Diagnostic Predictive Scores of Amyloid Cardiomyopathy in Patients with Heart Failure with Preserved Ejection Fraction and Left Ventricular Hypertrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
- Age ≥ 18 years;
- LV wall thickness ≥ 12 mm on transthoracic echocardiography (TTE) and evidence of preserved ejection fraction (>50%);
- Signs and symptoms of heart failure;
- Availability of a complete diagnostic work-up including
- A 12-lead electrocardiogram (ECG);
- Cardiac biomarkers [N-terminal pro–B-type natriuretic peptide (NT-proBNP) and high-sensitivity troponin];
- TTE with longitudinal strain analysis;
- Bone scintigraphy with 99mTc-DPD or 99mTc-HMDP;
- Serum and urine immunofixation and free light chain (FLC) assay.
2.2. Diagnostic Work-Up and Reference Standard
- Grade 2 or 3 myocardial uptake on bone scintigraphy;
- Absence of monoclonal protein on immunofixation;
- Normal FLC ratio.
- ATTR-positive: confirmed ATTR-CM (wild-type or variant);
- ATTR-negative: no evidence of ATTR-CM (including AL-CM and other LVH causes).
2.2.1. Electrocardiography and Echocardiography
2.2.2. Bone Scintigraphy and Immunological Testing
2.2.3. Scoring Systems Evaluated
- T-Amylo Score:
- ATTR-CM (Davies) Score:
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. General Characteristics of Study Population
3.2. ECG and Echocardiographic Findings
3.3. Biomarkers and Scintigraphy
3.4. Diagnostic Performance: T-Amylo vs. ATTR-CM (Davies)
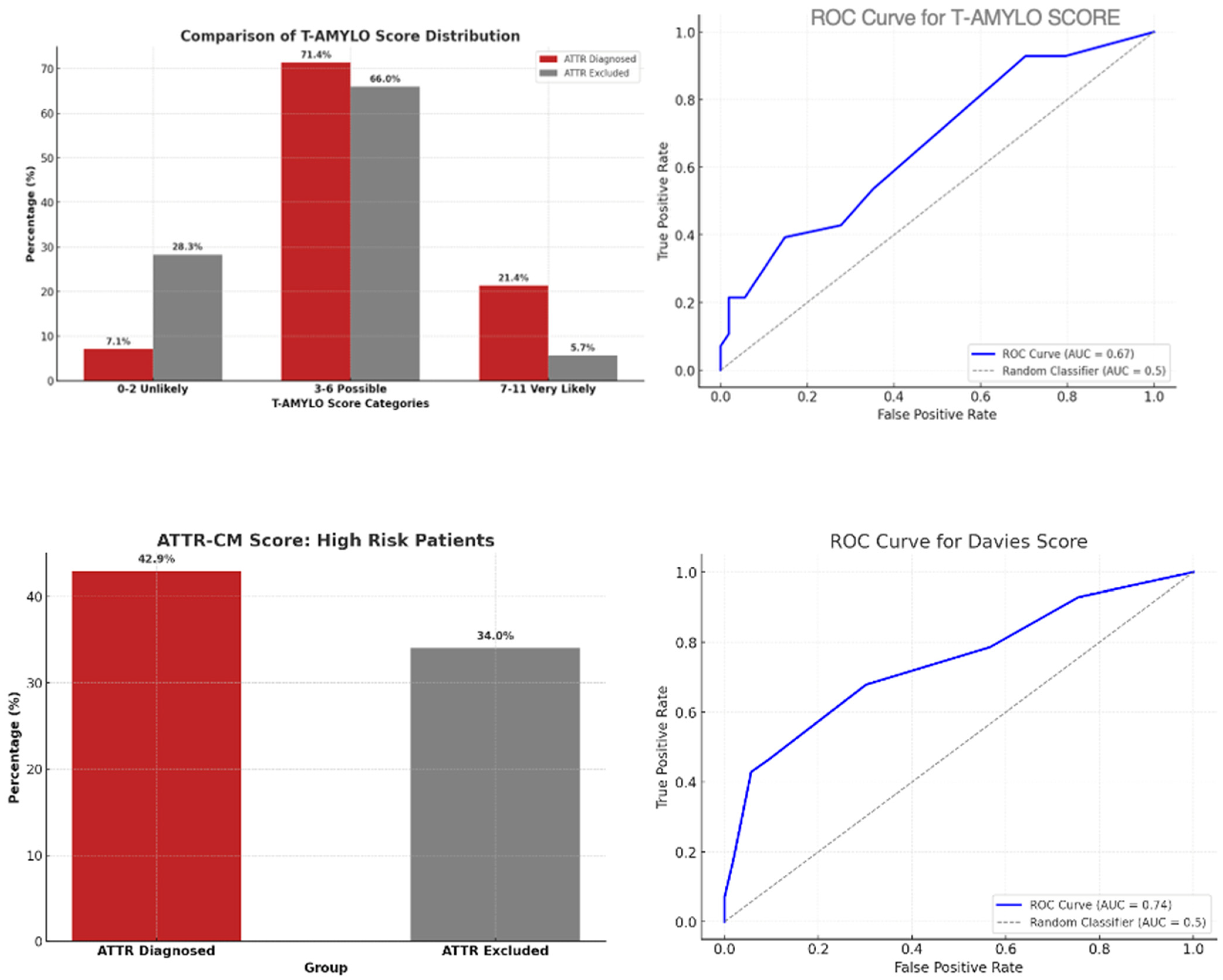
- T-Amylo: sensitivity 91.2%, specificity 65.0%, PPV 70.8%, NPV 89.7%, overall accuracy 77.0%.
- Davies: sensitivity 73.5%, specificity 85.0%, PPV 80.5%, NPV 79.1%, overall accuracy 79.7%.
- In the general population, T-Amylo was more sensitive, and Davies more specific.
- Agreement between T-Amylo and Davies was moderate, suggesting complementary diagnostic profiles.
- Dual-score strategies may optimize accuracy depending on the clinical setting.
4. Discussion
4.1. Comparison with Existing Literature
4.2. Clinical Implications
- Apply T-Amylo to screen and select patients for further evaluation;
- Use Davies to confirm high-probability cases before advanced imaging or biopsy.
4.3. Alignment with Evolving Diagnostic Frameworks
4.4. Limitations and Future Directions
- Prospective multicenter validation in more diverse populations (including women and non-Caucasian patients).
- Integration with advanced imaging (CMR extracellular volume, PET tracers) to refine intermediate-risk classification.
- Automated calculation of scores within electronic health records to trigger early referral.
- Prognostic studies to determine whether baseline score levels predict response to tafamidis or RNA silencing therapies.
5. Conclusions
- ATTRwt-CM is underdiagnosed in elderly patients with HFpEF and LVH.
- We compared T-Amylo and Davies scores in a consecutive real-world cohort.
- T-Amylo: sensitivity 91.2%, high NPV—ideal for initial screening.
- Davies: specificity 85.0%, high PPV—ideal for confirmation.
- Moderate agreement suggests complementary diagnostic roles.
- Sequential use of T-Amylo followed by Davies may optimize efficiency. This approach could reduce unnecessary imaging and expedite therapy.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Cardiac Amyloidosis. |
| HFpEF | Heart Failure with Preserved Ejection Fraction. |
| LVH | Left Ventricular Hypertrophy. |
| ATTRwt(-CM) | Wild-Type Transthyretin Cardiac Amyloidosis. |
| ATTRv | Variant Transthyretin Amyloidosis. |
| ATTR-CM | Transthyretin Cardiac Amyloidosis (generico). |
| AL-CM | Light-chain Cardiac Amyloidosis. |
| ECG | Electrocardiogram. |
| TTE | Transthoracic Echocardiography. |
| NT-proBNP | N-Terminal Pro-B-type Natriuretic Peptide. |
| GLS | Global Longitudinal Strain. |
| AUC | Area Under the Curve. |
| PPV | Positive Predictive Value. |
| NPV | Negative Predictive Value. |
References
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- González-López, E.; Gallego-Delgado, M.; Guzzo-Merello, G.; de Haro-Del Moral, F.J.; Cobo-Marcos, M.; Robles, C.; Bornstein, B.; Salas, C.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015, 36, 2585–2594. [Google Scholar] [CrossRef]
- Tubben, A.; Tingen, H.S.A.; Prakken, N.H.J.; van Empel, V.P.; Gorter, T.M.; Meems, L.M.; Manintveld, O.C.; Rienstra, M.; Tieleman, R.G.; Glaudemans, A.W.; et al. Prevalence of wild-type transthyretin amyloidosis in a prospective heart failure cohort with preserved and mildly reduced ejection fraction: Results of the Amylo-VIP-HF study. Eur. J. Heart Fail. 2024, 26, 695–698. [Google Scholar] [CrossRef]
- Healy, L.; Giblin, G.; Gray, A.; Starr, N.; Murphy, L.; O’SUllivan, D.; Kavanagh, E.; Howley, C.; Tracey, C.; Morrin, E.; et al. Prevalence of transthyretin cardiac amyloidosis in undifferentiated heart failure with preserved ejection fraction. ESC Heart Fail. 2025, 12, 1176–1182. [Google Scholar] [CrossRef]
- García-Pavía, P.; García-Pinilla, J.M.; Lozano-Bahamonde, A.; Yun, S.; García-Quintana, A.; Gavira-Gómez, J.J.; Aibar-Arregui, M.Á.; Barge-Caballero, G.; Villota, J.N.; Bernal, L.; et al. Prevalence of transthyretin cardiac amyloidosis in patients with heart failure with preserved ejection fraction: The PRACTICA study. Rev. Esp. Cardiol. (Engl. Ed.) 2025, 78, 301–310. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Maurer, M.S. Cardiac Amyloidosis Due to Transthyretin Protein: A Review. JAMA 2024, 331, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.A.; Peiró-Aventin, B.; Biagioni, G.; Tini, G.; Saturi, G.; Kronberger, C.; Achten, A.; Dobner, S.; Rijdt, W.P.T.; Gasperetti, A.; et al. Evaluation of the 2021 ESC recommendations for family screening in hereditary transthyretin cardiac amyloidosis. Eur. J. Heart Fail. 2024, 26, 2025–2034. [Google Scholar] [PubMed]
- Kittleson, M.M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Frantz, J.G.; Hershberger, R.E.; Maurer, M.S.; et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient With Cardiac Amyloidosis. JACC 2023, 81, 1076–1126, Erratum in JACC 2023, 81, 1135. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef]
- Kadakia, K.T.; Kittleson, M.M.; Krumholz, H.M. The Evolving Therapeutic Paradigm for Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2025, 85, 1907–1910. [Google Scholar] [CrossRef]
- Porcari, A.; Sinagra, G.; Gillmore, J.D.; Fontana, M.; Hawkins, P.N. Breakthrough advances enhancing care in ATTR amyloid cardiomyopathy. Eur. J. Intern. Med. 2024, 123, 29–36. [Google Scholar] [CrossRef]
- Maurer, M.S.; Kale, P.; Fontana, M.; Berk, J.L.; Grogan, M.; Gustafsson, F.; Hung, R.R.; Gottlieb, R.L.; Damy, T.; González-Duarte, A.; et al. Patisiran Treatment in Patients with Transthyretin Cardiac Amyloidosis. N. Engl. J. Med. 2023, 389, 1553–1565. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Judge, D.P.; Cappelli, F.; Fontana, M.; Garcia-Pavia, P.; Gibbs, S.; Grogan, M.; Hanna, M.; Hoffman, J.; Masri, A.; et al. Efficacy and Safety of Acoramidis in Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2024, 390, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Fontana, M.; Berk, J.L.; Gillmore, J.D.; Witteles, R.M.; Grogan, M.; Drachman, B.; Damy, T.; Garcia-Pavia, P.; Taubel, J.; Solomon, S.D.; et al. Vutrisiran in Patients with Transthyretin Amyloidosis with Cardiomyopathy. N. Engl. J. Med. 2025, 392, 33–44. [Google Scholar] [CrossRef]
- Fontana, M.; Solomon, S.D.; Kachadourian, J.; Walsh, L.; Rocha, R.; Lebwohl, D.; Smith, D.; Täubel, J.; Gane, E.J.; Pilebro, B.; et al. CRISPR-Cas9 Gene Editing with Nexiguran Ziclumeran for ATTR Cardiomyopathy. N. Engl. J. Med. 2024, 391, 2231–2241. [Google Scholar] [CrossRef]
- Arana-Achaga, X.; Goena-Vives, C.; Villanueva-Benito, I.; Solla-Ruiz, I.; Jimenez, A.R.; Gaspar, T.I.; Urreta-Barallobre, I.; Barge-Caballero, G.; Seijas-Marcos, S.; Cabrera, E.; et al. Development and Validation of a Prediction Model and Score for Transthyretin Cardiac Amyloidosis Diagnosis. JACC Cardiovasc. Imaging 2023, 16, 1567–1580. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.R.; Redfield, M.M.; Scott, C.G.; Minamisawa, M.; Grogan, M.; Dispenzieri, A.; Chareonthaitawee, P.; Shah, A.M.; Shah, S.J.; Wehbe, R.M.; et al. A Simple Score to Identify Increased Risk of Transthyretin Amyloid Cardiomyopathy in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2022, 7, 1036–1044. [Google Scholar] [CrossRef]
- Valentini, F.; Anselmi, F.; Metra, M.; Cavigli, L.; Giacomin, E.; Focardi, M.; Cameli, M.; Mondillo, S.; D’Ascenzi, F. Diagnostic and prognostic value of low QRS voltages in cardiomyopathies: Old but gold. Eur. J. Prev. Cardiol. 2022, 29, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Gorgels, A.; Hancock, E.W.; Josephson, M.; Kligfield, P.; et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part IV: The ST segment, T and U waves, and the QT interval: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 982–991. [Google Scholar]
- Cuddy, S.A.M.; Chetrit, M.; Jankowski, M.; Desai, M.; Falk, R.H.; Weiner, R.B.; Klein, A.L.; Phelan, D.; Grogan, M. Practical Points for Echocardiography in Cardiac Amyloidosis. J. Am. Soc. Echocardiogr. 2022, 35, A31–A40. [Google Scholar] [CrossRef]
- Burgos, L.M.; Spaccavento, A.; Ballari, F.N.; Seia, I.M.; Rodríguez, M.d.R.; Vila, R.C.B.; Elissamburu, P.; Meretta, A.H.; Diez, M.; Costabel, J.P. T-Amylo Score for the Diagnosis of Transthyretin Cardiac Amyloidosis in Patients With Acute Heart Failure. Int. J. Heart Fail. 2025, 7, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, G.S.; Onuegbu, C.; Espiche, C.; Scotti, A.; Ippolito, P.; Dwaah, H.; Gilman, J.; Tauras, J.; Schenone, A.L.; Slomka, P.J.; et al. Performance of Clinical Scoring Systems in the Diagnosis of Transthyretin Amyloid Cardiomyopathy in a Diverse Patient Cohort. J. Card. Fail. 2025, 31, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.; Lopez-Jimenez, F.; Cohen-Shelly, M.; Dispenzieri, A.; Attia, Z.I.; Abou Ezzedine, O.F.; Lin, G.; Kapa, S.; Borgeson, D.D.; Friedman, P.A.; et al. Artificial Intelligence-Enhanced Electrocardiogram for the Early Detection of Cardiac Amyloidosis. Mayo Clin. Proc. 2021, 96, 2768–2778, Erratum in Mayo Clin. Proc. 2023, 98, 211. [Google Scholar] [CrossRef]
- Slivnick, J.A.; Hawkes, W.; Oliveira, J.; Woodward, G.; Akerman, A.; Gomez, A.; Hamza, I.; Desai, V.K.; Barrett-O’kEefe, Z.; Grogan, M.; et al. Cardiac amyloidosis detection from a single echocardiographic video clip: A novel artificial intelligence-based screening tool. Eur. Heart J. 2025. advance online publication. [Google Scholar] [CrossRef] [PubMed]
| T-AMYLO Score | |
|---|---|
| Age ≥ 80 ys | 1 pt |
| IVSd thickness ≥ 16 mm | 2 pt |
| Low QRS voltage | 2 pt |
| Male gender | 3 pt |
| Carpal tunnel syndrome | 3 pt |
| High risk: 7–11 pt | |
| Bone scintigraphy and light chain analysis | |
| Intermediate risk: 3–6 pt | |
| Reconsider red flags | |
| Low risk: 0–2 pt | |
| Reconsider differential diagnosis of hypertrophic phenotype | |
| Adapted from [18]. | |
| ATTR-CM Score | |
| Age | |
| 60–69 | +2 pt |
| 70–79 | +3 pt |
| ≥80 | +4 pt |
| Male gender | +2 pt |
| Hypertension diagnosis | −1 pt |
| Ejection fraction (EF) < 60% | +1 pt |
| PWd thickness ≥ 12 mm | +1 pt |
| Relative wall thickness > 0.57 | +2 pt |
| High risk score ≥ 6 Require confirmatory bone scintigraphy | |
| Adapted from [19]. | |
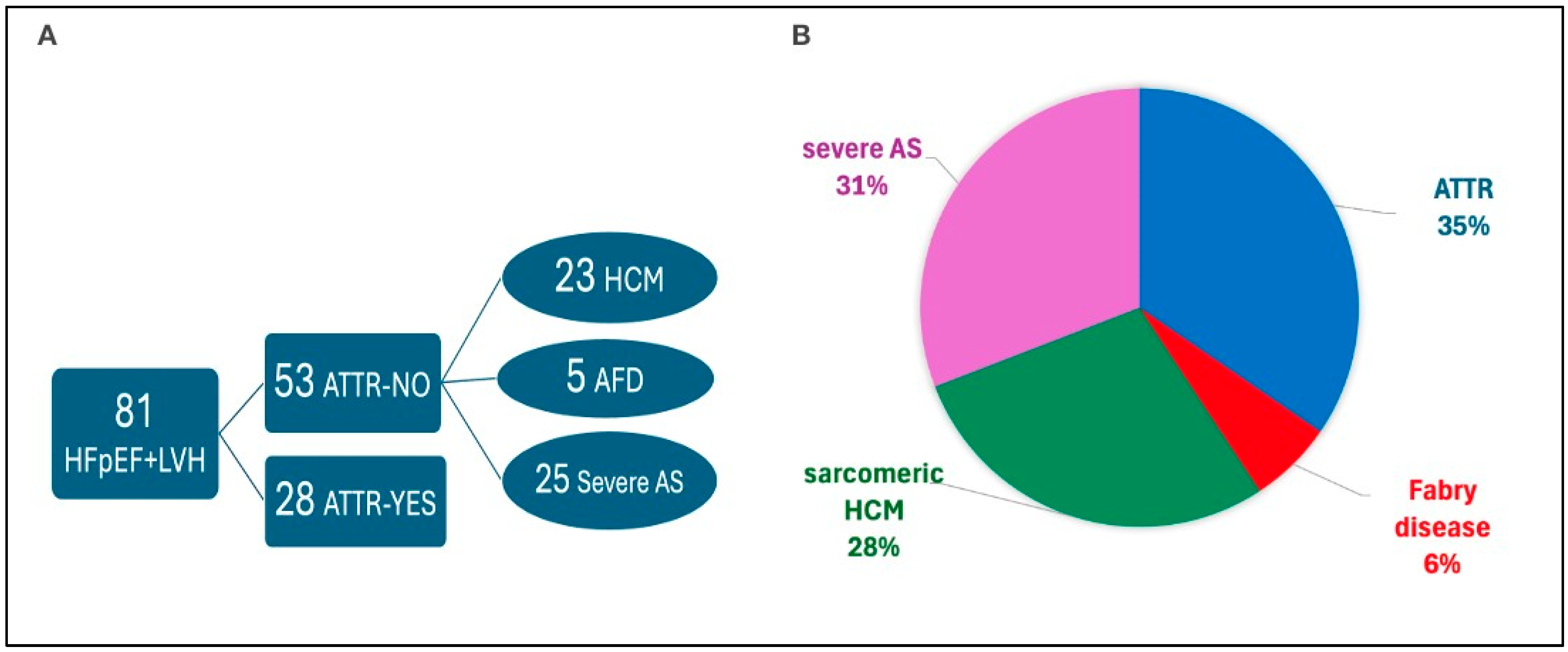
| Parameter | ALL N = 81 | ATTR Yes N = 28 | ATTR No N = 53 | p-Value |
|---|---|---|---|---|
| ATTR wild-type, n (%) | 18 (22.2) | 18 (64.3) | 0 (0) | <0.001 |
| ATTR variant, n (%) | 10 (12.3) | 10 (35.7) | 0 (0) | <0.001 |
| Age, years | 71.8 ± 12.7 | 69.2 ± 16.8 | 73.2 ± 9.8 | 0.176 |
| M, n (%) F, n (%) | 49 (60.5) 32 (39.5) | 19 (67.9) 9 (32.1) | 30 (56.6) 23 (43.4) | 0.032 |
| BSA, mq | 1.8 ± 0.26 | 1.8 ± 0.26 | 1.8 ± 0.26 | 0.884 |
| BMI, kg/mq | 27.3 ± 5.3 | 28.8 ± 4.4 | 27.6 ± 5.7 | 0.533 |
| NYHA 1, n (%) | 9 (11.1) | 7 (25.0) | 2 (3.8) | 0.003 |
| NYHA 2, n (%) | 56 (69.1) | 18 (64.3) | 38 (71.7) | 0.613 |
| NYHA 3, n (%) | 16 (19.8) | 3 (10.7) | 13 (24.5) | 0.137 |
| HR, bpm | 68.6 ± 11.0 | 67.3 ± 11.8 | 69.3 ± 10.5 | 0.438 |
| BPs, mmHg | 132.4 ± 15.1 | 132.1 ± 13.4 | 132.5 ± 16.1 | 0.928 |
| BPd, mmHg | 76.7 ± 10.3 | 79.8 ± 6.6 | 74.9 ± 11.5 | 0.043 |
| Hypertension, n (%) | 66 (81.5) | 22 (78.6) | 44 (83) | 0.624 |
| Diabetes, n (%) | 17 (21.0) | 4 (14.3) | 13 (24.5) | 0.281 |
| Dyslipidemia, n (%) | 42 (51.9) | 12 (42.9) | 30 (56.6) | 0.239 |
| Pharmacological therapy | ||||
| Beta-blockers, n (%) | 52 (64.2) | 14 (50) | 38 (71.7) | 0.052 |
| MRAs | 10 (12.3) | 7 (25) | 3 (5.7) | 0.012 |
| Amyodarone | 3 (3.7) | 0 (0) | 3 (5.7) | 0.199 |
| Other AAD | 1 (1.2) | 1 (3.6) | 0 (0) | 0.166 |
| ACEi/ARBs | 47 (58.0) | 15 (53.6) | 32 (60.4) | 0.555 |
| Diuretics | 43 (53.1) | 15 (53.6) | 28 (52.8) | 0.949 |
| Anticoagulants | 22 (27.2) | 9 (32.1) | 13 (24.5) | 0.463 |
| Antiplatelets | 34 (42.0) | 6 (21.4) | 28 (52.8) | 0.006 |
| ALL N = 81 | ATTR Yes N = 28 | ATTR No N = 53 | p-Value | |
|---|---|---|---|---|
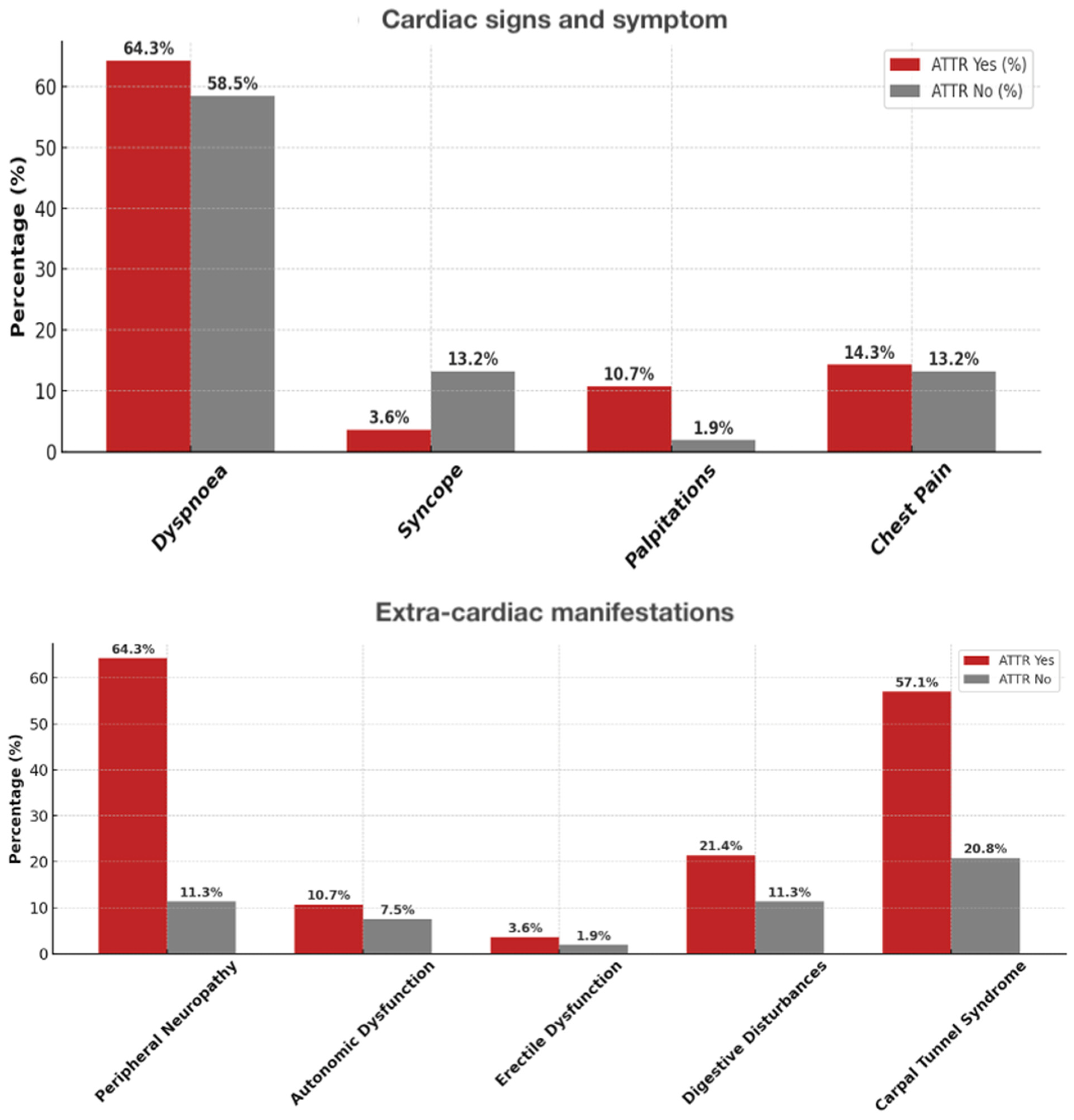
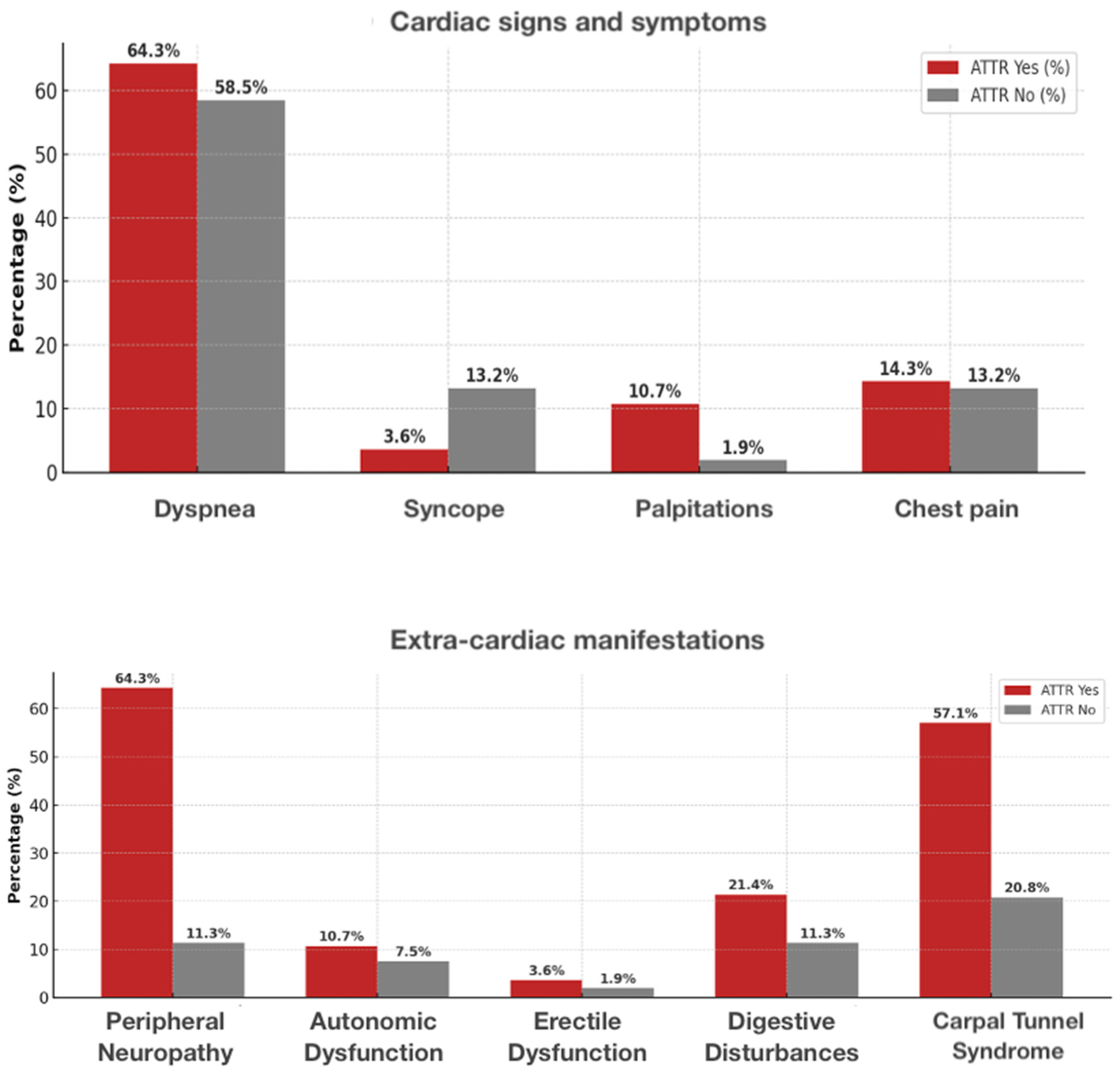
| Dyspnea (n, %) | 49 (60.5) | 18 (64.3) | 31 (58.5) | 0.641 |
| Syncope | 8 (9.9) | 1 (3.6) | 7 (13.2) | 0.166 |
| Palpitations | 4 (4.9) | 3 (10.7) | 1 (1.9) | 0.081 |
| Chest pain | 11 (13.6) | 4 (14.3) | 7 (13.2) | 0.892 |
| CAD | 9 (11.1) | 0 (0) | 9 (17.0) | 0.020 |
| PCI | 4 (4.9) | 0 (0) | 4 (7.5) | 0.136 |
| TAVR | 25 (30.9) | 0 (0) | 25 (47.2) | <0.001 |
| Scintigraphy done | 19 (23.5) | 18 (64.3) | 1 (1.9) | <0.001 |
| Perugini score | ||||
| 1, n (%) | 0 (0) | 0 (0) | ||
| 2, n (%) | 8 (9.9) | 8 (28.6) | ||
| 3, n (%) | 9 (11.1) | 9 (32.1) | ||
| BNP, pg/mL | 210 (99; 465) | 349 (100; 457) | 193 (96; 490) | 0.667 |
| NT-proBNP, pg/mL | 2225 (733; 3903) | 2226 (733; 3903) | ||
| Troponin, ng/mL | 25.5 (12.8; 49.8) | 40 (22; 71) | 19 (10; 30) | 0.060 |
| Extracardiac manifestations | ||||
| Peripheral neuropathy, n (%) | 24 (29.6) | 18 (64.3) | 6 (11.3) | <0.001 |
| Autonomic dysfunction | 7 (8.6) | 3 (10.7) | 4 (7.5) | 0.629 |
| Erectile dysfunction | 2 (2.5) | 1 (3.6) | 1 (1.9) | 0.642 |
| Incontinence | 8 (9.9) | 0 (0) | 8 (15.1) | 0.030 |
| Digestive disturbances | 12 (14.8) | 6 (21.4) | 6 (11.3) | 0.223 |
| Itch | 4 (4.9) | 0 (0) | 4 (7.5) | 0.136 |
| Carpal tunnel syndrome | 27 (33.3) | 16 (57.1) | 11 (20.8) | 0.001 |
| Lumbar stenosis | 3 (3.7) | 3 (10.7) | 0 (0) | 0.015 |
| Creatinine, mg/dl | 1 (0.9; 1.3) | 1.1 (0.9; 1.3) | 1.0 (0.9; 1.7) | 0.874 |
| eGFR, ml/min/mq | 59 ± 25 | 69 ± 21 | 53 ± 26 | 0.046 |
| Parameter | ALL N = 81 | ATTR Yes N = 28 | ATTR No N = 53 | p-Value |
|---|---|---|---|---|
| ECG and arrhythmias | ||||
| Holter done, n (%) | 24 (29.6) | 9 (32.1) | 15 (28.3) | 0.718 |
| NSVT | 4 (4.9) | 1 (3.6) | 3 (5.7) | 0.679 |
| AF | 23 (28.4) | 6 (21.4) | 17 (32.1) | 0.312 |
| PR duration, ms | 179.9 ± 42.7 | 194.6 ± 52.6 | 172.5 ± 35.3 | 0.058 |
| QRS duration, ms | 115.1 ± 25.5 | 113.1 ± 26.1 | 115.7 ± 25.6 | 0.742 |
| AVB I, n (%) | 18 (22.2) | 11 (39.3) | 7 (13.2) | 0.007 |
| AVB III | 2 (2.5) | 1 (3.6) | 1 (1.9) | 0.642 |
| LBBB | 5 (6.2) | 2 (7.1) | 3 (5.7) | 0.792 |
| RBBB | 21 (25.9) | 6 (21.4) | 15 (28.3) | 0.502 |
| LAFB | 3 (3.7) | 2 (7.1) | 1 (1.9) | 0.233 |
| Low QRS voltage | 6 (7.4) | 5 (17.9) | 1 (1.9) | 0.009 |
| Pseudo-infarction pattern | 5 (6.2) | 2 (7.1) | 3 (5.7) | 0.823 |
| PM implantation | 8 (9.9) | 3 (10.7) | 5 (9.4) | 0.854 |
| ICD implantation | 3 (3.7) | 0 (0) | 3 (5.7) | 0.199 |
| Echo parameters | ||||
| IVSd, mm | 14.0 ± 3.1 | 13.2 ± 3.2 | 14.5 ± 3.0 | 0.095 |
| LVPWd, mm | 12.0 ± 2.7 | 12.3 ± 2.6 | 11.8 ± 2.8 | 0.507 |
| LVMi, g/mq | 140.7 ± 40.5 | 136.8 ± 40.4 | 144.6 ± 41.0 | 0.483 |
| RWT | 0.51 ± 0.16 | 0.51 ± 0.18 | 0.51 ± 0.15 | 0.942 |
| EF, % | 60 ± 7 | 60 ± 4 | 60 ± 8 | 0.537 |
| E/A | 1.1 (0.82; 1.78) | 1.4 (0.97; 2.02) | 1.0 (0.66; 1.22) | 0.020 |
| E/e′ | 13.2 (11.1; 18.5) | 14.9 (11.5; 18.6) | 12.5 (10.1; 18.0) | 0.244 |
| TRVmax, m/s | 2.7 ± 0.5 | 2.7 ± 0.5 | 2.6 ± 0.6 | 0.601 |
| LAVi, ml/mq | 41.8 ± 17.0 | 35.5 ± 14.8 | 45.8 ± 17.4 | 0.015 |
| RVIDd, cm | 32.3 ± 4.45 | 34 ± 2.5 | 31.8 ± 4.7 | 0.220 |
| RAA, ml/mq | 21.13 ± 7.2 | 22.12 ± 6.8 | 20.5 ± 7.6 | 0.606 |
| TAPSE, mm | 2.1 (1.8; 2.3) | 1.8 (1.6; 2.0) | 2.2 (1.9; 2.4) | <0.001 |
| LV-GLS, % | −15 (−10; −19) | −13 (−10; −17) | −17.5 (−12.3; −19.7) | 0.004 |
| Apical sparing, n (%) | 16 (19.8) | 15 (53.6) | 1 (1.9) | <0.001 |
| Pericardial effusion, n (%) | 21 (25.9) | 11 (39.3) | 10 (18.9) | 0.046 |
| Aortic stenosis, n (%) | 30 (37.0) | 3 (10.7) | 27 (50.9) | <0.0001 |
| Severe, n (%) | 25 (30.9) | 0 (0) | 25 (47.2) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faro, D.C.; Romeo, F.; Losi, V.; Simonetti, D.; Capodanno, D.; Monte, I.P. Diagnostic Predictive Scores of Amyloid Cardiomyopathy in Patients with Heart Failure with Preserved Ejection Fraction and Left Ventricular Hypertrophy. J. Cardiovasc. Dev. Dis. 2025, 12, 417. https://doi.org/10.3390/jcdd12110417
Faro DC, Romeo F, Losi V, Simonetti D, Capodanno D, Monte IP. Diagnostic Predictive Scores of Amyloid Cardiomyopathy in Patients with Heart Failure with Preserved Ejection Fraction and Left Ventricular Hypertrophy. Journal of Cardiovascular Development and Disease. 2025; 12(11):417. https://doi.org/10.3390/jcdd12110417
Chicago/Turabian StyleFaro, Denise Cristiana, Fabrizia Romeo, Valentina Losi, Dario Simonetti, Davide Capodanno, and Ines Paola Monte. 2025. "Diagnostic Predictive Scores of Amyloid Cardiomyopathy in Patients with Heart Failure with Preserved Ejection Fraction and Left Ventricular Hypertrophy" Journal of Cardiovascular Development and Disease 12, no. 11: 417. https://doi.org/10.3390/jcdd12110417
APA StyleFaro, D. C., Romeo, F., Losi, V., Simonetti, D., Capodanno, D., & Monte, I. P. (2025). Diagnostic Predictive Scores of Amyloid Cardiomyopathy in Patients with Heart Failure with Preserved Ejection Fraction and Left Ventricular Hypertrophy. Journal of Cardiovascular Development and Disease, 12(11), 417. https://doi.org/10.3390/jcdd12110417







