Direct Axillary Artery Cannulation as Standard Perfusion Strategy in Minimally Invasive Coronary Artery Bypass Grafting
Abstract
1. Introduction
2. Methods
2.1. Patient Selection and Data Collection
2.2. Preoperative Evaluation
2.3. Anesthesia
2.4. Surgical Technique
2.5. Endpoints and Definitions
2.6. Ethical Standards and Consent Statement
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| BSA | body surface area |
| CABG | coronary artery bypass grafting |
| CPB | cardiopulmonary bypass |
| IQR | interquartile range |
| LVEF | left ventricular ejection fraction |
| NSTEMI | non-ST-elevation myocardial infarction |
| PCI | percutaneous coronary intervention |
| RAA | right axillary artery |
| TCRAT | total coronary revascularization via anterior thoracotomy |
| TOE | transoesophageal echocardiography |
References
- Sabik, J.F.; Lytle, B.W.; McCarthy, P.M.; Cosgrove, D.M. Axillary artery: An alternative site of arterial cannulation for patients with extensive aortic and peripheral vascular disease. J. Thorac. Cardiovasc. Surg. 1995, 109, 885–890; discussion 890–891. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.C.; Singer, R.L.; Manley, N.J.; Montesano, R.M. Cannulation of the axillary artery for cardiopulmonary bypass: Safeguards and pitfalls. Ann. Thorac. Surg. 2003, 75, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Schachner, T.; Vertacnik, K.; Laufer, G.; Bonatti, J. Axillary artery cannulation in surgery of the ascending aorta and the aortic arch. Eur. J. Cardiothorac. Surg. 2002, 22, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Villard, J.; Froment, J.C.; Milleret, R.; Dureau, G.; Amouroux, C.; Boivin, J.; Seffert, P.; Morel, J.J. Dissection aortique aiguë totale de type I. Intérêt de la perfusion artérielle par voie axillaire [Type I, complete, acute aortic dissection. Value of arterial perfusion by the axillary route (author’s transl)]. Ann. Chir. Thorac. Cardiovasc. 1976, 15, 133–135. (In French) [Google Scholar] [PubMed]
- Ohira, S.; Kai, M.; Goldberg, J.B.; Malekan, R.; Lansman, S.L.; Spielvogel, D. Direct Axillary Artery Cannulation for Aortic Surgery: Lessons from Contemporary Experiences. Ann. Thorac. Surg. 2022, 114, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Sabik, J.F.; Nemeh, H.; Lytle, B.W.; Blackstone, E.H.; Gillinov, A.M.; Rajeswaran, J.; Cosgrove, D.M. Cannulation of the axillary artery with a side graft reduces morbidity. Ann. Thorac. Surg. 2004, 77, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Puiu, P.C.; Pingpoh, C.; Beyersdorf, F.; Czerny, M.; Keyl, C.; Kreibich, M.; Kondov, S.; Rylski, B.; Zimmer, E.; Siepe, M. Direct Versus Side Graft Cannulation From the Right Axillary Artery in Thoracic Aortic Surgery. Ann. Thorac. Surg. 2021, 112, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Strauch, J.T.; Spielvogel, D.; Lauten, A.; Lansman, S.L.; McMurtry, K.; Bodian, C.A.; Griepp, R.B. Axillary artery cannulation: Routine use in ascending aorta and aortic arch replacement. Ann. Thorac. Surg. 2004, 78, 103–108; discussion 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ohira, S.; Malekan, R.; Kai, M.; Goldberg, J.B.; Spencer, P.J.; Lansman, S.L.; Spielvogel, D. Direct Axillary Artery Cannulation for Type A Dissection and Impact of Dissected Innominate Artery. Ann. Thorac. Surg. 2022, 113, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Schachner, T.; Nagiller, J.; Zimmer, A.; Laufer, G.; Bonatti, J. Technical problems and complications of axillary artery cannulation. Eur. J. Cardiothorac. Surg. 2005, 27, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Carino, D.; Mori, M.; Pang, P.Y.K.; Singh, M.; Elkinany, S.; Tranquilli, M.; Ziganshin, B.A.; Elefteriades, J.A. Direct axillary cannulation with open Seldinger-guided technique: Is it safe? Eur. J. Cardiothorac. Surg. 2018, 53, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Chamogeorgakis, T.; Lima, B.; Shafii, A.E.; Nagpal, D.; Pokersnik, J.A.; Navia, J.L.; Mason, D.; Gonzalez-Stawinski, G.V. Outcomes of axillary artery side graft cannulation for extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2013, 145, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Seldinger, S. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiol. 1953, 39, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Babliak, O.; Demianenko, V.; Melnyk, Y.; Revenko, K.; Pidgayna, L.; Stohov, O. Complete Coronary Revascularization via Left Anterior Thoracotomy. Innovations 2019, 14, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Dörge, H.; Sellin, C.; Belmenai, A.; Asch, S.; Eggebrecht, H.; Schächinger, V. Novel concept of routine total arterial coronary bypass grafting through a left anterior approach avoiding sternotomy. Heart Vessel. 2022, 37, 1299–1304. [Google Scholar] [CrossRef]
- Bonzel, T.; Schächinger, V.; Dörge, H. Description of a Heart Team approach to coronary revascularization its beneficial long-term effect on clinical events after PCI. Clin. Res. Cardiol. 2016, 105, 388–400. [Google Scholar] [CrossRef]
- Sousa-Uva, M.; Neumann, F.J.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. ESC Scientific Document Group 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. J. Cardiothorac. Surg. 2019, 55, 4–90. [Google Scholar] [CrossRef] [PubMed]
- Sellin, C.; Asch, S.; Belmenai, A.; Mourad, F.; Voss, M.; Dörge, H. Early Results of Total Coronary Revascularization via Left Anterior Thoracotomy. Thorac. Cardiovasc. Surg. 2023, 71, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.S.; Bassin, L.; Mathur, M.N. Liberal use of axillary artery cannulation for aortic and complex cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 755–758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Reuthebuch, O.; Schurr, U.; Hellermann, J.; Prêtre, R.; Künzli, A.; Lachat, M.; Turina, M.I. Advantages of subclavian artery perfusion for repair of acute type A dissection. Eur. J. Cardiothorac. Surg. 2004, 26, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.G.; Blackstone, E.H.; Rajeswaran, J.; Sabik, J.F., 3rd; Lytle, B.W.; Gonzalez-Stawinski, G.; Varvitsiotis, P.; Banbury, M.K.; McCarthy, P.M.; Pettersson, G.B.; et al. Does the arterial cannulation site for circulatory arrest influence stroke risk? Ann. Thorac. Surg. 2004, 78, 1274–1284; discussion 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Bassin, L.; Mathur, M.N. Axillary artery cannulation for aortic and complex cardiac surgery. Heart Lung Circ. 2010, 19, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, N.; Sherwood, J.T.; Schomisch, S.J.; Carino, J.L.; Markowitz, A.H. Axillary artery cannulation for cardiopulmonary bypass reduces cerebral microemboli. J. Thorac. Cardiovasc. Surg. 2004, 128, 386–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hillebrand, J.; Zheng, Z.; Ploss, A.; Herrmann, E.; Moritz, A.; Martens, S. Axillary artery cannulation provides balanced cerebral oxygenation. Heart Vessel. 2016, 31, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, J.; Konerding, M.A.; Koch, M.; Kaufmann, T.; Steinseifer, U.; Moritz, A.; Dzemali, O. Anatomic and flow dynamic considerations for safe right axillary artery cannulation. J. Thorac. Cardiovasc. Surg. 2013, 146, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Numata, S.; Itatani, K.; Kawajiri, H.; Yamazaki, S.; Kanda, K.; Yaku, H. Computational fluid dynamics simulation of the right subclavian artery cannulation. J. Thorac. Cardiovasc. Surg. 2017, 154, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Totaro, P.; Amoroso, F.; Musto, M.; Degani, A.; Pelenghi, S. Direct Right Axillary Artery Cannulation as First Choice Strategyduring Aortic Surgery Procedures: Results from a Single Experienced Surgical Centre. Heart Surg. Forum 2024, 27, E157–E161. [Google Scholar] [CrossRef]
- Mandel, J.L.; Yost, C.C.; Rosen, J.L.; Prochno, K.W.; Round, K.J.; Komlo, C.M.; Guy, T.S. An alternate approach: Percutaneous axillary cannulation for minimally invasive cardiac surgery. J. Card. Surg. 2022, 37, 5622–5625. [Google Scholar] [CrossRef] [PubMed]
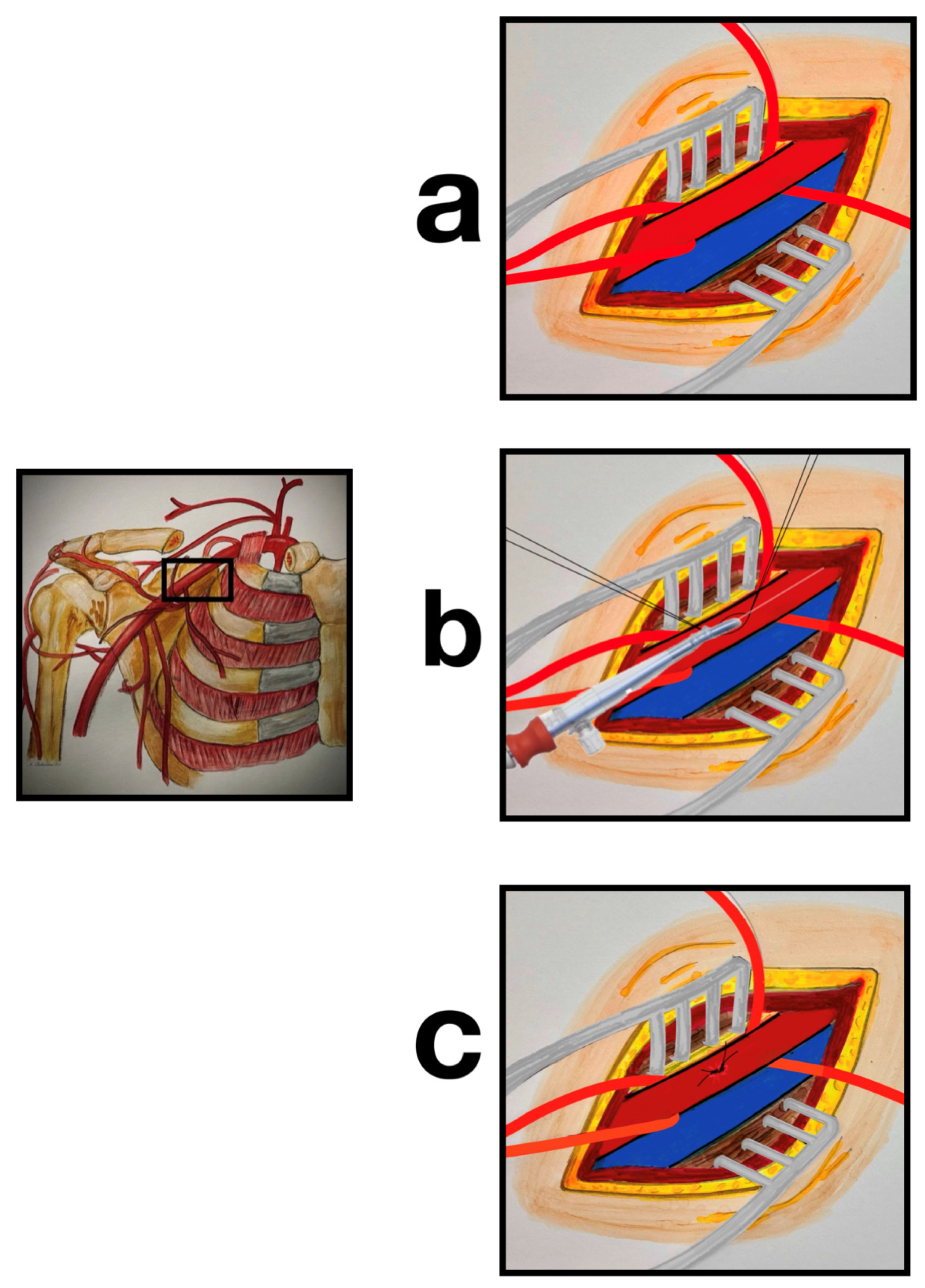
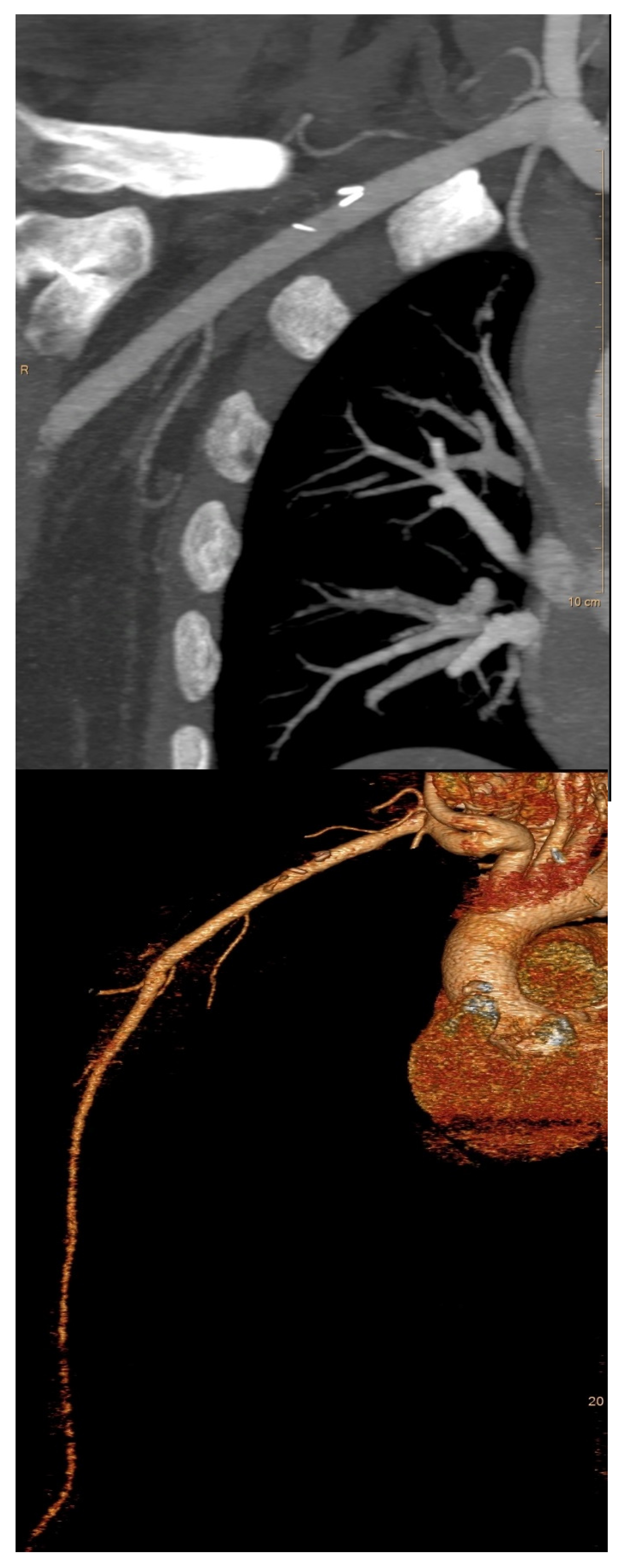
| Variables | N = 413 N (%) |
|---|---|
| Age (years) | 67.6 ± 9.9 (32–88) |
| ≥80 years | 54 (13.1%) |
| Male | 360 (87.2%) |
| BMI (kg/m2) | 28.4 ± 4.5 (18.0–42.6) |
| BMI ≥ 35 | 35 (8.5%) |
| BSA (m2) | 2.02 ± 0.2 (1.5–2.7) |
| Calculated cardiac output (L/min) | 4.8 ± 0.5 (3.5–6.5) |
| Diabetes mellitus | 145 (35.1%) |
| Chronic lung disease | 71 (17.2%) |
| Peripheral arterial disease | 147 (35.6%) |
| EuroSCORE II (%) | 3.0 ± 2.8 (0.4–29.6) |
| EuroSCORE II ≥ 4 | 101 (24.5%) |
| LVEF (%) | 48.9 ± 10.0 (10–65) |
| LVEF ≤ 30 | 33 (8.0%) |
| 2-vessel disease | 96 (23.2%) |
| 3-vessel disease | 317 (76.8%) |
| Left main stenosis > 50% | 133 (32.2%) |
| Recent NSTEMI | 170 (41.2%) |
| Prior PCI | 103 (24.9%) |
| Operative Data | N = 413 N (%) |
|---|---|
| Number of distal coronary anastomoses | 3.1 ± 0.8 (2–5) |
| Duration of (minutes) | |
| • CPB | 159 ± 41 (52–313) |
| • Aortic cross-clamping | 99 ± 32 (22–255) |
| • Operation | 330 ± 73 (145–705) |
| Cannula size | |
| ❖ 16 Fr | 143 (34.6%) |
| ➢ BSA | 2.0 ± 0.2 (1.5–2.4) |
| ➢ Calculated cardiac output (L/min) | 4.4 ± 0.3 (3.5–5.2) |
| ➢ Mean arterial line pressure at 100% cardiac output | 263 ± 52 (123–433) |
| ❖ 18 Fr | 264 (63.9%) |
| ➢ BSA | 2.0 ± 0.2 (1.5–2.7) |
| ➢ Calculated cardiac output (l/min) | 5.0 ± 0.4 (3.7–6.0) |
| ➢ Mean arterial line pressure at 100% cardiac output | 265 ± 55 (146–429) |
| ❖ 20 Fr | 6 (1.5%) |
| ➢ BSA | 2.1 ± 0.1 (1.9–2.2) |
| ➢ Calculated cardiac output (l/min) | 6.2 ± 0.2 (6.0–6.5) |
| ➢ Mean arterial line pressure at 100% cardiac output | 233 ± 34 (196–288) |
| Mean arterial line pressure at 100% cardiac output (mmHg) | 263 ± 53 (123–433) |
| Variables | N = 413 N (%) |
|---|---|
| Intraoperative cannulation-related events | |
| • Bleeding at cannulation site | 0 (0.0%) |
| • Intraoperative revision of the RAA (venous patch repair) | 2 (0.5%) |
| • Intraoperative aortic dissection | 0 (0.0%) |
| • Intraoperative dissection of the RAA | 0 (0.0%) |
| Perioperative CT angiography | 1 (0.2%) |
| Wound healing complications at cannulation site | 1 (0.2%) |
| • Superficial | 1 (0.2%) |
| • Deep | 0 (0.0%) |
| Variables | N = 397 | |
|---|---|---|
| Blood pressure | ||
| Right hand | ||
| • Systolic blood pressure (mmHg) | 128 ± 15 (90–179) | |
| • Diastolic blood pressure (mmHg) | 75 ± 10 (44–112) | |
| Left hand | ||
| • Systolic blood pressure (mmHg) | 129 ± 15 (92–176) | |
| • Diastolic blood pressure (mmHg) | 75 ± 11 (44–115) | |
| Systolic pressure gradient (mmHg) | Right > Left | Left > Right |
| • ∆ gradient < 10 | 171 (43.1%) | 155 (39.0%) |
| • ∆ gradient 11–20 | 28 (7.0%) | 30 (7.6%) |
| • ∆ gradient 21–25 | 6 (1.5%) | 7 (1.8%) |
| Feeling cold in the right hand | 0 (0.0%) | |
| Brachial plexus injury | ||
| • Transient numbness of the right hand/arm | 2 (0.5%) | |
| • Permanent numbness of the right hand/arm | 0 (0.0%) | |
| Re-intervention or surgical revision of the RAA | 0 (0.0%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellin, C.; Belmenai, A.; Demianenko, V.; Grossmann, M.; Dörge, H. Direct Axillary Artery Cannulation as Standard Perfusion Strategy in Minimally Invasive Coronary Artery Bypass Grafting. J. Cardiovasc. Dev. Dis. 2025, 12, 31. https://doi.org/10.3390/jcdd12010031
Sellin C, Belmenai A, Demianenko V, Grossmann M, Dörge H. Direct Axillary Artery Cannulation as Standard Perfusion Strategy in Minimally Invasive Coronary Artery Bypass Grafting. Journal of Cardiovascular Development and Disease. 2025; 12(1):31. https://doi.org/10.3390/jcdd12010031
Chicago/Turabian StyleSellin, Christian, Ahmed Belmenai, Volodymyr Demianenko, Marius Grossmann, and Hilmar Dörge. 2025. "Direct Axillary Artery Cannulation as Standard Perfusion Strategy in Minimally Invasive Coronary Artery Bypass Grafting" Journal of Cardiovascular Development and Disease 12, no. 1: 31. https://doi.org/10.3390/jcdd12010031
APA StyleSellin, C., Belmenai, A., Demianenko, V., Grossmann, M., & Dörge, H. (2025). Direct Axillary Artery Cannulation as Standard Perfusion Strategy in Minimally Invasive Coronary Artery Bypass Grafting. Journal of Cardiovascular Development and Disease, 12(1), 31. https://doi.org/10.3390/jcdd12010031






