Rethinking Childhood-Onset Hypertrophic Cardiomyopathy: A Review of Molecular Mechanisms and Unique Therapy Considerations
Abstract
1. Introduction
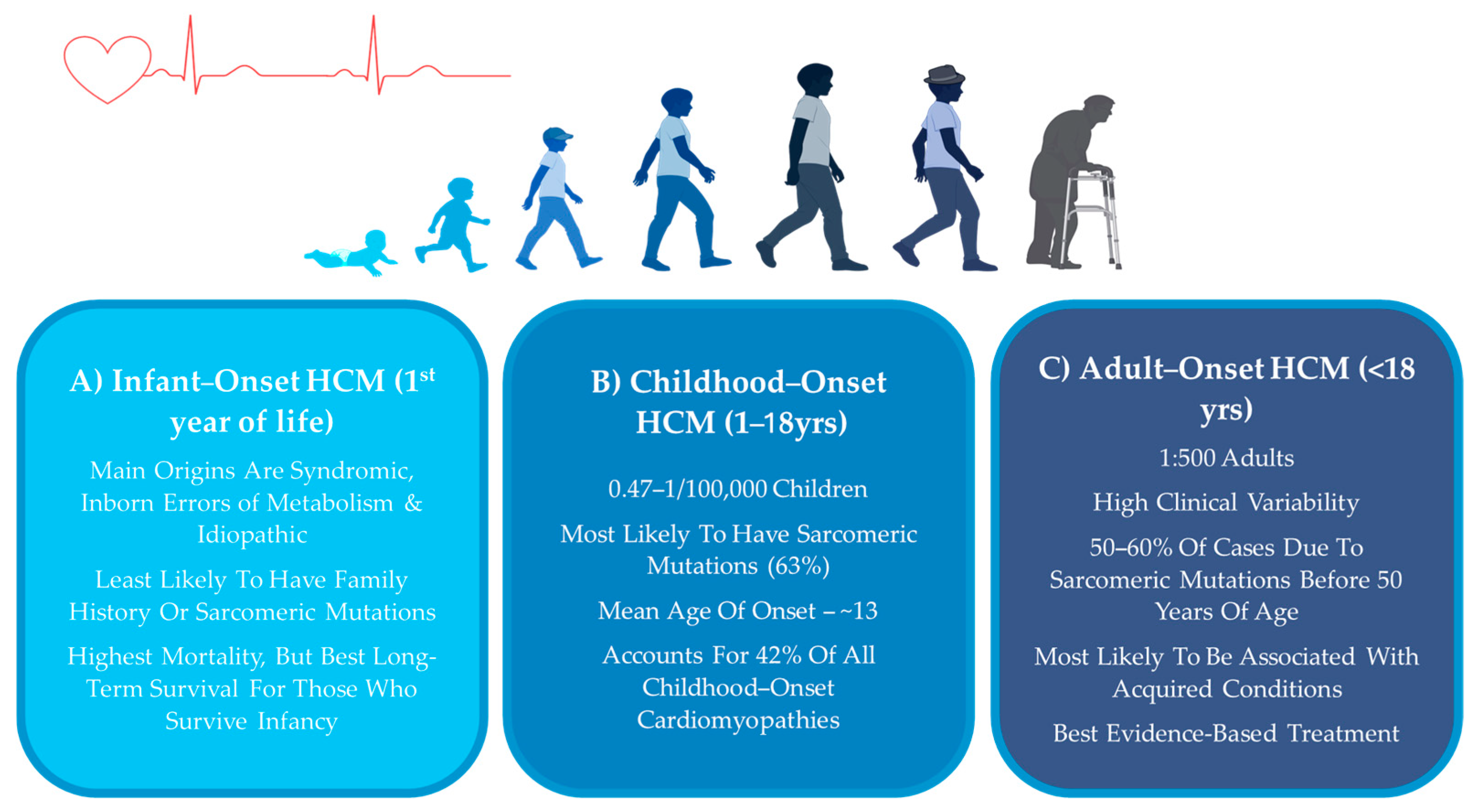
2. HCM Across the Lifespan
2.1. Adult-Onset HCM
2.2. Infantile HCM
2.3. Childhood-Onset HCM
3. HCM Symptoms in Children
4. Molecular Pathophysiology of Childhood-Onset HCM
4.1. Physiological vs. Pathological Cardiac Hypertrophy
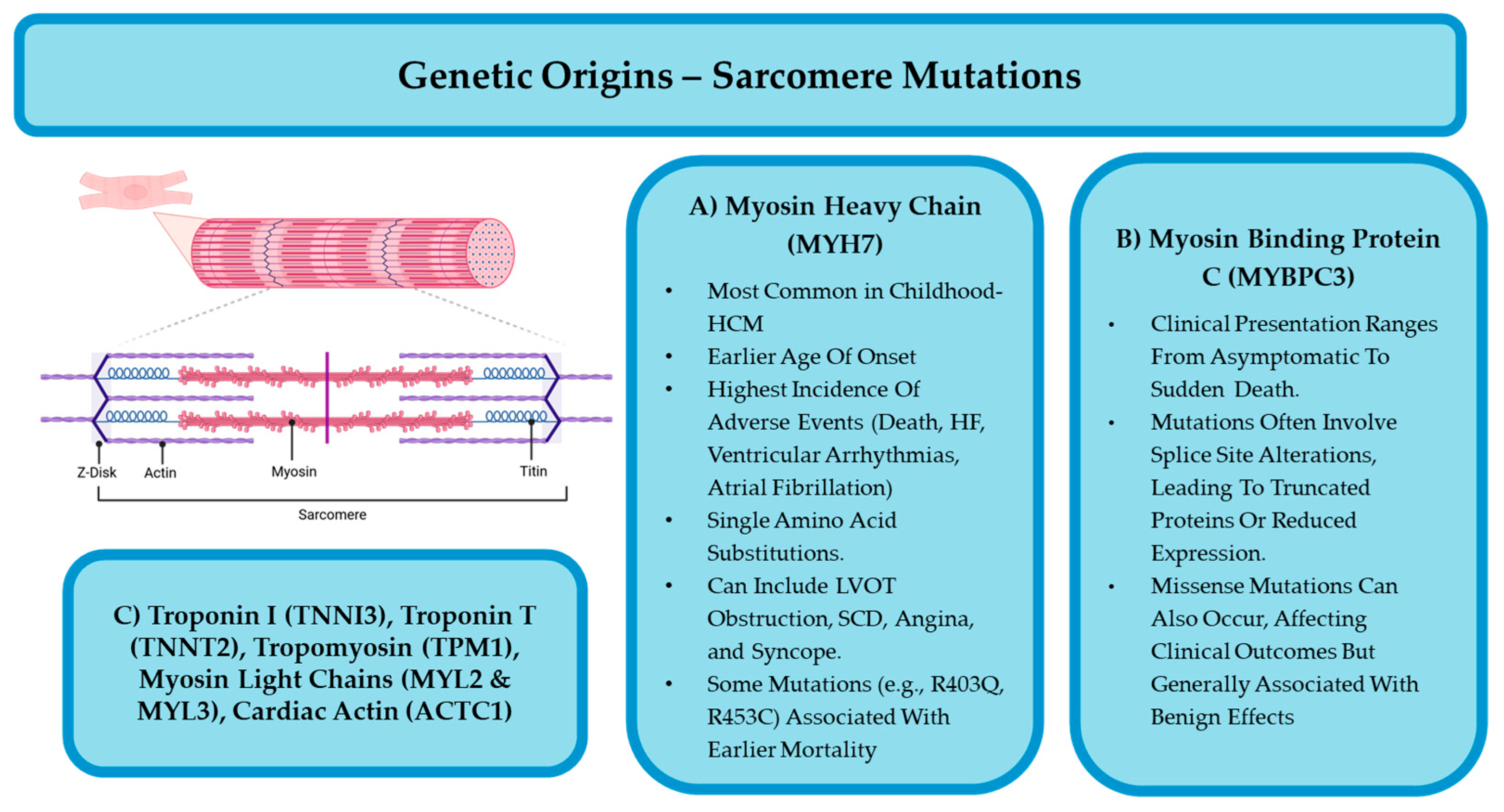
4.2. Sarcomeric and Non-Sarcomeric HCM
4.2.1. Sarcomeric HCM
4.2.2. Non-Sarcomeric HCM
5. Diagnostic Considerations in Children
6. HCM Treatment in Children
6.1. First-Line Pharmacological Treatments of Childhood-Onset HCM
6.1.1. Beta-Adrenergic Antagonists/Blockers
6.1.2. Calcium Channel Blockers
6.2. Second-Line Pharmacological Treatments of Childhood-Onset HCM
6.2.1. Renin–Angiotensin–Aldosterone System (RAAS) Suppressing Agents
6.2.2. Antiarrhythmic Agents
6.3. Surgical Interventions for Pediatric HCM
6.4. Lifestyle Management and Exercise Recommendations in Children with HCM
7. Children Who Progress to Heart Failure
8. Current and Future Research Areas
8.1. Recommendations for Genotype-Positive Phenotype-Negative HCM in Children
8.2. Management of Syndromic and Metabolic-Associated HCM
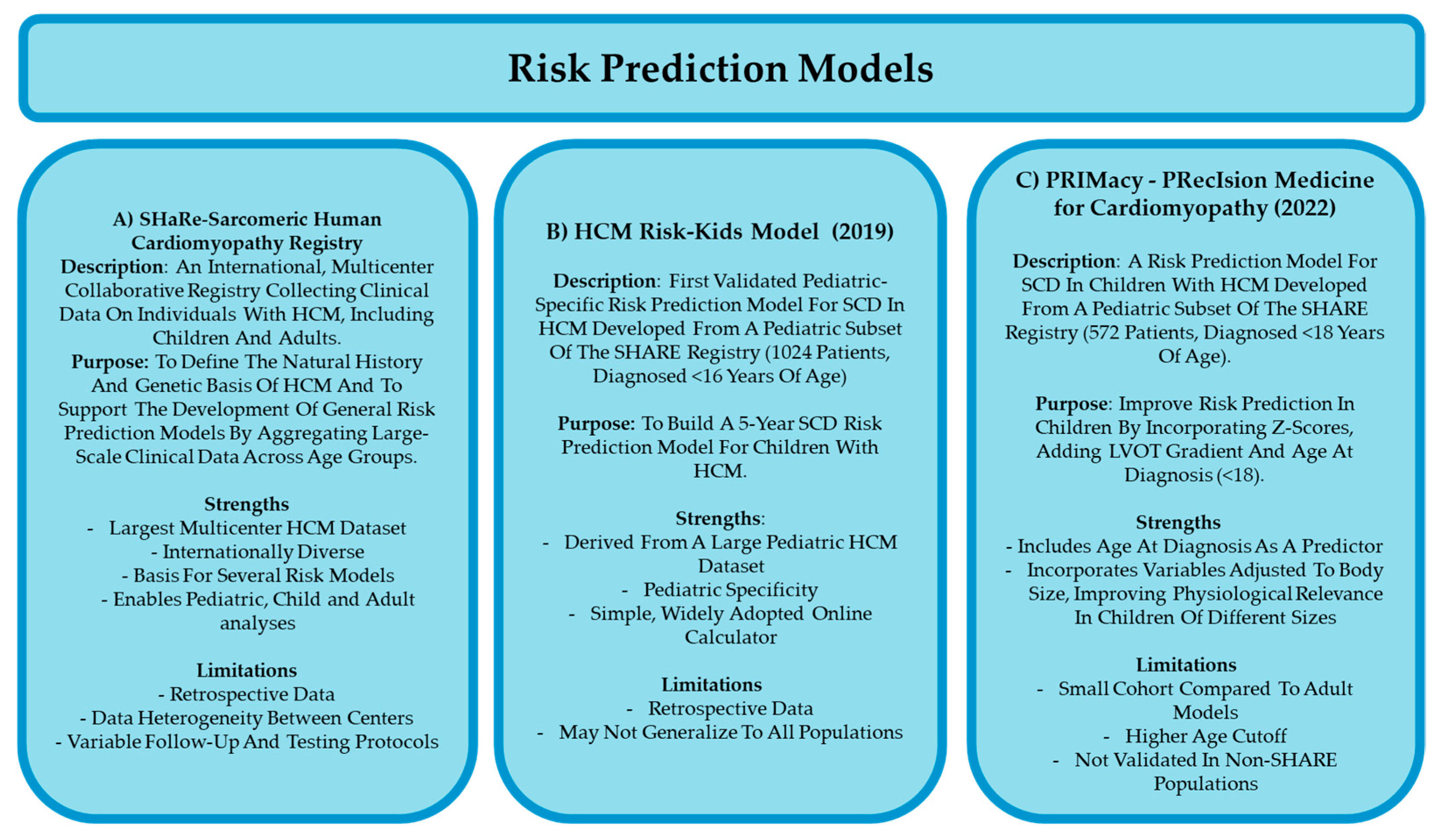
8.3. Risk Prediction Models of Childhood HCM
8.4. Future of HCM Research in Children
8.4.1. Preclinical Studies
8.4.2. Current Clinical Trials
8.4.3. Validation of Diagnostic/Prognostic Tools in Children
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin Converting Enzyme |
| AHA | American Heart Association (AHA) |
| ARBs | Angiotensin Receptor Blockers |
| CCBs | Calcium Channel Blockers |
| ECG | Electrocardiogram |
| ECHO | Echocardiography |
| ESC | European Society of Cardiology |
| HCM | Hypertrophic Cardiomyopathy |
| ICDs | Implantable Cardioverter-Defibrillators |
| LGE | Late Gadolinium Enhancement |
| LVOT | Left Ventricular Outflow Tract |
| MRI | Magnetic Resonance Imaging |
| MYBPC3 | Myosin-Binding Protein C3 |
| MYH7 | Beta-Myosin Heavy Chain |
| PRIMaCY | PRecIsion Medicine for Cardiomyopathy |
| RAAS | Renin–Angiotensin–Aldosterone System |
| SCD | Sudden Cardiac Death |
| SHaRe | Sarcomeric Human Cardiomyopathy Registry |
| TGF-beta | Transforming Growth Factor Beta |
| TNNI3 | Troponin I |
| TNNT2 | Troponin T |
| VANISH | Valsartan for Attenuating Disease Evolution in Early Sarcomeric Hypertrophic Cardiomyopathy |
References
- Marston, N.; Han, L.; Olivotto, I.; Day, S.M.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Ingles, J.; Semsarian, C.; Jacoby, D.; et al. Clinical Characteristics and Outcomes in Childhood-Onset Hypertrophic Cardiomyopathy. Eur. Heart J. 2021, 42, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.; Jeewa, A.; Khoury, M.; Cunningham, C.; George, K.; Conway, J. Unique Aspects of Hypertrophic Cardiomyopathy in Children. Can. J. Cardiol. 2024, 40, 907–920. [Google Scholar] [CrossRef]
- Gregor, P.; Čurila, K. Medical Treatment of Hypertrophic Cardiomyopathy–What Do We Know About It Today? Cor Vasa 2015, 57, e219–e224. [Google Scholar] [CrossRef]
- Arghami, A.; Dearani, J.; Said, S.; O’Leary, P.; Schaff, H. Hypertrophic Cardiomyopathy in Children. Ann. Cardiothorac. Surg. 2017, 6, 376–385. [Google Scholar] [CrossRef]
- Marian, J. Molecular Genetic Basis of Hypertrophic Cardiomyopathy. Circ. Res. 2021, 128, 1533–1553. [Google Scholar] [CrossRef]
- Repetti, G.; Toepfer, C.; Seidman, J.; Seidman, C. Novel Therapies for Prevention and Early Treatment of Cardiomyopathies: Now and in the Future. Circ. Res. 2019, 124, 1536–1550. [Google Scholar] [CrossRef]
- Tsatsopoulou, A.; Protonotarios, I.; Xylouri, Z.; Papagiannis, I.; Anastasakis, A.; Germanakis, I.; Patrianakos, A.; Nyktari, E.; Gavras, C.; Papadopoulos, G.; et al. Cardiomyopathies in Children: An Overview. Hell. J. Cardiol. 2023, 72, 43–56. [Google Scholar] [CrossRef]
- Kaski, J.P.; Norrish, G. Prevention of Sudden Cardiac Death in Childhood-Onset Hypertrophic Cardiomyopathy. Prog. Pediatr. Cardiol. 2021, 62, 101412. [Google Scholar] [CrossRef]
- Abou Alaiwi, S.; Roston, T.M.; Marstrand, P.; Claggett, B.L.; Parikh, V.N.; Helms, A.S.; Ingles, J.; Lampert, R.; Lakdawala, N.K.; Michels, M.; et al. Left Ventricular Systolic Dysfunction in Patients Diagnosed with Hypertrophic Cardiomyopathy During Childhood: Insights from the SHaRe Registry. Circulation 2023, 148, 394–404. [Google Scholar] [CrossRef]
- McKenna, W.J.; Crean, A.; Greenway, S.; Tadros, R.; Veselka, J.; Woo, A. Hypertrophic Cardiomyopathy: Evolution to the Present, Ongoing Challenges, and Opportunities. Can. J. Cardiol. 2024, 40, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Miyamoto, S.; Price, J.; Rossano, J.; Cabrera, A. Pediatric Heart Failure: An Evolving Public Health Concern. J. Pediatr. 2019, 218, 217–221. [Google Scholar] [CrossRef]
- Monda, E.; Rubino, M.; Lioncino, M.; Di Fraia, F.; Pacileo, R.; Verrillo, F.; Cirillo, A.; Caiazza, M.; Fusco, A.; Esposito, A.; et al. Hypertrophic Cardiomyopathy in Children: Pathophysiology, Diagnosis, and Treatment of Non-sarcomeric Causes. Front. Pediatr. 2021, 9, 632293. [Google Scholar] [CrossRef]
- Alromaihi, M.; Alrumaihi, F.; Alwanian, W.M.; Alharbi, H.O.; AlDuayji, N.N.; Alfifi, S.M.; Al-Doaiss, A.A.; Alshabrmi, F.M.; Thornbury, W.; Khan, S.U. A Multidimensional Approach to Understanding Genetic Diversity, Risk Stratification, and Personalized Interventions in Pediatric Hypertrophic Cardiomyopathy. Curr. Probl. Cardiol. 2025, 50, 103040. [Google Scholar] [CrossRef]
- Moak, J.P.; Kaski, J.P. Hypertrophic Cardiomyopathy in Children. Heart 2012, 98, 1044–1054. [Google Scholar] [CrossRef]
- Borrelli, F.; Losi, M.A.; Canciello, G.; Todde, G.; Perillo, E.F.; Ordine, L.; Frisso, G.; Esposito, G.; Lombardi, R. Sarcomeric versus Non-sarcomeric HCM. Cardiogenetics 2023, 13, 92–105. [Google Scholar] [CrossRef]
- Colan, S.D. Hypertrophic Cardiomyopathy in Childhood. Heart Fail. Clin. 2010, 6, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Fetisova, S.; Melnik, O.; Vasichkina, E.; Vershinina, T.; Kofeynikova, O.; Kozyreva, A.; Fomicheva, Y.; Sokolnikova, P.; Zhuk, S.; Pervunina, T.; et al. The Clinical and Genetic Spectrum of Pediatric Hypertrophic Cardiomyopathy Manifesting Before One Year of Age. Pediatr. Res. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Norrish, G.; Field, E.; Kaski, J.P. Childhood Hypertrophic Cardiomyopathy: A Disease of the Cardiac Sarcomere. Front. Pediatr. 2021, 9, 708679. [Google Scholar] [CrossRef] [PubMed]
- Östman-Smith, I. Beta-Blockers in Pediatric Hypertrophic Cardiomyopathies. Rev. Recent Clin. Trials 2014, 9, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Rehm, H.; Menesses, A.; McDonough, B.; Roberts, A.; Kucherlapati, R.; Towbin, J.; Seidman, J.; Seidman, C. Shared Genetic Causes of Cardiac Hypertrophy in Children and Adults. N. Engl. J. Med. 2008, 358, 1899–1908. [Google Scholar] [CrossRef]
- Oldfield, C.; Duhamel, T.; Dhalla, N. Mechanisms for the Transition from Physiological to Pathological Cardiac Hypertrophy. Can. J. Physiol. Pharmacol. 2019, 98, 74–84. [Google Scholar] [CrossRef]
- Ritter, O.; Neyses, L. The Molecular Basis of Myocardial Hypertrophy and Heart Failure. Trends Mol. Med. 2003, 9, 313–321. [Google Scholar] [CrossRef]
- Samak, M.; Fatullayev, J.; Sabashnikov, A.; Zeriouh, M.; Schmack, B.; Farag, M.; Popov, A.; Dohmen, P.; Choi, Y.; Wahlers, T.; et al. Cardiac Hypertrophy: An Introduction to Molecular and Cellular Basis. Med. Sci. Monit. Basic Res. 2016, 22, 75–79. [Google Scholar] [CrossRef]
- Marian, A.; Roberts, R. The Molecular Genetic Basis for Hypertrophic Cardiomyopathy. J. Mol. Cell. Cardiol. 2001, 33, 655–670. [Google Scholar] [CrossRef]
- Zhao, M.; He, X.; Min, X.; Yang, H.; Wu, W.; Zhong, J.; Xu, H.; Chen, J. Recent Clinical Updates of Hypertrophic Cardiomyopathy and Future Therapeutic Strategies. Rev. Cardiovasc. Med. 2025, 26, 25132. [Google Scholar] [CrossRef]
- Akhtar, M.; Elliott, P. The Genetics of Hypertrophic Cardiomyopathy. Glob. Cardiol. Sci. Pract. 2018, 3, 36. [Google Scholar]
- Ren, X.; Hensley, N.; Brady, M.; Gao, W. The Genetic and Molecular Bases for Hypertrophic Cardiomyopathy: The Role for Calcium Sensitization. J. Cardiothorac. Vasc. Anesth. 2018, 32, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Boczek, N.J.; Ye, D.; Jin, F.; Tester, D.J.; Huseby, A.; Bos, J.M.; Johnson, A.J.; Kanter, R.; Ackerman, M.J. Identification and Functional Characterization of a Novel CACNA1C-Mediated Cardiac Disorder Characterized by Prolonged QT Intervals with Hypertrophic Cardiomyopathy, Congenital Heart Defects, and Sudden Cardiac Death. Circ. Arrhythmia Electrophysiol. 2015, 8, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Seggewiss, H.; Rigopoulos, A. Management of Hypertrophic Cardiomyopathy in Children. Paediatr. Drugs 2003, 5, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Axelsson Raja, A.; Farhad, H.; Valente, A.M.; Couce, J.P.; Jefferies, J.L.; Bundgaard, H.; Zahka, K.; Lever, H.; Murphy, A.M.; Ashley, E.; et al. Prevalence and Progression of Late Gadolinium Enhancement in Children and Adolescents with Hypertrophic Cardiomyopathy. Circulation 2018, 138, 782–792. [Google Scholar] [CrossRef]
- Decampli, C.; Pourmoghadam, K.K. Hypertrophic Cardiomyopathy. In Pediatric Cardiac Surgery, 5th ed.; Mavroudis, C., Backer, C.L., Eds.; Wiley Blackwell: Oxford, UK, 2023; pp. 705–719. [Google Scholar]
- Tuohy, C.V.; Kaul, S.; Song, H.K.; Nazer, B.; Heitner, S.B. Hypertrophic Cardiomyopathy: The Future of Treatment. Eur. J. Heart Fail. 2020, 22, 228–240. [Google Scholar] [CrossRef]
- Grenier, M.A.; Fioravanti, J.; Truesdell, S.C.; Mendelsohn, A.M.; Vermilion, R.P.; Lipshultz, S.E. Angiotensin-Converting Enzyme Inhibitor Therapy for Ventricular Dysfunction in Infants, Children and Adolescents: A Review. Prog. Pediatr. Cardiol. 2000, 12, 91–111. [Google Scholar] [CrossRef]
- Stotter, B.R.; Ferguson, M.A. Should ACE Inhibitors and ARBs Be Used in Combination in Children? Pediatr. Nephrol. 2019, 34, 1521–1532. [Google Scholar] [CrossRef]
- Smeets, N.J.L.; Schreuder, M.F.; Dalinghaus, M.; Male, C.; Lagler, F.B.; Walsh, J.; Laer, S.; de Wildt, S.N. Pharmacology of Enalapril in Children: A Review. Drug Discov. Today 2020, 25, 1957–1970. [Google Scholar] [CrossRef]
- Cantarutti, N.; Mencarelli, E.; Di Marzio, S.; Cicenia, M.; Battipaglia, I.; Ingrasciotta, G.; D’Anna, C.; Franceschini, A.; Drago, F.; Amodeo, A.; et al. Efficacy and Safety of ACE Inhibitors in Pediatric Hypertrophic Cardiomyopathy. J. Heart Lung Transplant. 2025, 44, S636. [Google Scholar] [CrossRef]
- Vissing, C.R.; Axelsson Raja, A.; Day, S.M.; Russell, M.W.; Zahka, K.; Lever, H.M.; Pereira, A.C.; Colan, S.D.; Margossian, R.; Murphy, A.M.; et al. Cardiac Remodeling in Subclinical Hypertrophic Cardiomyopathy: The VANISH Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 1083–1088. [Google Scholar] [CrossRef]
- Ho, C.Y.; Day, S.M.; Axelsson, A.; Russell, M.W.; Zahka, K.; Lever, H.M.; Pereira, A.C.; Colan, S.D.; Margossian, R.; Murphy, A.M.; et al. Valsartan in Early-Stage Hypertrophic Cardiomyopathy: A Randomized Phase 2 Trial. Nat. Med. 2021, 27, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Lesser, J.R.; Garberich, R.F.; McGriff, D.M.; Maron, M.S. Hypertrophic Cardiomyopathy in Children, Adolescents, and Young Adults Associated with Low Cardiovascular Mortality with Contemporary Management Strategies. Circulation 2016, 133, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.M.; Zenker, M.; Boleti, O.; Norrish, G.; Russell, M.; Meisner, J.K.; Peng, D.M.; Prendiville, T.; Kleinmahon, J.; Kantor, P.F.; et al. Impact of MEK Inhibition on Childhood RASopathy-Associated Hypertrophic Cardiomyopathy. JACC Basic Transl. Sci. 2025, 10, 152–166. [Google Scholar] [CrossRef]
- Norrish, G.; Ding, T.; Field, E.; Ziólkowska, L.; Olivotto, I.; Limongelli, G.; Anastasakis, A.; Weintraub, R.; Biagini, E.; Ragni, L.; et al. Development of a Novel Risk Prediction Model for Sudden Cardiac Death in Childhood Hypertrophic Cardiomyopathy (HCM Risk-Kids). JAMA Cardiol. 2019, 4, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Norrish, G.; Qu, C.; Field, E.; Cervi, E.; Khraiche, D.; Klaassen, S.; Ojala, T.H.; Sinagra, G.; Yamazawa, H.; Marrone, C.; et al. External Validation of the HCM Risk-Kids Model for Predicting Sudden Cardiac Death in Childhood Hypertrophic Cardiomyopathy. Eur. J. Prev. Cardiol. 2022, 29, 678–686. [Google Scholar] [CrossRef]
- Boleti, O.D.; Roussos, S.; Norrish, G.; Field, E.; Oates, S.; Tollit, J.; Nepali, G.; Bhole, V.; Uzun, O.; Daubeney, P.E.F.; et al. Sudden cardiac death in childhood RASopathy-associated hypertrophic cardiomyopathy: Validation of the HCM Risk-Kids model and predictors of events. Int. J. Cardiol. 2023, 393, 131405. [Google Scholar] [CrossRef] [PubMed]
- Norrish, G.; Protonotarios, A.; Stec, M.; Boleti, O.; Field, E.; Cervi, E.; Elliott, P.M.; Kaski, J.P. Performance of the PRIMaCY Sudden Death Risk Prediction Model for Childhood Hypertrophic Cardiomyopathy: Implications for Implantable Cardioverter-Defibrillator Decision-Making. Europace 2023, 25, euad330. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Lakdawala, N.K.; Cirino, A.L.; Lipshultz, S.E.; Sparks, E.; Abbasi, S.A.; Kwong, R.Y.; Antman, E.M.; Semsarian, C.; González, A.; et al. Diltiazem treatment for pre-clinical hypertrophic cardiomyopathy sarcomere mutation carriers: A pilot randomized trial to modify disease expression. JACC Heart Fail. 2015, 3, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.T.; Fan, S.W.D. Prevalence and Clinical Significance of Late Gadolinium Enhancement in Children and Adolescents with Hypertrophic Cardiomyopathy: A Systematic Review and Meta-analysis. Cardiol. Young 2024, 34, 1456–1465. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menzies, C.; Dolinsky, V.W. Rethinking Childhood-Onset Hypertrophic Cardiomyopathy: A Review of Molecular Mechanisms and Unique Therapy Considerations. J. Cardiovasc. Dev. Dis. 2025, 12, 374. https://doi.org/10.3390/jcdd12100374
Menzies C, Dolinsky VW. Rethinking Childhood-Onset Hypertrophic Cardiomyopathy: A Review of Molecular Mechanisms and Unique Therapy Considerations. Journal of Cardiovascular Development and Disease. 2025; 12(10):374. https://doi.org/10.3390/jcdd12100374
Chicago/Turabian StyleMenzies, Caitlin, and Vernon W. Dolinsky. 2025. "Rethinking Childhood-Onset Hypertrophic Cardiomyopathy: A Review of Molecular Mechanisms and Unique Therapy Considerations" Journal of Cardiovascular Development and Disease 12, no. 10: 374. https://doi.org/10.3390/jcdd12100374
APA StyleMenzies, C., & Dolinsky, V. W. (2025). Rethinking Childhood-Onset Hypertrophic Cardiomyopathy: A Review of Molecular Mechanisms and Unique Therapy Considerations. Journal of Cardiovascular Development and Disease, 12(10), 374. https://doi.org/10.3390/jcdd12100374







