Hypoxia-Induced Pulmonary Injury—Adrenergic Blockade Attenuates Nitrosative Stress, and Proinflammatory Cytokines but Not Pulmonary Edema
Abstract
1. Introduction
2. Materials and Methods
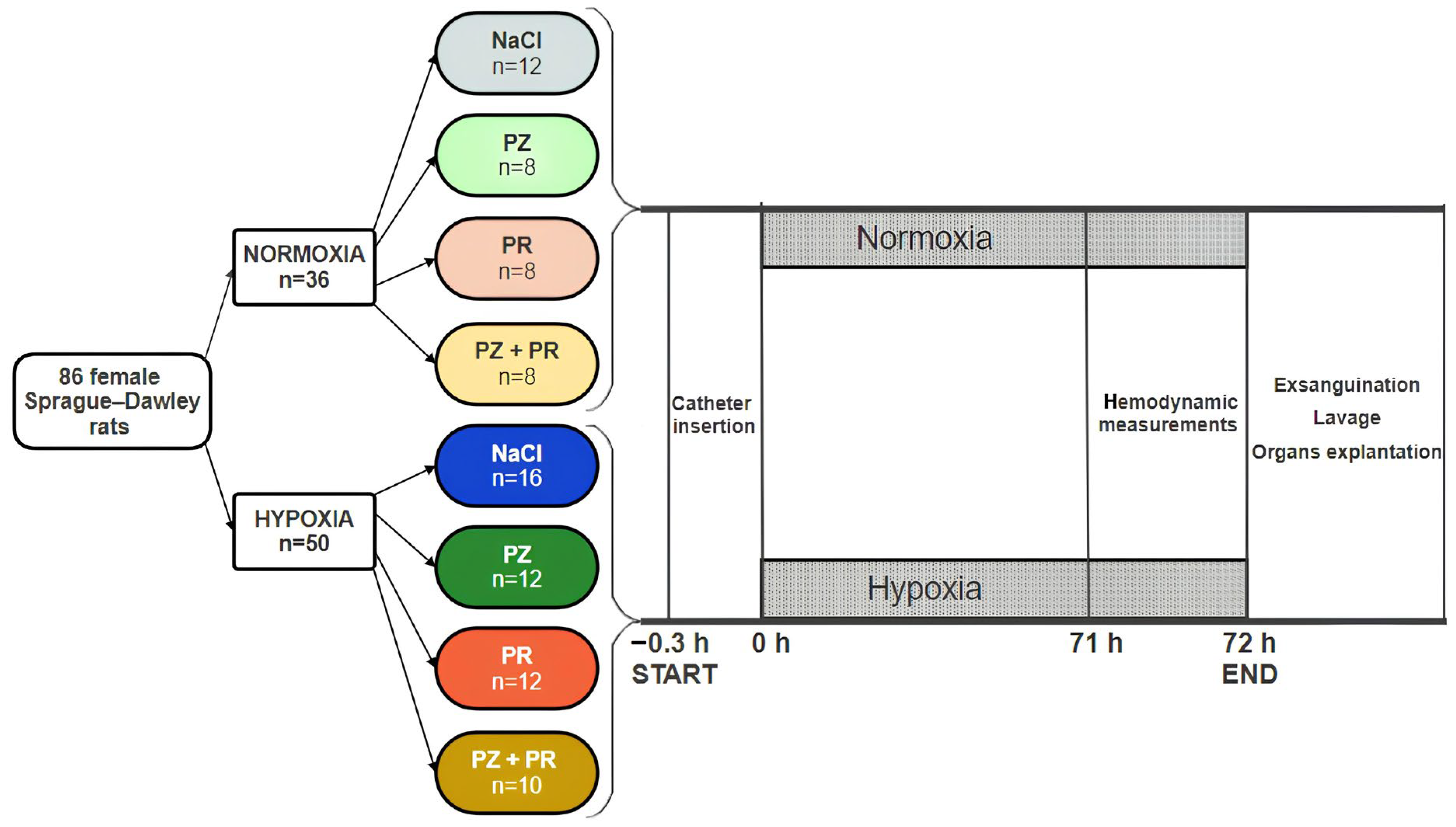
2.1. Animal Model
2.2. Study Protocol
2.3. Hemodynamic Measurements
2.4. Sampling of Materials
2.5. Lung Histology
2.6. Immunohistochemistry
2.7. Lung Wet-to-Dry Weight Ratio
2.8. BAL Cytology
2.9. Statistical Analysis
3. Results
3.1. Hemodynamic Results
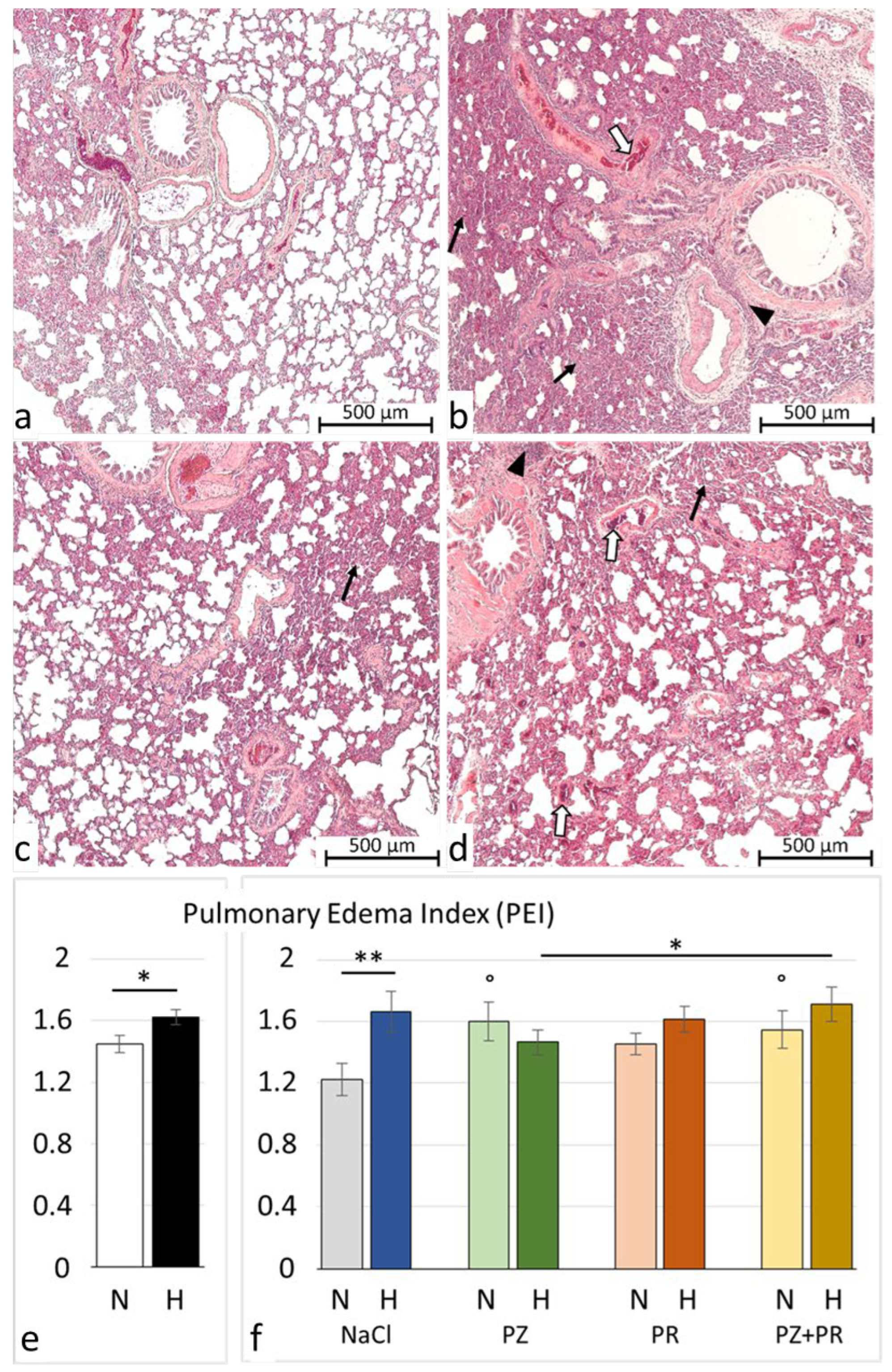
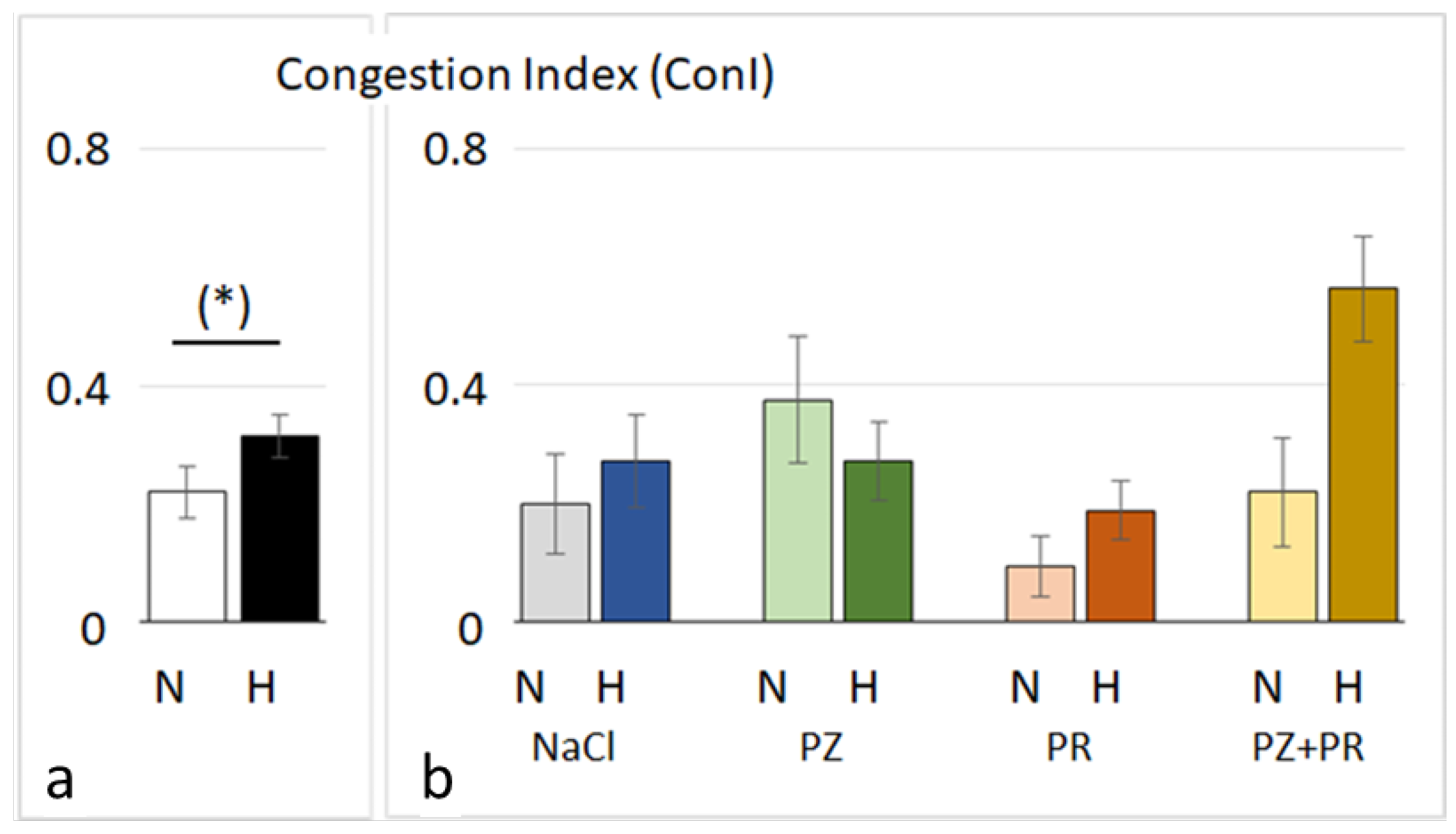
3.2. Lung Histology and W/D Ratio
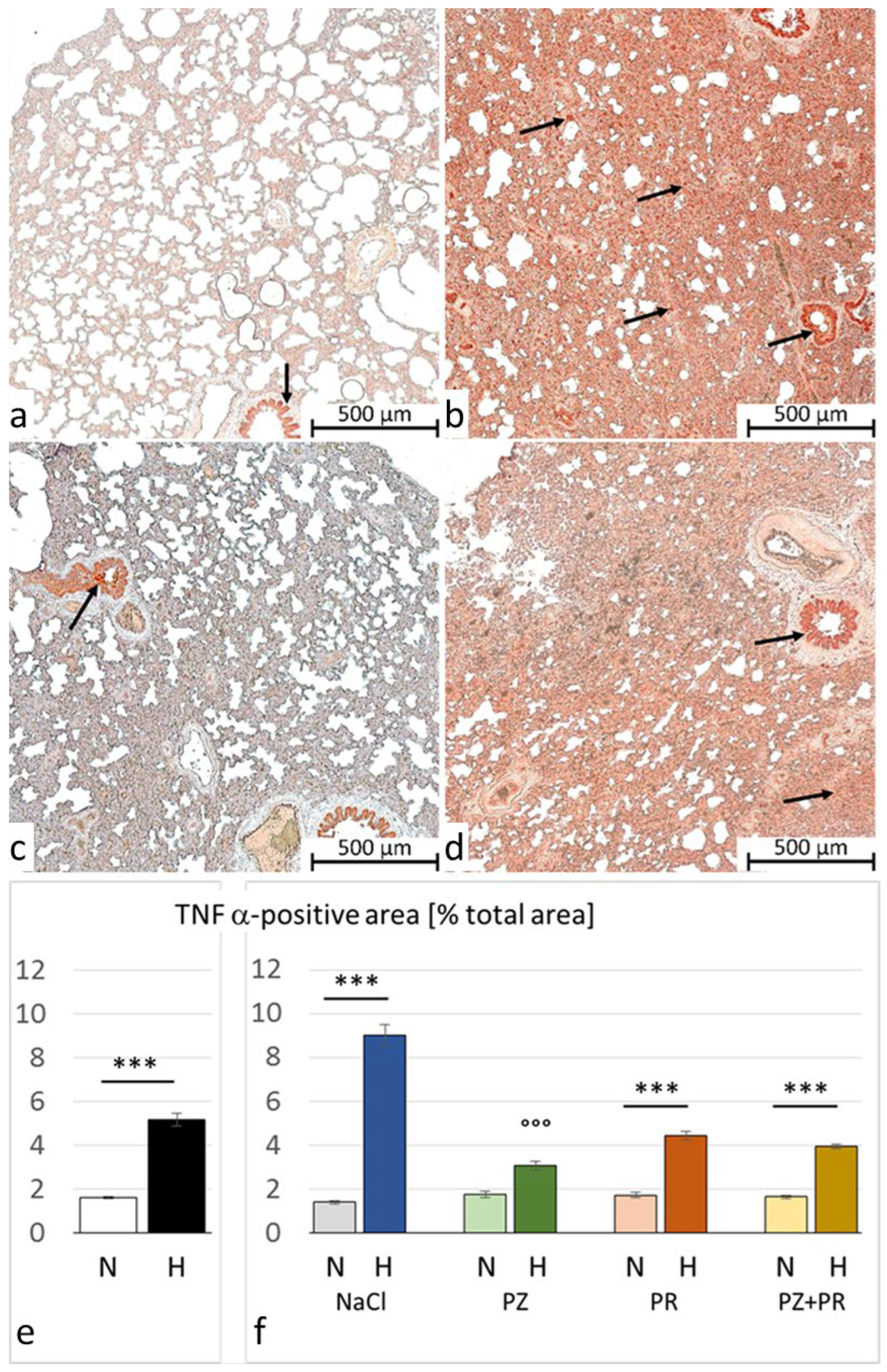
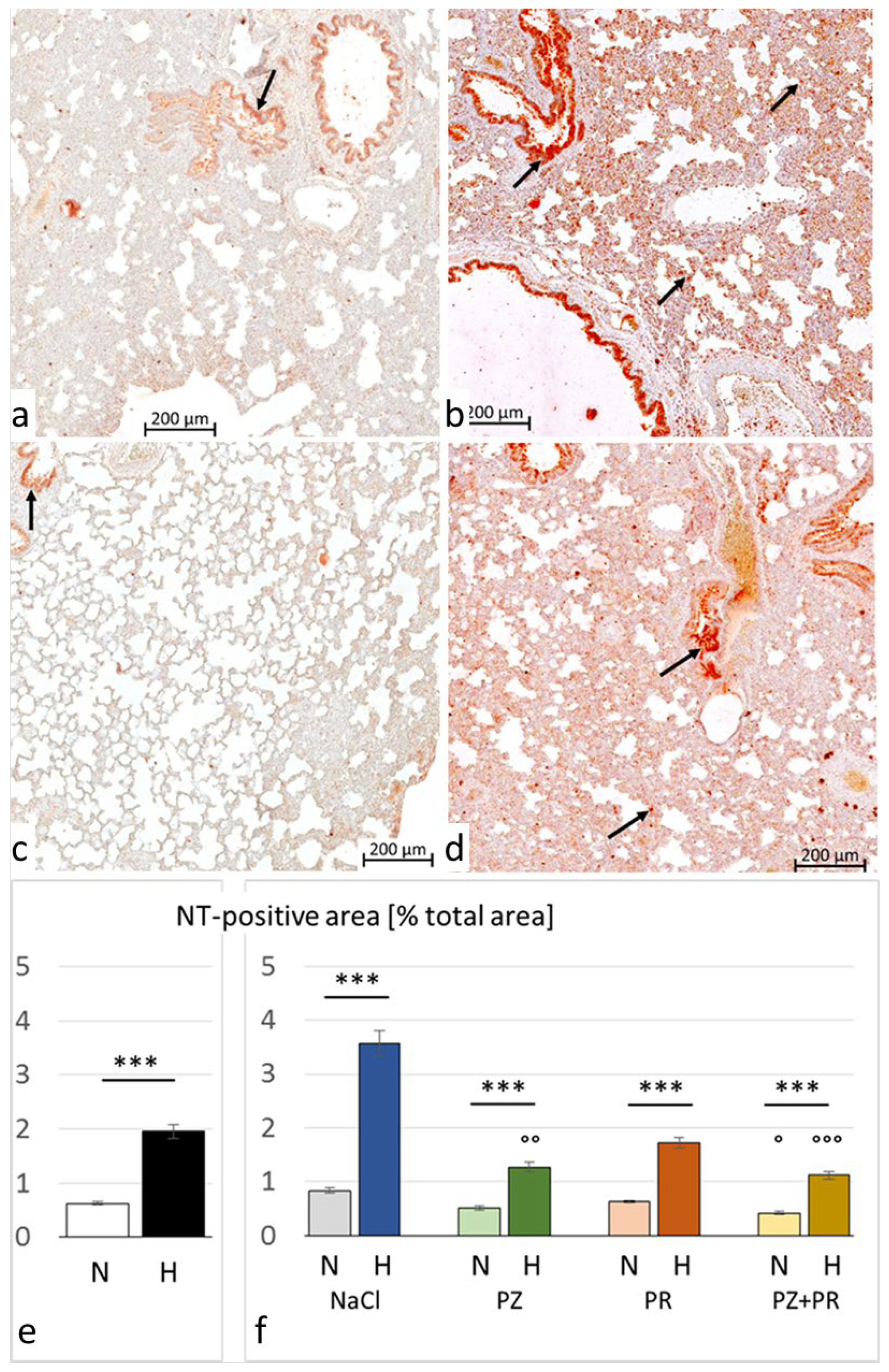
3.3. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | adrenergic blockade |
| ALI/ARDS | acute lung injury/acute respiratory distress syndrome |
| BAL | bronchoalveolar lavage |
| BSA | bovine serum albumin |
| CI | cardiac index |
| ConI | congestion index |
| COPD | chronic obstructive pulmonary disease |
| DAP | diastolic aortic pressure |
| dP/dtmax | maximal velocity of increase in pressure |
| dP/dtmin | maximal velocity of decrease in pressure |
| edP | end-diastolic pressure |
| H | normobaric hypoxia |
| HAPE | high-altitude pulmonary edema |
| HPV | hypoxic pulmonary vasoconstriction |
| HR | heart rate |
| IL | interleukin |
| LV | left ventricle/ventricular |
| MAP | mean aortic pressure |
| N | normoxia |
| NaCl | sodium chloride |
| NE | norepinephrine |
| NF-κB | nuclear factor-κB |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NT | nitrotyrosine |
| PE | pulmonary edema |
| PEI | pulmonary edema index |
| PR | propranolol |
| PZ | prazosin |
| RV | right ventricle/ventricular |
| SP | systolic peak pressure |
| SV | stroke volume |
| TNF | tumor necrosis factor |
| TPR | total peripheral resistance |
| W/D ratio | wet-to-dry weight ratio |
References
- Bärtsch, P.; Mairbäurl, H.; Maggiorini, M.; Swenson, E.R. Physiological aspects of high-altitude pulmonary edema. J. Appl. Physiol. 2005, 98, 1101–1110. [Google Scholar] [CrossRef]
- Luks, A.M.; Swenson, E.R.; Bärtsch, P. Acute high-altitude sickness. Eur. Respir. Rev. 2017, 26, 160096. [Google Scholar] [CrossRef]
- Teboul, J.L.; Douguet, D.; Mercat, A.; Depret, J.; Richard, C.; Zelter, M. Effects of catecholamines on the pulmonary venous bed in sheep. Crit. Care Med. 1998, 26, 1569–1575. [Google Scholar] [CrossRef]
- Maggiorini, M.; Mélot, C.; Pierre, S.; Pfeiffer, F.; Greve, I.; Sartori, C.; Lepori, M.; Hauser, M.; Scherrer, U.; Naeije, R. High-altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation 2001, 103, 2078–2083. [Google Scholar] [CrossRef]
- Gao, Y.; Raj, J.U. Role of veins in regulation of pulmonary circulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L213–L226. [Google Scholar] [CrossRef]
- Hopkins, S.R.; Levin, D.L. Heterogeneous pulmonary blood flow in response to hypoxia: A risk factor for high altitude pulmonary edema? Respir. Physiol. Neurobiol. 2006, 151, 217–228. [Google Scholar] [CrossRef]
- Younes, M.; Bshouty, Z.; Ali, J. Longitudinal distribution of pulmonary vascular resistance with very high pulmonary blood flow. J. Appl. Physiol. 1987, 62, 344–358. [Google Scholar] [CrossRef]
- Hultgren, H.N.; Grover, R.F.; Hartley, L.H. Abnormal circulatory responses to high altitude in subjects with a previous history of high-altitude pulmonary edema. Circulation 1971, 44, 759–770. [Google Scholar] [CrossRef]
- Grünig, E.; Mereles, D.; Hildebrandt, W.; Swenson, E.R.; Kübler, W.; Kuecherer, H.; Bärtsch, P. Stress Doppler echocardiography for identification of susceptibility to high altitude pulmonary edema. J. Am. Coll. Cardiol. 2000, 35, 980–987. [Google Scholar] [CrossRef]
- West, J.B.; Colice, G.L.; Lee, Y.J.; Namba, Y.; Kurdak, S.S.; Fu, Z.; Ou, L.C.; Mathieu-Costello, O. Pathogenesis of high-altitude pulmonary oedema: Direct evidence of stress failure of pulmonary capillaries. Eur. Respir. J. 1995, 8, 523–529. [Google Scholar] [CrossRef]
- West, J.B.; Mathieu-Costello, O. Structure, strength, failure, and remodeling of the pulmonary blood-gas barrier. Annu. Rev. Physiol. 1999, 61, 543–572. [Google Scholar] [CrossRef]
- Bölter, C.; Gabriel, P.; Appelt, P.; Salameh, A.; Schierle, K.; Rassler, B. Effects of Adrenergic Agonists and Antagonists on Cardiopulmonary Function During Normobaric Hypoxia in Rat. Front. Physiol. 2019, 10, 860. [Google Scholar] [CrossRef]
- Appelt, P.; Gabriel, P.; Bölter, C.; Fiedler, N.; Schierle, K.; Salameh, A.; Rassler, B. Left ventricular depression and pulmonary edema in rats after short-term normobaric hypoxia: Effects of adrenergic blockade and reduced fluid load. Pflug. Arch. 2021, 473, 1723–1735. [Google Scholar] [CrossRef]
- Berger, M.M.; Hesse, C.; Dehnert, C.; Siedler, H.; Kleinbongard, P.; Bardenheuer, H.J.; Kelm, M.; Bärtsch, P.; Haefeli, W.E. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 2005, 172, 763–767. [Google Scholar] [CrossRef]
- Bärtsch, P.; Gibbs, J.S. Effect of altitude on the heart and the lungs. Circulation 2007, 116, 2191–2202. [Google Scholar] [CrossRef]
- Scherrer, U.; Rexhaj, E.; Jayet, P.Y.; Allemann, Y.; Sartori, C. New insights in the pathogenesis of high-altitude pulmonary edema. Prog. Cardiovasc. Dis. 2010, 52, 485–492. [Google Scholar] [CrossRef]
- Jourd’heuil, D.; Jourd’heuil, F.L.; Kutchukian, P.S.; Musah, R.A.; Wink, D.A.; Grisham, M.B. Reaction of superoxide and nitric oxide with peroxynitrite. Implications for peroxynitrite-mediated oxidation reactions in vivo. J. Biol. Chem. 2001, 276, 28799–28805. [Google Scholar] [CrossRef]
- Hurst, J.K. Whence nitrotyrosine? J. Clin. Investig. 2002, 109, 1287–1289. [Google Scholar] [CrossRef]
- Bowers, R.; Cool, C.; Murphy, R.C.; Tuder, R.M.; Hopken, M.W.; Flores, S.C.; Voelkel, N.F. Oxidative stress in severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2004, 169, 764–769. [Google Scholar] [CrossRef]
- Salameh, A.; Zöbisch, H.; Schröder, B.; Vigelahn, J.; Jahn, M.; Abraham, G.; Seeger, J.; Dähnert, I.; Dhein, S. Effects of Hypoxia and Acidosis on Cardiac Electrophysiology and Hemodynamics. Is NHE-Inhibition by Cariporide Still Advantageous? Front. Physiol. 2020, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Radak, Z. High altitude and free radicals. J. Sports Sci. Med. 2004, 3, 64–69. [Google Scholar] [PubMed]
- Sarada, S.; Himadri, P.; Mishra, C.; Geetali, P.; Ram, M.S.; Ilavazhagan, G. Role of oxidative stress and NFkB in hypoxia-induced pulmonary edema. Exp. Biol. Med. 2008, 233, 1088–1098. [Google Scholar] [CrossRef]
- Bailey, D.M.; Dehnert, C.; Luks, A.M.; Menold, E.; Castell, C.; Schendler, G.; Faoro, V.; Gutowski, M.; Evans, K.A.; Taudorf, S.; et al. High-altitude pulmonary hypertension is associated with a free radical-mediated reduction in pulmonary nitric oxide bioavailability. J. Physiol. 2010, 588, 4837–4847. [Google Scholar] [CrossRef]
- Gao, H.; Tian, Y.; Wang, W.; Yao, D.; Zheng, T.; Meng, Q. Levels of interleukin-6; superoxide dismutase and malondialdehyde in the lung tissue of a rat model of hypoxia-induced acute pulmonary edema. Exp. Ther. Med. 2016, 11, 993–997. [Google Scholar] [CrossRef]
- Paul, S.; Arya, A.; Gangwar, A.; Bhargava, K.; Ahmad, Y. Size restricted silymarin suspension evokes integrated adaptive response against acute hypoxia exposure in rat lung. Free Radic. Biol. Med. 2016, 96, 139–151. [Google Scholar] [CrossRef]
- Kubo, K.; Hanaoka, M.; Hayano, T.; Miyahara, T.; Hachiya, T.; Hayasaka, M.; Koizumi, T.; Fujimoto, K.; Kobayashi, T.; Honda, T. Inflammatory cytokines in BAL fluid and pulmonary hemodynamics in high-altitude pulmonary edema. Respir. Physiol. 1998, 111, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Madjdpour, C.; Jewell, U.R.; Kneller, S.; Ziegler, U.; Schwendener, R.; Booy, C.; Kläusli, L.; Pasch, T.; Schimmer, R.C.; Beck-Schimmer, B. Decreased alveolar oxygen induces lung inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L360–L367. [Google Scholar] [CrossRef]
- Kowalleck, U.; Ahmed, M.A.A.; Koedel, J.; Schierle, K.; Salameh, A.; Rassler, B. Relaxin does not prevent development of hypoxia-induced pulmonary edema in rats. Pflug. Arch. 2022, 474, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Swenson, E.R.; Maggiorini, M.; Mongovin, S.; Gibbs, J.S.; Greve, I.; Mairbäurl, H.; Bärtsch, P. Pathogenesis of high-altitude pulmonary edema: Inflammation is not an etiologic factor. JAMA 2002, 287, 2228–2235. [Google Scholar] [CrossRef]
- Johnson, T.S.; Young, J.B.; Landsberg, L. Sympathoadrenal responses to acute and chronic hypoxia in the rat. J. Clin. Investig. 1983, 71, 1263–1272. [Google Scholar] [CrossRef]
- Xie, A.; Skatrud, J.B.; Puleo, D.S.; Morgan, B.J. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J. Appl. Physiol. 2001, 91, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Sander, M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J. Physiol. 2003, 546, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Duplain, H.; Vollenweider, L.; Delabays, A.; Nicod, P.; Bärtsch, P.; Scherrer, U. Augmented sympathetic activation during short-term hypoxia and high-altitude exposure in subjects susceptible to high-altitude pulmonary edema. Circulation 1999, 99, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Šedý, J.; Kuneš, J.; Zicha, J. Pathogenetic Mechanisms of Neurogenic Pulmonary Edema. J. Neurotrauma 2015, 32, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Rachfalska, N.; Putowski, Z.; Krzych, Ł.J. Distant Organ Damage in Acute Brain Injury. Brain Sci. 2020, 10, 1019. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Chen, S.J.; Yang, B.S.; Lü, S.M.; Zhu, M.; Xu, Y.F.; Chen, J.; Cai, H.W.; Mao, W. Recurrence of non-cardiogenic pulmonary edema and sustained hypotension shock in cystic pheochromocytoma. J. Zhejiang Univ. Sci. B 2017, 18, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Y-Hassan, S.; Falhammar, H. Cardiovascular Manifestations and Complications of Pheochromocytomas and Paragangliomas. J. Clin. Med. 2020, 9, 2435. [Google Scholar] [CrossRef] [PubMed]
- Rassler, B.; Reissig, C.; Briest, W.; Tannapfel, A.; Zimmer, H.G. Catecholamine-induced pulmonary edema and pleural effusion in rats--alpha- and beta-adrenergic effects. Respir. Physiol. Neurobiol. 2003, 135, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Rassler, B.; Marx, G.; Schierle, K.; Zimmer, H.G. Catecholamines can induce pulmonary remodeling in rats. Cell. Physiol. Biochem. 2012, 30, 1134–1147. [Google Scholar] [CrossRef]
- Rassler, B.; Barth, W.; Zimmer, H.G. Transient pleural effusion in norepinephrine-stimulated rats. Basic Res. Cardiol. 2001, 96, 471–477. [Google Scholar] [CrossRef]
- Salameh, A.; Dhein, S.; Blanke, K.; Rastan, A.; Hiyasat, B.; Dietze, A.; Sobiraij, A.; Dähnert, I.; Janousek, J. Right or left ventricular pacing in young minipigs with chronic atrioventricular block: Long-term in vivo cardiac performance; morphology; electrophysiology; and cellular biology. Circulation 2012, 125, 2578–2587. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dhein, S.; Grassl, M.; Gerdom, M.; Vollroth, M.; Bakhtiary, F.; von Salisch, S.; Krämer, K.; Sobiraj, A.; Kostelka, M.; Mohr, F.W.; et al. Organ-protective effects on the liver and kidney by minocycline in small piglets undergoing cardiopulmonary bypass. Naunyn Schmiedeberg’s Arch. Pharmacol. 2015, 388, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- de Blic, J.; Midulla, F.; Barbato, A.; Clement, A.; Dab, I.; Eber, E.; Green, C.; Grigg, J.; Kotecha, S.; Kurland, G.; et al. Bronchoalveolar lavage in children. ERS Task Force on bronchoalveolar lavage in children. European Respiratory Society. Eur. Respir. J. 2000, 15, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Negrini, D.; Passi, A.; de Luca, G.; Miserocchi, G. Pulmonary interstitial pressure and proteoglycans during development of pulmonary edema. Am. J. Physiol. 1996, 270, H2000–H2007. [Google Scholar] [CrossRef] [PubMed]
- Richalet, J.P. CrossTalk opposing view: Barometric pressure, independent of PO2, is not the forgotten parameter in altitude physiology and mountain medicine. J. Physiol. 2020, 598, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Deten, A.; Millar, H.; Zimmer, H.G. Catheterization of pulmonary artery in rats with an ultraminiature catheter pressure transducer. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2212–H2217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, Z.; Godana, D.; Li, A.; Rodriguez, B.; Gu, C.; Tang, H.; Minshall, R.D.; Huang, W.; Chen, J. Echocardiographic assessment of right ventricular function in experimental pulmonary hypertension. Pulm. Circ. 2019, 9, 2045894019841987. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiang, R.; Peng, X.; Dong, Q.; Li, D.; Yu, G.; Xiao, L.; Qin, S.; Huang, W. Transection of the cervical sympathetic trunk inhibits the progression of pulmonary arterial hypertension via ERK-1/2 Signalling. Respir. Res. 2019, 20, 121. [Google Scholar] [CrossRef]
- Hartmann, G.; Tschöp, M.; Fischer, R.; Bidlingmaier, C.; Riepl, R.; Tschöp, K.; Hautmann, H.; Endres, S.; Toepfer, M. High altitude increases circulating interleukin-6; interleukin-1 receptor antagonist and C-reactive protein. Cytokine 2000, 12, 246–252. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Hoidal, J.R.; Mukherjee, T.K. Role of TNFalpha in pulmonary pathophysiology. Respir. Res. 2006, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- El Alam, S.; Pena, E.; Aguilera, D.; Siques, P.; Brito, J. Inflammation in Pulmonary Hypertension and Edema Induced by Hypobaric Hypoxia Exposure. Int. J. Mol. Sci. 2022, 23, 12656. [Google Scholar] [CrossRef]
- Rashid, M.; Fahim, M.; Kotwani, A. Efficacy of tadalafil in chronic hypobaric hypoxia-induced pulmonary hypertension: Possible mechanisms. Fundam. Clin. Pharmacol. 2013, 27, 271–278. [Google Scholar] [CrossRef]
- Burke, D.L.; Frid, M.G.; Kunrath, C.L.; Karoor, V.; Anwar, A.; Wagner, B.D.; Strassheim, D.; Stenmark, K.R. Sustained hypoxia promotes the development of a pulmonary artery-specific chronic inflammatory microenvironment. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L238–L250. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Yun, J.H.; Bhunia, A.; Fijalkowska, I. Hypoxia and chronic lung disease. J. Mol. Med. 2007, 85, 1317–1324. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Sarma, J.V.; Day, D.E.; Lentsch, A.B.; Huber-Lang, M.S.; Ward, P.A. Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PLoS ONE 2009, 4, e4414. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Sautner, T. Immunomodulatory effects of vasoactive catecholamines. Wien. Klin. Wochenschr. 2002, 114, 752–761. [Google Scholar]
- Rassler, B. Role of α- and β-adrenergic mechanisms in the pathogenesis of pulmonary injuries characterized by edema; inflammation and fibrosis. Cardiovasc. Hematol. Disord. Drug Targets 2013, 13, 197–207. [Google Scholar] [CrossRef]
- Pongratz, G.; Straub, R.H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 2014, 16, 504. [Google Scholar] [CrossRef]
- Rassler, B.; Reissig, C.; Briest, W.; Tannapfel, A.; Zimmer, H.G. Pulmonary edema and pleural effusion in norepinephrine-stimulated rats–hemodynamic or inflammatory effect? Mol. Cell. Biochem. 2003, 250, 55–63. [Google Scholar] [CrossRef]
- Rassler, B.; Rohling, M.A.; Reissig, C.; Briest, W.; Tannapfel, A.; Zimmer, H.G. Involvement of cytokines and inflammation in catecholamine-induced pulmonary injury in rats. Vasc. Dis. Prev. 2005, 2, 1–9. [Google Scholar] [CrossRef]
- van den Tweel, E.R.; Nijboer, C.; Kavelaars, A.; Heijnen, C.J.; Groenendaal, F.; van Bel, F. Expression of nitric oxide synthase isoforms and nitrotyrosine formation after hypoxia-ischemia in the neonatal rat brain. J. Neuroimmunol. 2005, 167, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.C.; Liu, H.; Pinkas, G.A.; Thompson, L.P. Chronic hypoxia increases peroxynitrite, MMP9 expression, and collagen accumulation in fetal guinea pig hearts. Pediatr. Res. 2012, 71, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Lau, W.B.; Yuan, Y.; Booth, D.; Li, J.J.; Scalia, R.; Preston, K.; Gao, E.; Koch, W.; et al. Adiponectin inhibits tumor necrosis factor-α-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation. Circ. Res. 2014, 114, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Ferro, T.J.; Hocking, D.C.; Johnson, A. Tumor necrosis factor-alpha alters pulmonary vasoreactivity via neutrophil-derived oxidants. Am. J. Physiol. 1993, 265, L462–L471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Doerschuk, C.M. Neutrophil-induced changes in the biomechanical properties of endothelial cells: Roles of ICAM-1 and reactive oxygen species. J. Immunol. 2000, 164, 6487–6494. [Google Scholar] [CrossRef] [PubMed]
- Corda, S.; Laplace, C.; Vicaut, E.; Duranteau, J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am. J. Respir. Cell. Mol. Biol. 2001, 24, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Citro, R.; Schneider, B.; Morel, O.; Ghadri, J.R.; Templin, C.; Omerovic, E. Pathophysiology of Takotsubo Syndrome: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 902–921. [Google Scholar] [CrossRef] [PubMed]
- Surikow, S.Y.; Nguyen, T.H.; Stafford, I.; Chapman, M.; Chacko, S.; Singh, K.; Licari, G.; Raman, B.; Kelly, D.J.; Zhang, Y.; et al. Nitrosative Stress as a Modulator of Inflammatory Change in a Model of Takotsubo Syndrome. JACC Basic Transl. Sci. 2018, 3, 213–226. [Google Scholar] [CrossRef]
- Schäper, J.; Wagner, A.; Enigk, F.; Brell, B.; Mousa, S.A.; Habazettl, H.; Schäfer, M. Regional sympathetic blockade attenuates activation of intestinal macrophages and reduces gut barrier failure. Anesthesiology 2013, 118, 134–142. [Google Scholar] [CrossRef]
- Mortola, J.P.; Saiki, C. Ventilatory response to hypoxia in rats: Gender differences. Respir. Physiol. 1996, 106, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Dayton, A.; Exner, E.C.; Bukowy, J.D.; Stodola, T.J.; Kurth, T.; Skelton, M.; Greene, A.S.; Cowley, A.W., Jr. Breaking the Cycle: Estrous Variation Does Not Require Increased Sample Size in the Study of Female Rats. Hypertension 2016, 68, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
| Cohort | Normoxic Cohort: | Hypoxic Cohort: | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | N-NaCl | N-PZ | N-PR | N-PZ+PR | H-NaCl | H-PZ | H-PR | H-PZ+PR |
| LVSP [mmHg] | 106.9 ± 3.7 | 99.4 ± 2.4 | ||||||
| 123.3 ± 3.4 | 99.9 ± 5.5 °° | 102.2 ± 11.9 ° | 92.5 ± 5.2 °°° | 103.5 ± 3.8 ** | 96.8 ± 4.4 | 95.5 ± 6.3 | 101.3 ± 5.5 | |
| LV dP/dtmax [mmHg/s] | 8748 ± 494 | 7142 ± 364 ** | ||||||
| 10,419 ± 622 | 9912 ± 933 | 6943 ± 1029 °° | 6804 ± 908 °° | 8365 ± 569 * | 7798 ± 755 | 5838 ± 551 °° | 5893 ± 858 ° | |
| LV dP/dtmin [mmHg/s] | −9534 ± 541 | −8437 ± 413 | ||||||
| −11,896 ± 468 | −9328 ± 1170 ° | −8433 ± 1307 °° | −7136 ± 927 °°° | −10,176 ± 614 | −9178 ± 801 | −6957 ± 689 °° | −6439 ± 788 °°° | |
| Stroke volume [μL] | 194.7 ± 6.9 | 165.5 ± 6.5 ** | ||||||
| 215.3 ± 7.2 | 211.7 ± 6.8 | 167.4 ± 18.8 ° | 172.4 ± 13.2 ° | 185.6 ± 11.6 * | 152.9 ± 8.8 ° ** | 143.5 ± 18.5 ° | 164.8 ± 12.3 | |
| LV edP [mmHg] | 5.74 ± 0.34 | 6.97 ± 0.25 *** | ||||||
| 5.93 ± 0.54 | 6.50 ± 1.13 | 5.04 ± 0.54 | 5.52 ± 0.67 | 6.38 ± 0.35 | 7.06 ± 0.48 | 7.10 ± 0.34 | 7.69 ± 0.86 | |
| RVSP [mmHg] | 27.9 ± 0.7 | 30.8 ± 0.9 * | ||||||
| 30.9 ± 0.9 | 26.8 ± 1.1 | 27.7 ± 1.6 | 24.7 ± 1.0 ° | 34.3 ± 1.5 | 29.6 ± 1.9 | 28.3 ± 0.8 | 29.1 ± 2.6 | |
| RV dP/dtmax [mmHg/s] | 2201 ± 138 | 2096 ± 119 | ||||||
| 2307 ± 183 | 2466 ± 280 | 2542 ± 444 | 1511 ± 66 | 2458 ± 192 | 2350 ± 247 | 1638 ± 99 | 1646 ± 309 ° | |
| RV dP/dtmin [mmHg/s] | −1777 ± 114 | −1692 ± 81 | ||||||
| −2098 ± 174 | −1815 ± 214 | −1503 ± 129 | −1503 ± 315 | −1928 ± 137 | −1824 ± 181 | −1377 ± 80 | −1469 ± 191 | |
| Heart rate [min−1] | 426.0 ± 7.7 | 390.0 ± 6.2 *** | ||||||
| 441.8 ± 8.1 | 458.1 ± 11.2 | 423.9 ± 9.3 | 374.5 ± 21.1 °°° | 410.4 ± 8.3 * | 407.4 ± 11.1 | 359.6 ± 11.9 *** °°° | 367.7 ± 13.9 °° | |
| Cardiac index [ml min−1 kg−1] | 337.4 ± 14.5 | 264.8 ± 9.6 *** | ||||||
| 387.4 ± 11.8 | 360.2 ± 38.6 | 302.3 ± 27.7 °° | 276.5 ± 25.9 °°° | 312.7 ± 15.6 ** | 258.7 ± 12.9 *** ° | 243.4 ± 18.0 °° | 245.0 ± 14.7 °° | |
| MAP [mmHg] | 96.4 ± 3.5 | 91.0 ± 2.4 | ||||||
| 109.6 ± 3.5 | 91.0 ± 5.7 | 92.7 ± 11.6 | 84.7 ± 5.0 | 91.4 ± 3.6 | 91.2 ± 4.8 | 87.1 ± 5.9 | 95.2 ± 5.7 | |
| TPR [mmHg·min·kg·s−1] | 0.30 ± 0.01 | 0.38 ± 0.03 * | ||||||
| 0.29 ± 0.01 | 0.26 ± 0.02 | 0.33 ± 0.07 | 0.31 ± 0.03 | 0.30 ± 0.02 | 0.35 ± 0.03 | 0.42 ± 0.08 | 0.40 ± 0.04 | |
| Cohort | Normoxic Cohort: | Hypoxic Cohort: | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | N-NaCl | N-PZ | N-PR | N-PZ+PR | H-NaCl | H-PZ | H-PR | H-PZ+PR |
| W/D ratio | 4.82 (4.73; 4.96) | 5.03 (4.83; 5.42) ** | ||||||
| 4.96 (4.80; 5.59) | 4.77 (4.68; 4.82) | 4.85 (4.66; 4.98) | 4.79 (4.68; 4.92) | 4.99 (4.82; 5.44) | 5.01 (4.97; 5.16) | 5.43 4.94; 5.74) | 4.93 (4.73; 5.14) | |
| Pleural fluid volume [mL] | 0.18 (0.10; 1.68) | 0.20 (0.10; 1.23) | ||||||
| 0.10 (0.05; 0.16) | 1.90 (0.13; 5.25) | 0.35 (0.08; 1.68) | 0.63 (0.16; 3.68) | 0.13 (0.04; 0.55) | 0.20 (0.08; 2.10) | 0.20 (0.10; 0.80) | 0.23 (0.10; 0.13) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riha, I.; Salameh, A.; Hoschke, A.; Raffort, C.; Koedel, J.; Rassler, B. Hypoxia-Induced Pulmonary Injury—Adrenergic Blockade Attenuates Nitrosative Stress, and Proinflammatory Cytokines but Not Pulmonary Edema. J. Cardiovasc. Dev. Dis. 2024, 11, 195. https://doi.org/10.3390/jcdd11070195
Riha I, Salameh A, Hoschke A, Raffort C, Koedel J, Rassler B. Hypoxia-Induced Pulmonary Injury—Adrenergic Blockade Attenuates Nitrosative Stress, and Proinflammatory Cytokines but Not Pulmonary Edema. Journal of Cardiovascular Development and Disease. 2024; 11(7):195. https://doi.org/10.3390/jcdd11070195
Chicago/Turabian StyleRiha, Isabel, Aida Salameh, Annekathrin Hoschke, Coralie Raffort, Julia Koedel, and Beate Rassler. 2024. "Hypoxia-Induced Pulmonary Injury—Adrenergic Blockade Attenuates Nitrosative Stress, and Proinflammatory Cytokines but Not Pulmonary Edema" Journal of Cardiovascular Development and Disease 11, no. 7: 195. https://doi.org/10.3390/jcdd11070195
APA StyleRiha, I., Salameh, A., Hoschke, A., Raffort, C., Koedel, J., & Rassler, B. (2024). Hypoxia-Induced Pulmonary Injury—Adrenergic Blockade Attenuates Nitrosative Stress, and Proinflammatory Cytokines but Not Pulmonary Edema. Journal of Cardiovascular Development and Disease, 11(7), 195. https://doi.org/10.3390/jcdd11070195






