Virtual Stenting Based on Fractional Flow Reserve Derived from Computed Tomography in Predicting Post-Percutaneous Coronary Intervention Functional Outcomes: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
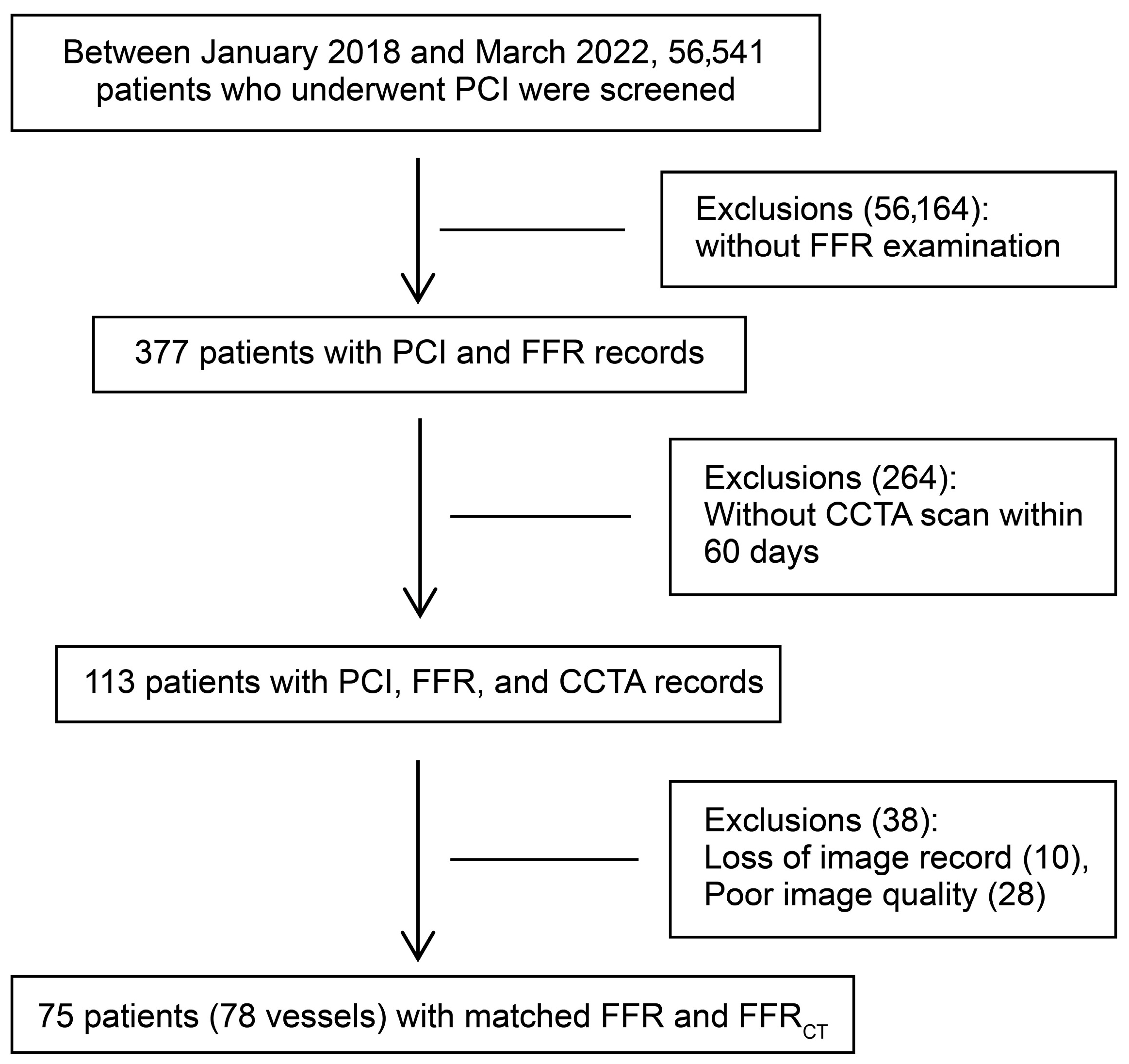
2.1. Study Population and Design
2.2. Clinical Data-Collection Methods
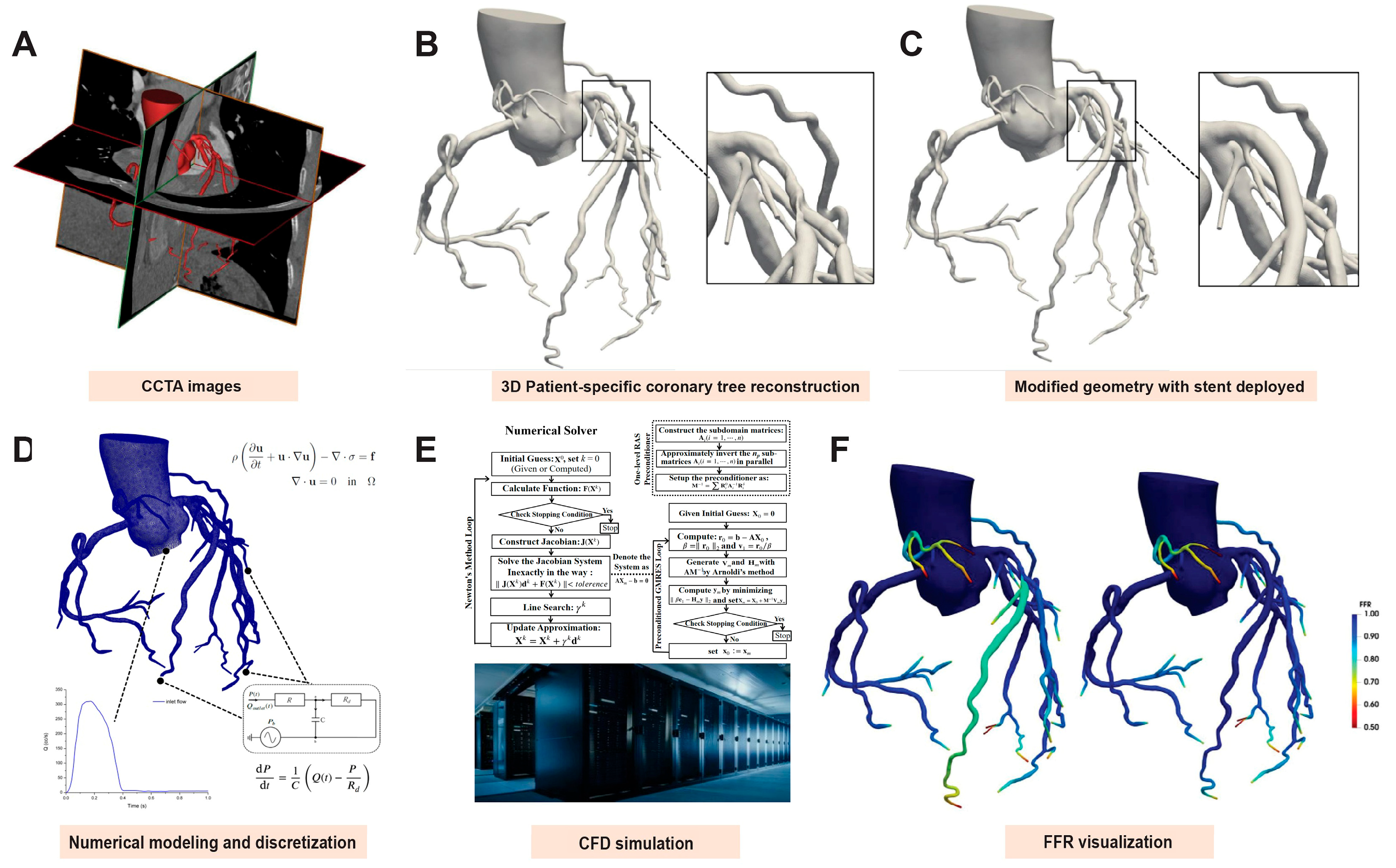
2.3. Coronary Reconstruction and Virtual Stent Deployment
2.4. Non-Invasive FFR Analysis
2.5. Statistical Analysis
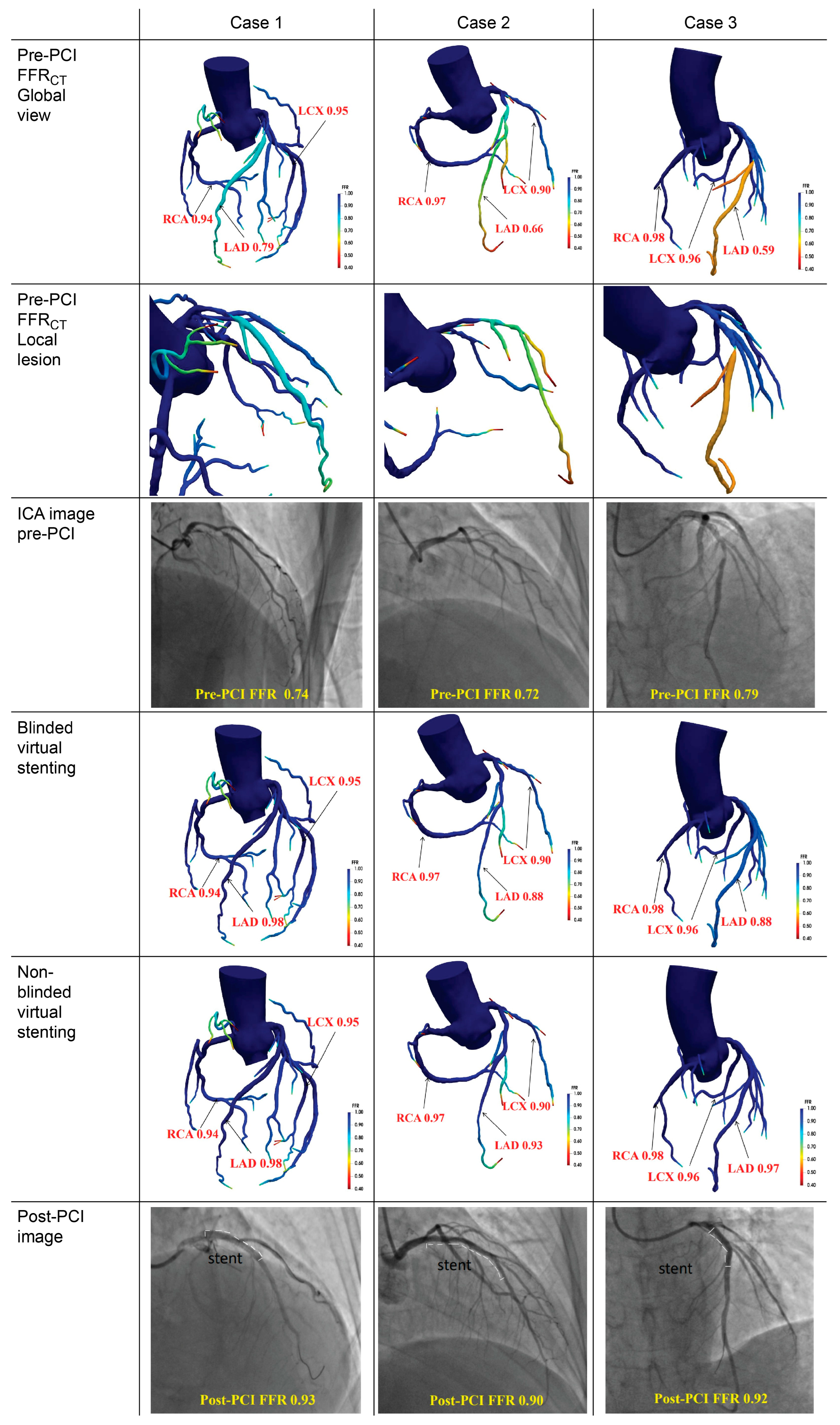
3. Results
3.1. Baseline Characteristics
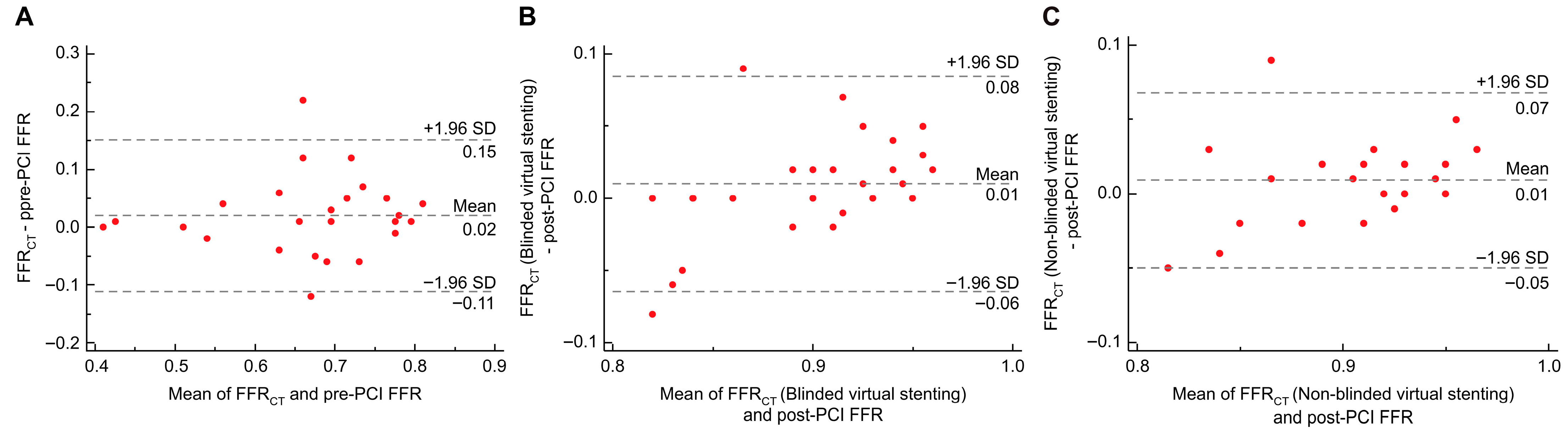
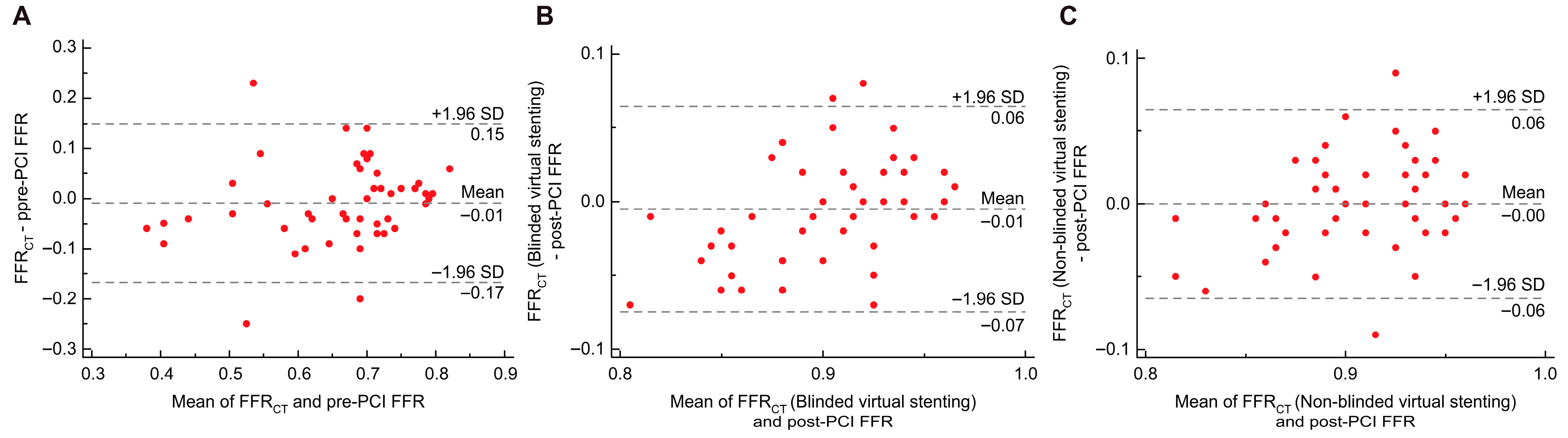
3.2. Overall Agreement Analysis
3.3. Diagnostic Efficacy and Cohort-Stratified Validation
3.3.1. Diagnostic Efficacy
3.3.2. Cohort-Stratified Validation
3.4. Regression Models and Error Analysis
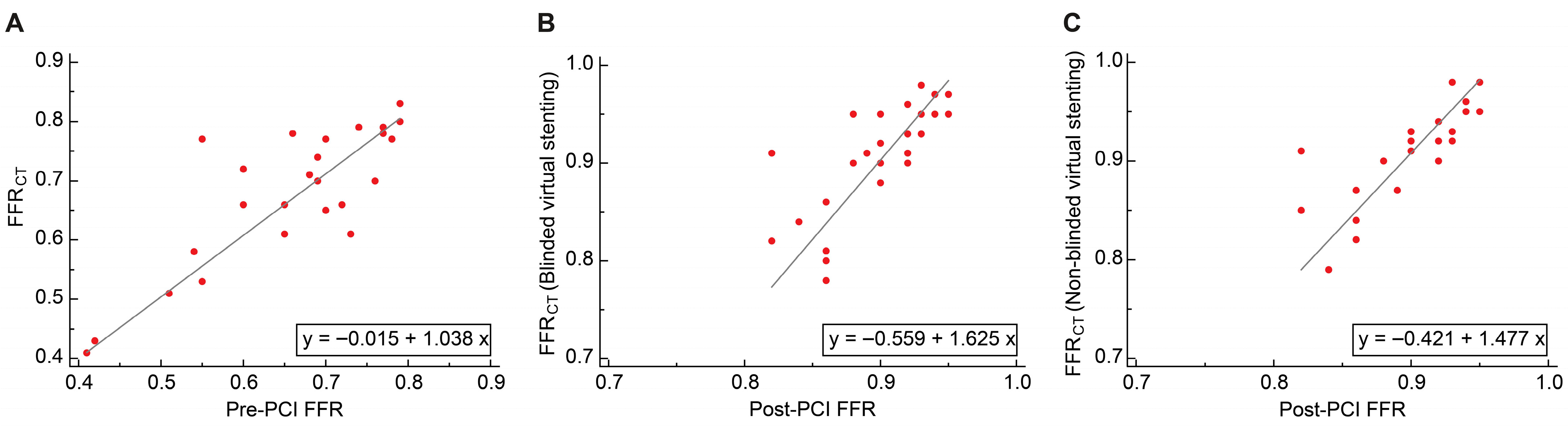
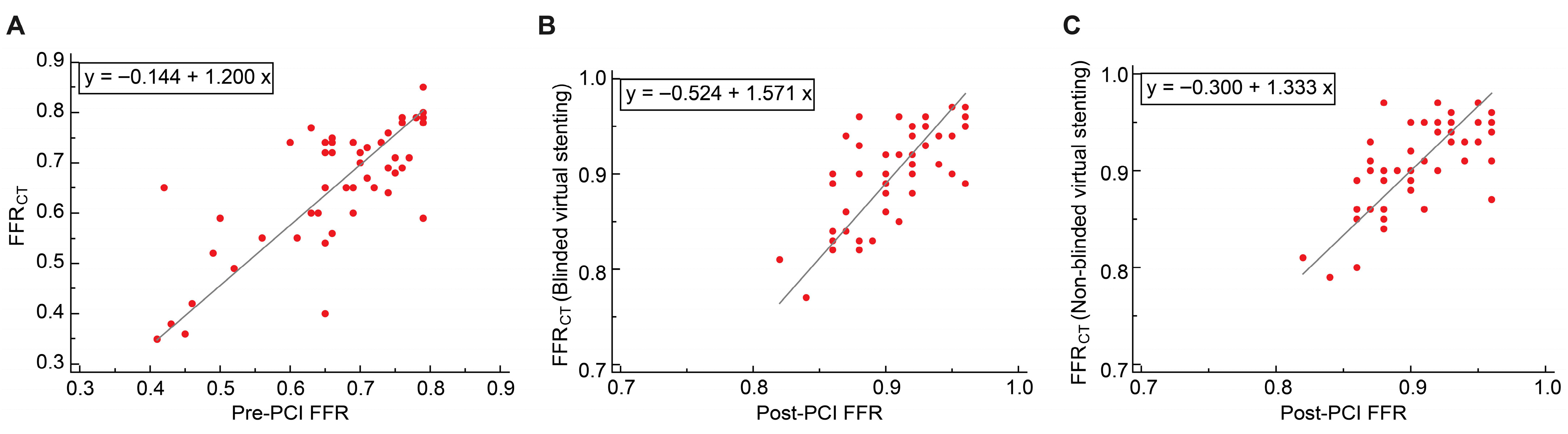
3.4.1. Passing–Bablok Regression
3.4.2. RMSE Analysis
4. Discussion
4.1. Innovativeness and Strengths
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICA | Invasive coronary angiography |
| PCI | Percutaneous coronary intervention |
| FFR | Fractional flow reserve |
| CFD | Computational fluid dynamics |
| CCTA | Coronary computed tomography angiography |
| FFRCT | Fractional flow reserve derived from computed tomography |
| ICC | Intraclass correlation coefficient |
| RMSE | Root mean square error |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| QFR | Quantitative flow ratio |
| vFFR | Virtual fractional flow reserve |
| OCT | Optical coherence tomography |
| IVUS | Intravascular ultrasound |
References
- Pijls, N.H.; van Schaardenburgh, P.; Manoharan, G.; Boersma, E.; Bech, J.W.; van’t Veer, M.; Bär, F.; Hoorntje, J.; Koolen, J.; Wijns, W.; et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J. Am. Coll. Cardiol. 2007, 49, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Pijls, N.H.; Fearon, W.F.; Tonino, P.A.; Siebert, U.; Ikeno, F.; Bornschein, B.; van’t Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J. Am. Coll. Cardiol. 2010, 56, 177–184. [Google Scholar] [CrossRef]
- De Bruyne, B.; Pijls, N.H.; Kalesan, B.; Barbato, E.; Tonino, P.A.; Piroth, Z.; Jagic, N.; Möbius-Winkler, S.; Rioufol, G.; Witt, N.; et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Layland, J.; Oldroyd, K.G.; Curzen, N.; Sood, A.; Balachandran, K.; Das, R.; Junejo, S.; Ahmed, N.; Lee, M.M.; Shaukat, A.; et al. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: The British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur. Heart J. 2015, 36, 100–111. [Google Scholar] [CrossRef]
- Engstrøm, T.; Kelbæk, H.; Helqvist, S.; Høfsten, D.E.; Kløvgaard, L.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamäki, K.; Clemmensen, P.; et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): An open-label, randomised controlled trial. Lancet 2015, 386, 665–671. [Google Scholar] [CrossRef]
- Smits, P.C.; Abdel-Wahab, M.; Neumann, F.J.; Boxma-de Klerk, B.M.; Lunde, K.; Schotborgh, C.E.; Piroth, Z.; Horak, D.; Wlodarczak, A.; Ong, P.J.; et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N. Engl. J. Med. 2017, 376, 1234–1244. [Google Scholar] [CrossRef]
- Koo, B.K.; Erglis, A.; Doh, J.H.; Daniels, D.V.; Jegere, S.; Kim, H.S.; Dunning, A.; DeFrance, T.; Lansky, A.; Leipsic, J.; et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J. Am. Coll. Cardiol. 2011, 58, 1989–1997. [Google Scholar] [CrossRef]
- Min, J.K.; Leipsic, J.; Pencina, M.J.; Berman, D.S.; Koo, B.K.; van Mieghem, C.; Erglis, A.; Lin, F.Y.; Dunning, A.M.; Apruzzese, P.; et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. J. Am. Med. Assoc. 2012, 308, 1237–1245. [Google Scholar] [CrossRef]
- Nørgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.S.; Pontone, G.; Hlatky, M.A.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: The prospective longitudinal trial of FFR(CT): Outcome and resource impacts study. Eur. Heart J. 2015, 36, 3359–3367. [Google Scholar] [CrossRef]
- Fairbairn, T.A.; Nieman, K.; Akasaka, T.; Nørgaard, B.L.; Berman, D.S.; Raff, G.; Hurwitz-Koweek, L.M.; Pontone, G.; Kawasaki, T.; Sand, N.P.; et al. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: Lessons from the ADVANCE Registry. Eur. Heart J. 2018, 39, 3701–3711. [Google Scholar] [CrossRef]
- Kim, K.H.; Doh, J.H.; Koo, B.K.; Min, J.K.; Erglis, A.; Yang, H.M.; Park, K.W.; Lee, H.Y.; Kang, H.J.; Kim, Y.J.; et al. A novel noninvasive technology for treatment planning using virtual coronary stenting and computed tomography-derived computed fractional flow reserve. J. Am. Coll. Cardiol. Cardiovasc. Interv. 2014, 7, 72–78. [Google Scholar] [CrossRef]
- Sonck, J.; Nagumo, S.; Norgaard, B.L.; Otake, H.; Ko, B.; Zhang, J.; Mizukami, T.; Maeng, M.; Andreini, D.; Takahashi, Y.; et al. Clinical validation of a virtual planner for coronary interventions based on coronary CT angiography. J. Am. Coll. Cardiol. Cardiovasc. Imaging 2022, 15, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Bom, M.J.; Schumacher, S.P.; Driessen, R.S.; van Diemen, P.A.; Everaars, H.; de Winter, R.W.; van de Ven, P.M.; van Rossum, A.C.; Sprengers, R.W.; Verouden, N.J.W.; et al. Non-invasive procedural planning using computed tomography-derived fractional flow reserve. Catheter. Cardiovasc. Interv. 2021, 97, 614–622. [Google Scholar] [CrossRef]
- Gaur, S.; Achenbach, S.; Leipsic, J.; Mauri, L.; Bezerra, H.G.; Jensen, J.M.; Bøtker, H.E.; Lassen, J.F.; Nørgaard, B.L. Rationale and design of the HeartFlowNXT (HeartFlow analysis of coronary blood flow using CT angiography: NeXt sTeps) study. J. Cardiovasc. Comput. Tomogr. 2013, 7, 279–288. [Google Scholar] [CrossRef]
- Abbara, S.; Blanke, P.; Maroules, C.D.; Cheezum, M.; Choi, A.D.; Han, B.K.; Marwan, M.; Naoum, C.; Norgaard, B.L.; Rubinshtein, R.; et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2016, 10, 435–449. [Google Scholar] [CrossRef]
- Leipsic, J.; Abbara, S.; Achenbach, S.; Cury, R.; Earls, J.P.; Mancini, G.J.; Nieman, K.; Pontone, G.; Raff, G.L. SCCT guidelines for the interpretation and reporting of coronary CT angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 2014, 8, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Toth, G.G.; Johnson, N.P.; Jeremias, A.; Pellicano, M.; Vranckx, P.; Fearon, W.F.; Barbato, E.; Kern, M.J.; Pijls, N.H.; De Bruyne, B. Standardization of fractional flow reserve measurements. J. Am. Coll. Cardiol. 2016, 68, 742–753. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J. Am. Coll. Cardiol. 2011, 58, e44–e122. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wu, B.; Cheng, Z.; Shiu, W.S.; Liu, J.; Liu, L.; Wang, Y.; Wang, X.; Cai, X.C. A parallel non-nested two-level domain decomposition method for simulating blood flows in cerebral artery of stroke patient. Int. J. Numer. Methods Biomed. Eng. 2020, 36, e3392. [Google Scholar] [CrossRef]
- Segers, P.; Stergiopulos, N.; Westerhof, N.; Wouters, P.; Kolh, P.; Verdonck, P. Systemic and pulmonary hemodynamics assessed with a lumped-parameter heart-arterial interaction model. J. Eng. Math. 2003, 47, 185–199. [Google Scholar] [CrossRef]
- Vignon-Clementel, I.E.; Figueroa, C.A.; Jansen, K.E.; Taylor, C.A. Outflow boundary conditions for 3D simulations of non-periodic blood flow and pressure fields in deformable arteries. Comput. Methods Biomech. Biomed. Eng. 2010, 13, 625–640. [Google Scholar] [CrossRef]
- Yan, Z.; Yao, Z.; Guo, W.; Shang, D.; Chen, R.; Liu, J.; Cai, X.C.; Ge, J. Impact of pressure wire on fractional flow reserve and hemodynamics of the coronary arteries: A computational and clinical study. IEEE Trans. Biomed. Eng. 2023, 70, 1683–1691. [Google Scholar] [CrossRef]
- Pijls, N.H.; Klauss, V.; Siebert, U.; Powers, E.; Takazawa, K.; Fearon, W.F.; Escaned, J.; Tsurumi, Y.; Akasaka, T.; Samady, H.; et al. Coronary pressure measurement after stenting predicts adverse events at follow-up: A multicenter registry. Circulation 2002, 105, 2950–2954. [Google Scholar] [CrossRef]
- Johnson, N.P.; Tóth, G.G.; Lai, D.; Zhu, H.; Açar, G.; Agostoni, P.; Appelman, Y.; Arslan, F.; Barbato, E.; Chen, S.L.; et al. Prognostic value of fractional flow reserve: Linking physiologic severity to clinical outcomes. J. Am. Coll. Cardiol. 2014, 64, 1641–1654. [Google Scholar] [CrossRef]
- Rimac, G.; Fearon, W.F.; De Bruyne, B.; Ikeno, F.; Matsuo, H.; Piroth, Z.; Costerousse, O.; Bertrand, O.F. Clinical value of post-percutaneous coronary intervention fractional flow reserve value: A systematic review and meta-analysis. Am. Heart J. 2017, 183, 1–9. [Google Scholar] [CrossRef]
- Rubimbura, V.; Guillon, B.; Fournier, S.; Amabile, N.; Chi Pan, C.; Combaret, N.; Eeckhout, E.; Kibler, M.; Silvain, J.; Wijns, W.; et al. Quantitative flow ratio virtual stenting and post stenting correlations to post stenting fractional flow reserve measurements from the DOCTORS (Does Optical Coherence Tomography Optimize Results of Stenting) study population. Catheter. Cardiovasc. Interv. 2020, 96, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Gosling, R.C.; Morris, P.D.; Silva Soto, D.A.; Lawford, P.V.; Hose, D.R.; Gunn, J.P. Virtual coronary intervention: A treatment planning tool based upon the angiogram. J. Am. Coll. Cardiol. Cardiovasc. Imaging 2019, 12, 865–872. [Google Scholar] [CrossRef]
- Castiello, D.S.; Buongiorno, F.; Manzi, L.; Narciso, V.; Forzano, I.; Florimonte, D.; Sperandeo, L.; Canonico, M.E.; Avvedimento, M.; Paolillo, R.; et al. Procedural and antithrombotic therapy optimization in patients with atrial fibrillation undergoing percutaneous coronary intervention: A narrative review. J. Cardiovasc. Dev. Dis. 2025, 12, 142. [Google Scholar] [CrossRef] [PubMed]

| Number of cases | 75 |
| Number of vessels | 78 |
| Age, years | 59.76 ± 8.77 |
| Male | 56 (74.7%) |
| BMI | 25.79 ± 3.25 |
| Hypertension | 44 (58.7%) |
| Diabetes mellitus | 16 (21.3%) |
| Hyperlipidemia | 38 (50.7%) |
| Current tobacco use | 33 (44%) |
| Prior PCI (Non-target vessel) | 1 (1.3%) |
| LVEF (%) | 65 (61–67) |
| Systolic blood pressure (mmHg) | 131.80 ± 15.60 |
| Diastolic blood pressure (mmHg) | 76.44 ± 9.42 |
| Heart rate (beats per minute) | 68 (64–76) |
| Creatinine (µmol/L) | 69.60 ± 12.02 |
| LDL-C (mmol/L) | 2.15 (1.73–2.88) |
| Time interval between CCTA and PCI (days) | 6 (2–11) |
| Target vessel | |
| LAD-PCI | 58 (74.4%) |
| LCX-PCI | 7 (9%) |
| RCA-PCI | 13 (16.7%) |
| Actual stent length (mm) | 26.5 (18–37.25) |
| Virtual stent length (mm) | 23 (17–30) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Ren, Y.; Li, J.; Zhang, M.; Zhang, L.; Chen, R.; Liu, J.; Yan, Z.; Song, X. Virtual Stenting Based on Fractional Flow Reserve Derived from Computed Tomography in Predicting Post-Percutaneous Coronary Intervention Functional Outcomes: A Retrospective Cohort Study. J. Cardiovasc. Dev. Dis. 2025, 12, 373. https://doi.org/10.3390/jcdd12090373
Zhao H, Ren Y, Li J, Zhang M, Zhang L, Chen R, Liu J, Yan Z, Song X. Virtual Stenting Based on Fractional Flow Reserve Derived from Computed Tomography in Predicting Post-Percutaneous Coronary Intervention Functional Outcomes: A Retrospective Cohort Study. Journal of Cardiovascular Development and Disease. 2025; 12(9):373. https://doi.org/10.3390/jcdd12090373
Chicago/Turabian StyleZhao, Han, Yanlong Ren, Jiang Li, Mingduo Zhang, Lijun Zhang, Rongliang Chen, Jia Liu, Zhengzheng Yan, and Xiantao Song. 2025. "Virtual Stenting Based on Fractional Flow Reserve Derived from Computed Tomography in Predicting Post-Percutaneous Coronary Intervention Functional Outcomes: A Retrospective Cohort Study" Journal of Cardiovascular Development and Disease 12, no. 9: 373. https://doi.org/10.3390/jcdd12090373
APA StyleZhao, H., Ren, Y., Li, J., Zhang, M., Zhang, L., Chen, R., Liu, J., Yan, Z., & Song, X. (2025). Virtual Stenting Based on Fractional Flow Reserve Derived from Computed Tomography in Predicting Post-Percutaneous Coronary Intervention Functional Outcomes: A Retrospective Cohort Study. Journal of Cardiovascular Development and Disease, 12(9), 373. https://doi.org/10.3390/jcdd12090373







