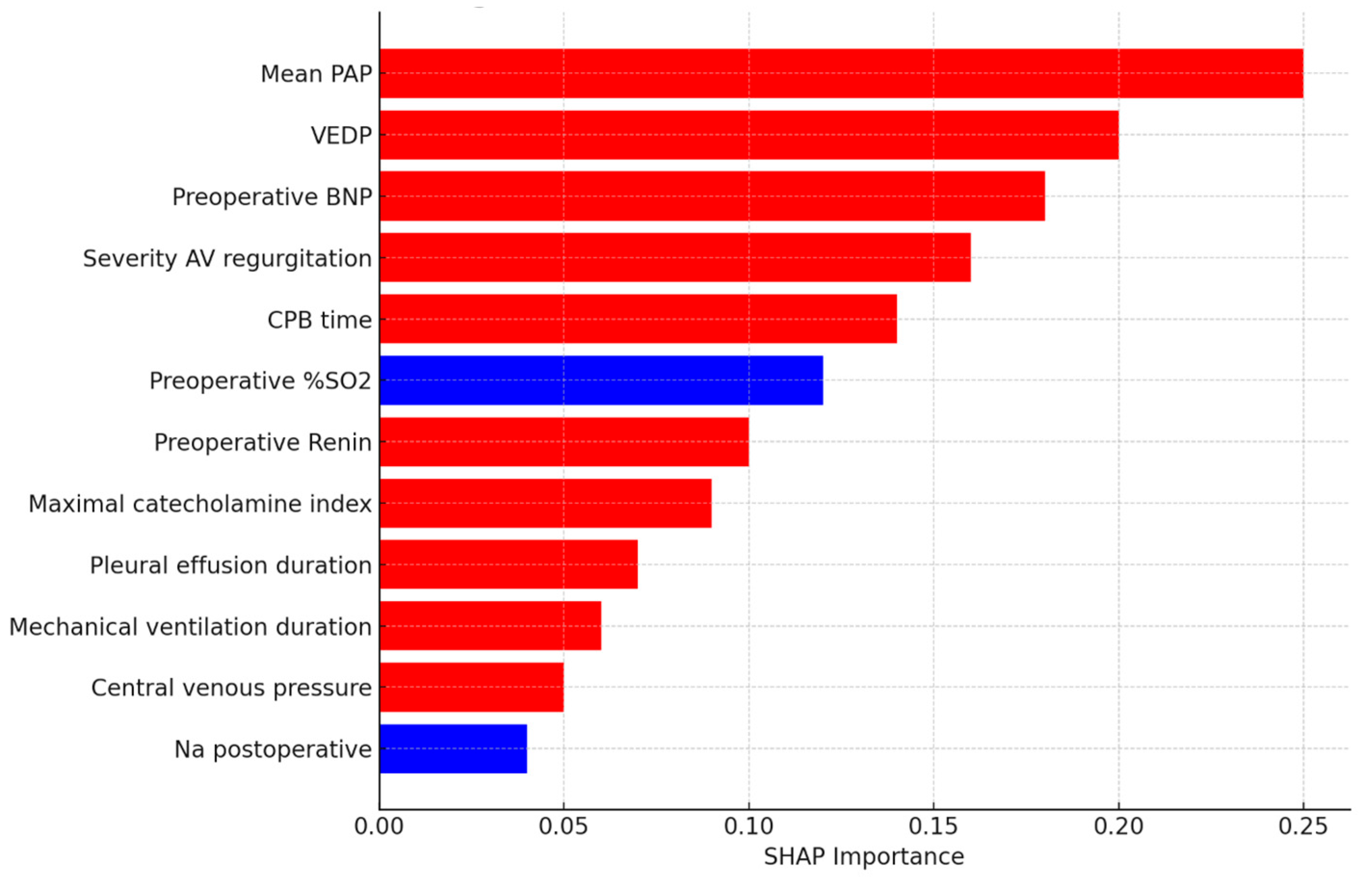
3.3. Feature Importance and Interpretability
3.4. Application Interface for Clinical Utility
4. Discussion
5. Limitations of This Study
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ANP | Atrial Natriuretic Peptide |
| AoSat | Aortic Oxygen Saturation |
| AV | Atrioventricular |
| AVV | Atrioventricular Valve |
| AVVR | Atrioventricular Valve Regurgitation |
| BNP | Brain Natriuretic Peptide |
| CHD | Congenital Heart Disease |
| CI | Confidence Interval |
| CPB | Cardiopulmonary Bypass |
| DNN | Deep Neural Network |
| DL | Deep Learning |
| DHCA | Deep Hypothermic Circulatory Arrest |
| EDVP/VEDP | Ventricular End-Diastolic Pressure |
| GAN | Generative Adversarial Network |
| HLHS | Hypoplastic Left Heart Syndrome |
| ICU | Intensive Care Unit |
| LOS | Length of Stay |
| LV | Left Ventricle |
| ML | Machine Learning |
| Na, K, Cl, TCO2 | Sodium, Potassium, Chloride, Total Carbon Dioxide |
| OSF | Open Science Framework |
| PAP | Pulmonary Artery Pressure |
| Pasat | Pulmonary Artery Oxygen Saturation |
| PVsat | Pulmonary Vein Oxygen Saturation |
| Qp/Qs | Pulmonary-to-Systemic Blood Flow Ratio |
| RA_pr | Right Atrial Pressure |
| ROC-AUC/AUC-ROC | Receiver Operating Characteristic—Area Under the Curve |
| SD | Standard Deviation |
| SHAP | SHapley Additive exPlanations |
| SMOTE | Synthetic Minority Over-sampling Technique |
| SVCsat | Superior Vena Cava Oxygen Saturation |
| STS-EACTS | Society of Thoracic Surgeons—European Association for Cardio-Thoracic Surgery |
| TPG | Transpulmonary Gradient |
Appendix A
| Section | Reported | Location in Manuscript |
| Title/Abstract | Yes | Title identifies the study as an AI-based prediction model; the Abstract reports the objectives, data, methods, and performance. (Abstract, p.1) |
| Background/Rationale | Yes | Clear description of clinical problem and need for better prediction tools. (Introduction) |
| Objectives | Yes | Explicit aim to develop and validate a DNN to predict post-Fontan mortality. (Abstract, Introduction) |
| Source of Data | Yes | Single-center retrospective dataset, 230 patients, 2010–2024. (Methods: Study Population) |
| Participants | Yes | Inclusion/exclusion criteria, demographics, and clinical characteristics are described. (Methods: Study Population, Results: Patient Characteristics) |
| Outcome | Yes | Primary outcome: postoperative mortality. Defined and recorded. (Methods: Study Population, Results) |
| Predictors | Yes | Comprehensive list of pre-, intra-, and postoperative variables, biochemical, and hemodynamic. (Methods: Variables) |
| Sample Size | Yes | 230 patients, 12 mortality events. (Methods: Study Population, Results) |
| Missing Data | Yes | Multiple imputation was used. (Methods: Study Population) |
| Statistical Analysis Methods | Yes | DNN architecture, preprocessing (SMOTE), cross-validation, calibration. (Methods: Model Development, Validation, Statistical Analysis) |
| Model Performance | Yes | Accuracy, AUC, precision, recall, F1, and calibration are presented with CIs. (Results: Model Performance) |
| Model Development | Yes | Details of hyperparameters, dropout, TensorFlow/Keras. (Methods: Model Development) |
| Model Validation | Yes | 5-fold stratified CV, calibration, and subgroup analysis. (Methods, Results) |
| Model Explainability | Yes | SHAP analysis for feature importance. (Methods: Interpretability, Results: SHAP) |
| Results–Participants | Yes | Flow of patients described; baseline demographics provided. (Results: Patient Characteristics) |
| Results–Model | Yes | DNN outperforms other models; detailed performance metrics are provided. (Results) |
| Results–Features | Yes | Key predictors identified by SHAP: PAP, VEDP, BNP, AVVR, and CPB time. (Results: Feature Importance) |
| Limitations | Yes | Single-center, small events, need for external validation. (Discussion: Limitations) |
| Interpretation | Yes | Comparison with literature, discussion of clinical implications. (Discussion) |
| Implementation/Clinical Use | Yes | The Streamlit tool was developed for practical use. (Results: Application Interface, Discussion) |
| Reproducibility | Yes | Code to be made available post-publication. (Future Directions, Conclusion) |
| Ethics | Yes | Ethical approval with consent waiver, anonymized data. (Methods: Study Population) |
| Fairness/Bias | Yes | Subgroup analyses by sex and age; bias addressed, but could be expanded. (Methods: Validation, Results) |
| Funding | Yes | Materials and Methods under Study Population and Data Collection |
| Registration/Protocol | Yes | Planned and registered. Materials and Methods. |
| Conclusions | Yes | Summarizes findings, emphasizes interpretability, and clinical potential. (Conclusion) |
References
- van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar]
- Gewillig, M.; Brown, S.C. The Fontan circulation after 45 years: Update in physiology. Heart 2016, 102, 1081–1086. [Google Scholar]
- Iyengar, A.J.; Winlaw, D.S.; Galati, J.C.; Celermajer, D.S.; Wheaton, G.R.; Gentles, T.L.; Grigg, L.E.; Weintraub, R.G.; Bullock, A.; Justo, R.N.; et al. Trends in Fontan surgery and risk factors for early adverse outcomes after Fontan surgery: The Australia and New Zealand Fontan Registry experience. J. Thorac. Cardiovasc. Surg. 2014, 148, 566–575. [Google Scholar]
- Kay, W.A.; Moe, T.; Suter, B.; Tennancour, A.; Chan, A.; Krasuski, R.A.; Zaidi, A.N. Long-Term Consequences of the Fontan Procedure and How to Manage Them. Prog. Cardiovasc. Dis. 2018, 61, 365–376. [Google Scholar]
- Pundi, K.N.; Johnson, J.N.; Dearani, J.A.; Pundi, K.N.; Li, Z.; Hinck, C.A.; Dahl, S.H.; Cannon, B.C.; O’Leary, P.W.; Driscoll, D.J.; et al. 40-Year Follow-Up After the Fontan Operation: Long-Term Outcomes of 1,052 Patients. J. Am. Coll. Cardiol. 2015, 66, 1700–1710. [Google Scholar]
- Jacobs, J.P.; O’bRien, S.M.; Pasquali, S.K.; Gaynor, J.W.; Mayer, J.E., Jr.; Karamlou, T.; Welke, K.F.; Filardo, G.; Han, J.M.; Kim, S.; et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database Mortality Risk Model: Part 2-Clinical Application. Ann. Thorac. Surg. 2015, 100, 1063–1068; discussion 8–70. [Google Scholar]
- Benedetto, U.; Dimagli, A.; Sinha, S.; Cocomello, L.; Gibbison, B.; Caputo, M.; Gaunt, T.; Lyon, M.; Holmes, C.; Angelini, G.D. Machine learning improves mortality risk prediction after cardiac surgery: Systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2022, 163, 2075–2087.e9. [Google Scholar]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar]
- Gearhart, A.; Anjewierden, S.; Buddhe, S.; Tandon, A. Review of the Current State of Artificial Intelligence in Pediatric Cardiovascular Magnetic Resonance Imaging. Children 2025, 12, 416. [Google Scholar]
- Mayourian, J.; El-Bokl, A.; Lukyanenko, P.; La Cava, W.G.; Geva, T.; Valente, A.M.; Triedman, J.K.; Ghelani, S.J. Electrocardiogram-based deep learning to predict mortality in paediatric and adult congenital heart disease. Eur. Heart J. 2025, 46, 856–868. [Google Scholar]
- Venkatesh, P.; Gao, H.; Abudayyeh, I.; Pai, R.G.; Varadarajan, P. Contemporary Management of the Failing Fontan. J. Clin. Med. 2024, 13, 3049. [Google Scholar]
- d’Udekem, Y.; Xu, M.Y.; Galati, J.C.; Lu, S.; Iyengar, A.J.; Konstantinov, I.E.; Wheaton, G.R.; Ramsay, J.M.; Grigg, L.E.; Millar, J.; et al. Predictors of survival after single-ventricle palliation: The impact of right ventricular dominance. J. Am. Coll. Cardiol. 2012, 59, 1178–1185. [Google Scholar]
- Vatche Bahudian, J.V. Generating Synthetic Data for Machine Learning Models from the Pediatric Heart Network Fontan I Dataset. Congenit. Heart Dis. 2025, 20, 115–127. [Google Scholar]
- Jenkins, K.J. Risk adjustment for congenital heart surgery: The RACHS-1 method. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2004, 7, 180–184. [Google Scholar]
- Jalali, A.; Lonsdale, H.; Do, N.; Peck, J.; Gupta, M.; Kutty, S.; Ghazarian, S.R.; Jacobs, J.P.; Rehman, M.; Ahumada, L.M. Deep Learning for Improved Risk Prediction in Surgical Outcomes. Sci. Rep. 2020, 10, 9289. [Google Scholar]

| Variable | Value |
|---|---|
| Age (months) | 18.13 ± 4.47 |
| HLHS | 134 (58.3%) |
| Dominant ventricle; right | 144 (62.6%) |
| AVV regurgitation | 136 (59.1%) |
| Pulmonary stenosis/distortion | 36 (15.7%) |
| Preoperative oxygen saturation (%) | 85.43 ± 5.79 |
| Right atrial pressure (mmHg) | 5.00 [4.40–5.40] |
| Systemic pressure (mmHg) | 65.70 [62.60–67.60] |
| Pulmonary artery pressure (mmHg) | 10.80 [10.40–11.20] |
| Ventricular end-diastolic pressure (mmHg) | 7.90 [7.00–8.20] |
| Transpulmonary gradient (mmHg) | 3.00 [2.60–3.55] |
| Pulmonary-to-systemic flow ratio | 0.70 [0.62–0.74] |
| Superior vena cava saturation (%) | 61.40 [58.60–62.80] |
| Pulmonary artery saturation (%) | 61.90 [59.40–63.15] |
| Aortic saturation (%) | 84.00 [82.00–86.40] |
| Pulmonary vein saturation (%) | 97.60 [97.20–98.00] |
| Cardiopulmonary bypass time (min) | 85.05 ± 13.35 |
| Ultrafiltration volume removed (mL) | 447.50 [420.00–455.00] |
| Ultrafiltration end hematocrit (%) | 44.40 [44.40–45.00] |
| ICU stay (days) | 3.00 [2.00–5.00] |
| Hospital stay (days) | 10.00 [7.80–18.00] |
| Pleural effusion duration (days) | 3.00 [0.00–8.00] |
| Mechanical ventilation (hours) | 9.00 [6.90–17.75] |
| Cardiac ICU stay (hours) | 27.95 [26.60–54.12] |
| Discharge oxygen saturation (%) | 86.00 [82.00–90.00] |
| Time to extubation (hours) | 5.00 [4.00–6.60] |
| Baseline (Day 0) | |
|---|---|
| Variable | Value |
| Sodium (mmol/L) | 136.80 [135.80–137.20] |
| Potassium (mmol/L) | 4.20 [4.10–4.20] |
| Chloride (mmol/L) | 103.00 [101.85–103.60] |
| Total CO2 (mmol/L) | 18.80 [18.40–19.20] |
| Renin activity (ng/mL/h) | 199.94 [175.35–246.69] |
| B-type natriuretic peptide (pg/mL) | 29.02 [25.30–44.30] |
| Angiotensin II (pg/mL) | 162.72 [108.37–165.93] |
| Vasopressin (pg/mL) | 12.10 [2.84–12.43] |
| Atrial natriuretic peptide (pg/mL) | 13.95 [8.69–16.14] |
| Day 1 Postoperative | |
| Variable | Value |
| Sodium (mmol/L) | 134.40 [133.80–135.40] |
| Potassium (mmol/L) | 3.75 [3.60–4.00] |
| Chloride (mmol/L) | 97.40 [97.20–97.95] |
| Total CO2 (mmol/L) | 22.00 [21.60–22.95] |
| Renin activity (ng/mL/h) | 166.16 [120.92–202.46] |
| B-type natriuretic peptide (pg/mL) | 126.91 [112.42–152.57] |
| Angiotensin II (pg/mL) | 87.07 [42.98–103.50] |
| Vasopressin (pg/mL) | 4.65 [3.08–9.12] |
| Atrial natriuretic peptide (pg/mL) | 18.83 [8.48–26.22] |
| Day 7 Postoperative | |
| Variable | Value |
| Sodium (mmol/L) | 134.40 [134.00–135.00] |
| Potassium (mmol/L) | 4.50 [4.30–4.60] |
| Chloride (mmol/L) | 97.20 [96.45–98.00] |
| Total CO2 (mmol/L) | 23.00 [22.20–24.60] |
| Renin activity (ng/mL/h) | 182.07 [100.62–373.20] |
| B-type natriuretic peptide (pg/mL) | 139.02 [103.40–157.28] |
| Angiotensin II (pg/mL) | 58.11 [49.08–82.89] |
| Vasopressin (pg/mL) | 3.29 [3.07–4.31] |
| Atrial natriuretic peptide (pg/mL) | 50.06 [16.94–131.80] |
| Model | Accuracy (%) | Precision (%) | Recall/ Sensitivity (%) | Specificity (%) | F1-Score (%) | AUC-ROC (95% CI) |
|---|---|---|---|---|---|---|
| Logistic Regression | 82.3 | 74.5 | 78.0 | 84.0 | 76.2 | 0.85 (0.79–0.91) |
| Decision Tree | 78.5 | 71.2 | 76.5 | 80.3 | 73.8 | 0.81 (0.74–0.88) |
| Random Forest | 86.8 | 78.9 | 84.5 | 88.4 | 81.6 | 0.89 (0.83–0.94) |
| Gradient Boosting | 88.6 | 81.4 | 86.2 | 90.2 | 83.7 | 0.91 (0.86–0.96) |
| Deep Neural Network | 91.5 | 83.3 | 90.9 | 92.5 | 87.0 | 0.94 (0.88–0.99) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolcz, J.; Budzynska, A.; Stefaniak, J.; Szydlak, R.; Kononowicz, A.A. Prediction of Postoperative Mortality After Fontan Procedure: A Clinical Prediction Model Study Using Deep Learning Artificial Intelligence Techniques. J. Cardiovasc. Dev. Dis. 2025, 12, 420. https://doi.org/10.3390/jcdd12110420
Kolcz J, Budzynska A, Stefaniak J, Szydlak R, Kononowicz AA. Prediction of Postoperative Mortality After Fontan Procedure: A Clinical Prediction Model Study Using Deep Learning Artificial Intelligence Techniques. Journal of Cardiovascular Development and Disease. 2025; 12(11):420. https://doi.org/10.3390/jcdd12110420
Chicago/Turabian StyleKolcz, Jacek, Anna Budzynska, Justyna Stefaniak, Renata Szydlak, and Andrzej A. Kononowicz. 2025. "Prediction of Postoperative Mortality After Fontan Procedure: A Clinical Prediction Model Study Using Deep Learning Artificial Intelligence Techniques" Journal of Cardiovascular Development and Disease 12, no. 11: 420. https://doi.org/10.3390/jcdd12110420
APA StyleKolcz, J., Budzynska, A., Stefaniak, J., Szydlak, R., & Kononowicz, A. A. (2025). Prediction of Postoperative Mortality After Fontan Procedure: A Clinical Prediction Model Study Using Deep Learning Artificial Intelligence Techniques. Journal of Cardiovascular Development and Disease, 12(11), 420. https://doi.org/10.3390/jcdd12110420







