Electrocardiographic Changes with Age in Japanese Patients with Noonan Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Electrocardiogram
2.3. Mutation Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendez, H.M.; Opitz, J.M. Noonan syndrome: A review. Am. J. Med. Genet. 1985, 21, 493–506. [Google Scholar] [CrossRef]
- Roberts, A.E.; Allanson, J.E.; Tartaglia, M.; Gelb, B.D. Noonan syndrome. Lancet 2013, 381, 333–342. [Google Scholar] [CrossRef]
- Baban, A.; Olivini, N.; Lepri, F.R.; Calì, F.; Mucciolo, M.; Digilio, M.C.; Calcagni, G.; di Mambro, C.; Dallapiccola, B.; Adorisio, R.; et al. SOS1 mutations in Noonan syndrome: Cardiomyopathies and not only congenital heart defects! Report of six patients including two novel variants and literature review. Am. J. Med. Genet. A 2019, 179, 2083–2090. [Google Scholar] [CrossRef]
- Prendiville, T.W.; Gauvreau, K.; Tworog-Dube, E.; Patkin, L.; Kucherlapati, R.S.; Roberts, A.E.; Lacro, R.V. Cardiovascular disease in Noonan syndrome. Arch. Dis. Child. 2014, 99, 629–634. [Google Scholar] [CrossRef]
- Gelb, B.D.; Roberts, A.E.; Tartaglia, M. Cardiomyopathies in Noonan syndrome and the other RASopathies. Prog. Pediatr. Cardiol. 2015, 39, 13–19. [Google Scholar] [CrossRef]
- Vos, E.; Leenders, E.; Werkman, S.R.; Udink Ten Cate, F.E.A.; Draaisma, J.M.T. The added value of the electrocardiogram in Noonan syndrome. Cardiol. Young 2022, 32, 936–943. [Google Scholar] [CrossRef]
- Raaijmakers, R.; Noordam, C.; Noonan, J.A.; Croonen, E.A.; van der Burgt, C.J.; Draaisma, J.M. Are ECG abnormalities in Noonan syndrome characteristic for the syndrome? Eur. J. Pediatr. 2008, 167, 1363–1367. [Google Scholar] [CrossRef]
- Croonen, E.A.; van der Burgt, I.; Kapusta, L.; Draaisma, J.M. Electrocardiography in Noonan syndrome PTPN11 gene mutation—Phenotype characterization. Am. J. Med. Genet. A 2008, 146A, 350–353. [Google Scholar] [CrossRef]
- Sanchez-Cascos, A. The Noonan syndrome. Eur. Heart J. 1983, 4, 223–229. [Google Scholar] [CrossRef]
- Bertola, D.R.; Kim, C.A.; Sugayama, S.M.; Albano, L.M.; Wagenführ, J.; Moysés, R.L.; Gonzalez, C.H. Cardiac findings in 31 patients with Noonan’s syndrome. Arq. Bras. Cardiol. 2000, 75, 409–412. [Google Scholar] [CrossRef]
- Van der Burgt, I. Noonan syndrome. Orphanet J. Rare Dis. 2007, 2, 4. [Google Scholar] [CrossRef]
- Davignon, A.; Rautaharju, P.M.; Boisselle, E.B.; Soumis, F.; Mégélas, M.; Choquette, A. Normal ECG standards for infants and children. Pediatr. Cardiol. 1980, 1, 123–131. [Google Scholar] [CrossRef]
- Park, M.K. Pediatric Cardiology for Practitioners, 6th ed.; Elsevier: Philadelphia, PA, USA, 2014. [Google Scholar]
- Park, M.K.; Guntheroth, W.G. How to Read Pediatric ECGs, 4th ed.; Mosby: Philadelphia, PA, USA, 2006. [Google Scholar]
- Ichikawa, Y.; Kuroda, H.; Ikegawa, T.; Kawai, S.; Ono, S.; Kim, K.S.; Yanagi, S.; Kurosawa, K.; Aoki, Y.; Ueda, H. Cardiac features of Noonan syndrome in Japanese patients. Cardiol. Young 2023, 33, 564–569. [Google Scholar] [CrossRef]
- Narumi, Y.; Aoki, Y.; Niihori, T.; Neri, G.; Cave, H.; Verloes, A.; Nava, C.; Kavamura, M.I.; Okamoto, N.; Kurosawa, K.; et al. Molecular and clinical characterization of cardio-facio-cutaneous (CFC) syndrome: Overlapping clinical manifestations with Costello syndrome. Am. J. Med. Genet. A 2007, 143A, 799–807. [Google Scholar] [CrossRef]
- Nishimura, N.; Murakami, H.; Hayashi, T.; Sato, H.; Kurosawa, K. Multiple craniosynostosis and facial dysmorphisms with homozygous IL11RA variant caused by maternal uniparental isodisomy of chromosome 9. Congenit. Anom. 2020, 60, 153–155. [Google Scholar] [CrossRef]
- Umeki, I.; Niihori, T.; Abe, T.; Kanno, S.I.; Okamoto, N.; Mizuno, S.; Kurosawa, K.; Nagasaki, K.; Yoshida, M.; Ohashi, H.; et al. Delineation of LZTR1 mutation-positive patients with Noonan syndrome and identification of LZTR1 binding to RAF1-PPP1CB complexes. Hum. Genet. 2019, 138, 21–35. [Google Scholar] [CrossRef]
- Saarel, E.V.; Granger, S.; Kaltman, J.R.; Minich, L.L.; Tristani-Firouzi, M.; Kim, J.J.; Ash, K.; Tsao, S.S.; Berul, C.I.; Stephenson, E.A.; et al. Electrocardiograms in Healthy North American Children in the Digital Age. Circ. Arrhythm. Electrophysiol. 2018, 11, e005808. [Google Scholar] [CrossRef]
- Chou, F.S.; Johnson, A.J.; Ghimire, L.V. The Significance of Left Axis Deviation in the Pediatric Population: A Meta-Analysis. Pediatr. Cardiol. 2019, 40, 677–684. [Google Scholar] [CrossRef]
- Mustard, W.T.; Jain, S.C.; Trusler, G.A. Pulmonary stenosis in the first year of life. Br. Heart J. 1968, 30, 255–257. [Google Scholar] [CrossRef]
- Niwa, K.; Warita, N.; Sunami, Y.; Shimura, A.; Tateno, S.; Sugita, K. Prevalence of arrhythmias and conduction disturbances in large population-based samples of children. Cardiol. Young 2004, 14, 68–74. [Google Scholar] [CrossRef]
- Fretz, E.B.; Rosenberg, H.C. Diagnostic value of ECG patterns of right ventricular hypertrophy in children. Can. J. Cardiol. 1993, 9, 829–832. [Google Scholar] [PubMed]
- Bourdillon, P.J. Electrocardiography: Right ventricular hypertrophy. Br. J. Hosp. Med. 1978, 20, 498–501, 504. [Google Scholar] [PubMed]
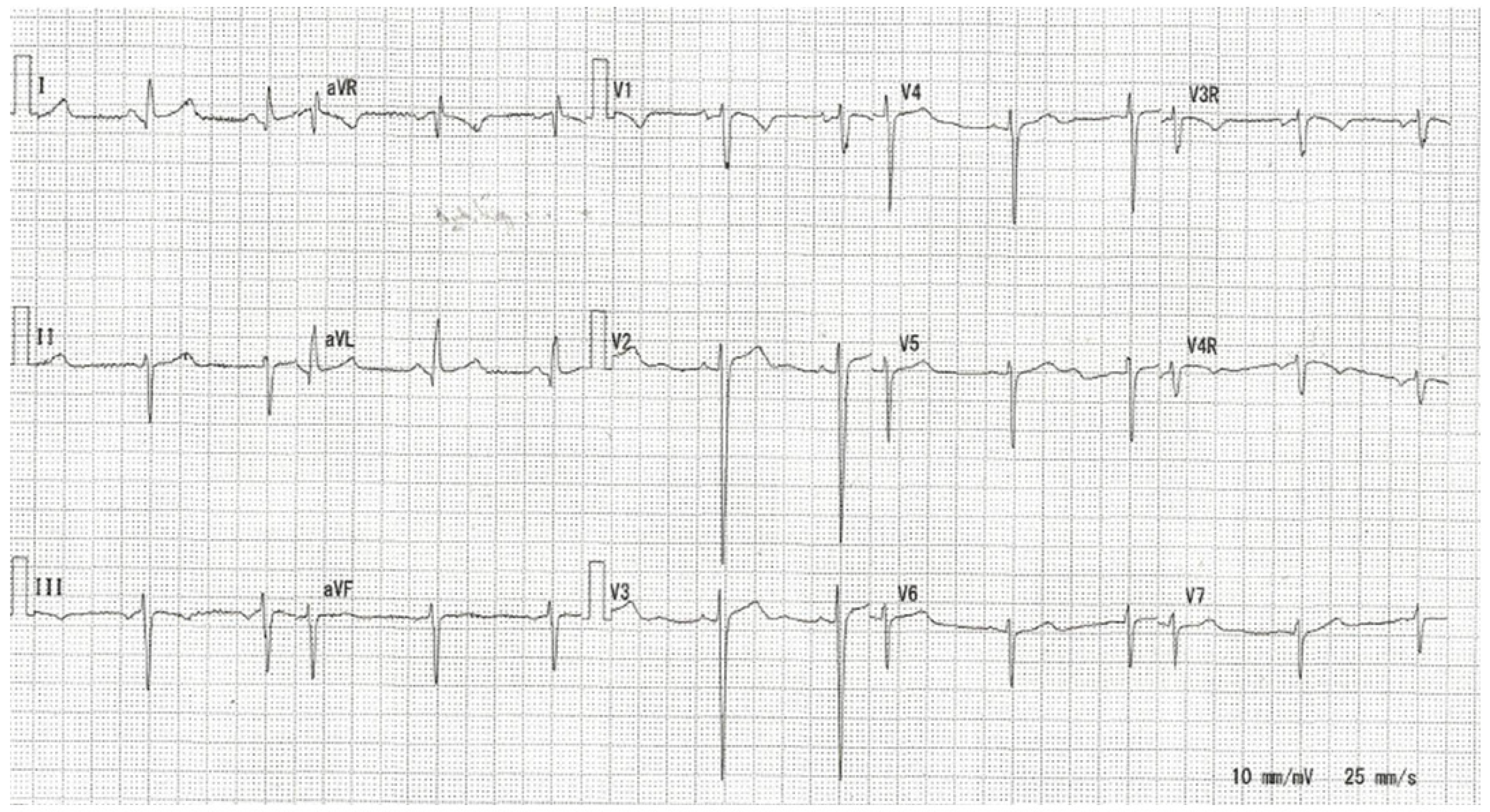
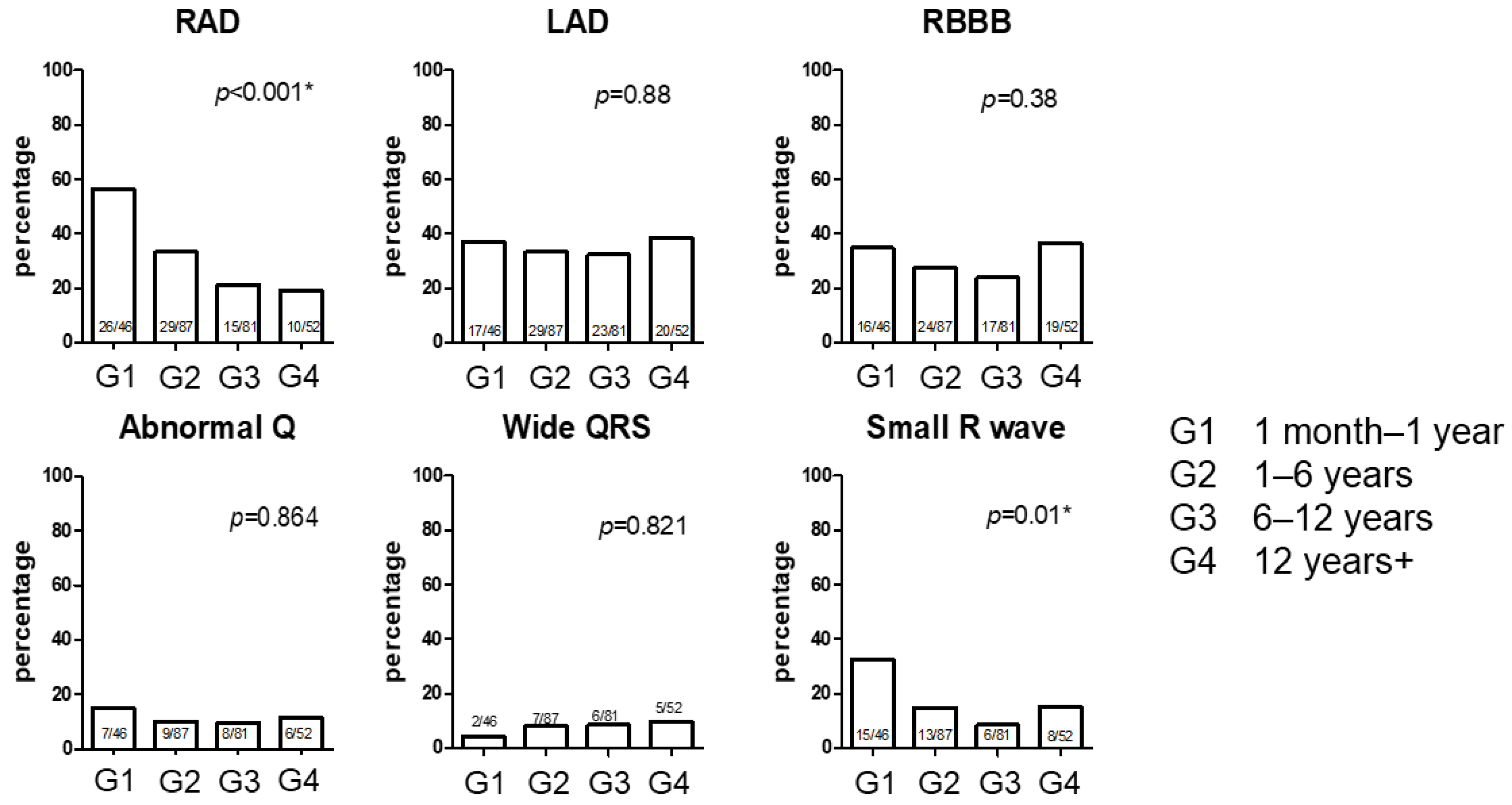
| Group | 1 (1 Month–1 Year) | 2 (1–6 Years) | 3 (6–12 Years) | 4 (>12 Years) | p | Total |
|---|---|---|---|---|---|---|
| n | 46 | 87 | 71 | 52 | 112 | |
| Age (years) (SD) | 0.42 (0.26) | 4.04 (1.29) | 8.76 (1.72) | 15.35 (3.34) | ||
| Sex (men) (%) | 25 (54.3) | 48 (55.1) | 38 (53.5) | 30 (57.6) | 67 (59.8) | |
| HCM (%) | 14 (30.4) | 20 (23.0) | 17 (23.9) | 15 (28.8) | 0.732 | 22 (19.6) |
| PS (%) | 29 (63.0) | 44 (50.6) | 35 (40.3) | 25 (48.1) | 0.417 | 53 (47.3) |
| ASD (%) | 15 (32.6) | 21 (24.1) | 16 (22.5) | 12 (23.1) | 0.632 | 28 (25.0) |
| VSD (%) | 3 (6.5) | 4 (4.6) | 5 (7.0) | 4 (7.7) | 0.86 | 6 (5.3) |
| CoA (%) | 0 (0.0) | 1 (1.1) | 1 (1.4) | 0 (0.0) | 0.458 | 1 (0.9) |
| TOF (%) | 1 (2.2) | 1 (1.1) | 1 (1.4) | 1 (1.9) | 1 | 1 (0.9) |
| AVSD (%) | 1 (2.2) | 1 (1.1) | 1 (1.4) | 1 (1.9) | 1 | 1 (0.9) |
| PDA (%) | 2 (4.3) | 2 (2.3) | 2 (2.8) | 0 (0.0) | 0.521 | 2 (1.8) |
| Coronary stenosis (%) | 1 (2.2) | 1 (1.1) | 1 (1.4) | 1 (1.9) | 1 | 2 (1.8) |
| AR (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0.382 | 1 (0.9) |
| Total CHD (%) | 41 (89.1) | 66 (75.8) | 53 (74.6) | 42 (80.8) | 0.38 | 81 (72.3) |
| Group | 1 (1 Month−1 Year) | 2 (1−6 Years) | 3 (6−12 Years) | 4 (>12 Years) | p |
|---|---|---|---|---|---|
| n | 46 | 87 | 71 | 52 | |
| HR (beats/min) | 140.00 (15.63) | 107.38 (22.06) | 82.14 (15.58) | 76.21 (15.38) | <0.001 |
| PR (ms) | 0.12 (0.01) | 0.14 (0.02) | 0.14 (0.02) | 0.15 (0.03) | <0.001 |
| QT (ms) | 0.28 (0.02) | 0.32 (0.04) | 0.37 (0.04) | 0.39 (0.03) | <0.001 |
| QTcB (ms) | 0.43 (0.03) | 0.43 (0.03) | 0.43 (0.03) | 0.43 (0.03) | 0.315 |
| QTcF (ms) | 0.38 (0.02) | 0.39 (0.03) | 0.41 (0.03) | 0.42 (0.02) | <0.001 |
| QRS (ms) | 0.10 (0.13) | 0.08 (0.01) | 0.09 (0.01) | 0.11 (0.11) | 0.151 |
| Axis (degree) | 51.02 (123.88) | 46.18 (99.71) | 35.92 (81.63) | 30.75 (86.04) | 0.682 |
| R/S V6 | 1.54 (3.02) | 3.27 (4.82) | 4.78 (4.95) | 3.74 (3.83) | 0.002 |
| SV1 (mV) | 0.96 (0.84) | 1.13 (1.13) | 1.32 (1.29) | 1.05 (0.91) | 0.32 |
| RV1 (mV) | 1.09 (0.63) | 0.70 (0.58) | 0.60 (0.54) | 0.66 (0.82) | <0.001 |
| SV6 (mV) | 0.76 (0.48) | 0.56 (0.60) | 0.46 (0.71) | 0.68 (1.06) | 0.119 |
| RV6 (mV) | 0.57 (0.43) | 0.76 (0.68) | 0.89 (0.64) | 0.90 (0.58) | 0.02 |
| Q in V6 (mV) | 0.04 (0.09) | 0.09 (0.44) | 0.11 (0.60) | 0.16 (0.79) | 0.745 |
| Q in AVF (mV) | 0.07 (0.13) | 0.06 (0.16) | 0.04 (0.10) | 0.07 (0.20) | 0.472 |
| Abnormal Q (%) | 7 (15.2) | 9 (10.3) | 8 (11.3) | 6 (11.5) | 0.864 |
| ST elevation (%) | 0 (0.0) | 3 (3.4) | 2 (2.8) | 2 (3.8) | 0.71 |
| ST depression (%) | 1 (2.2) | 4 (4.6) | 5 (7.0) | 2 (3.8) | 0.733 |
| Negative T (%) | 1 (2.2) | 4 (4.6) | 6 (8.5) | 1 (1.9) | 0.365 |
| AV block (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.8) | 0.072 |
| PAC (%) | 1 (2.2) | 2 (2.3) | 1 (1.4) | 0 (0.0) | 0.836 |
| PVC (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.9) | 0.383 |
| LAD; Group 1 | RAD; Group 1 | RBBB; Group 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | p | Positive | Negative | p | Positive | Negative | p | |
| N of patient | 17 | 29 | 26 | 20 | 16 | 30 | |||
| HCM | 6 | 8 | 0.741 | 7 | 7 | 0.077 | 3 | 11 | 0.812 |
| ASD | 9 | 6 | 0.471 | 6 | 9 | 0.203 | 6 | 9 | 0.203 |
| PS | 10 | 19 | 0.411 | 18 | 11 | 0.368 | 10 | 19 | 1 |
| Total CHD | 15 | 26 | 1 | 24 | 17 | 0.639 | 13 | 28 | 0.324 |
| Total intervention | 6 | 12 | 0.647 | 12 | 6 | 0.364 | 8 | 10 | 0.347 |
| Small R; Group 1 | Abnormal Q; Group 1 | Wide QRS; Group 1 | |||||||
| positive | negative | p | positive | Negative | p | positive | negative | p | |
| N of patient | 15 | 31 | 7 | 39 | 2 | 44 | |||
| HCM | 8 | 6 | 0.0377 | 2 | 12 | 1 | 1 | 13 | - |
| ASD | 5 | 10 | 1 | 0 | 15 | 0.0782 | 0 | 15 | - |
| PS | 9 | 20 | 1 | 4 | 25 | 1 | 1 | 28 | - |
| Total CHD | 14 | 27 | 1 | 7 | 32 | 0.572 | 2 | 39 | - |
| Total intervention | 8 | 10 | 0.208 | 2 | 16 | 1 | 2 | 16 | - |
| T Wave in V1 | |||
|---|---|---|---|
| Positive | Negative | p | |
| N of patient | 18 | 35 | |
| HCM | 6 | 10 | 0.667 |
| ASD | 2 | 9 | 0.295 |
| PS | 15 | 16 | 0.0098 |
| Total CHD | 17 | 29 | 0.651 |
| Total intervention | 13 | 6 | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichikawa, Y.; Kuroda, H.; Ikegawa, T.; Kawai, S.; Ono, S.; Kim, K.-S.; Yanagi, S.; Kurosawa, K.; Aoki, Y.; Iwamoto, M.; et al. Electrocardiographic Changes with Age in Japanese Patients with Noonan Syndrome. J. Cardiovasc. Dev. Dis. 2024, 11, 10. https://doi.org/10.3390/jcdd11010010
Ichikawa Y, Kuroda H, Ikegawa T, Kawai S, Ono S, Kim K-S, Yanagi S, Kurosawa K, Aoki Y, Iwamoto M, et al. Electrocardiographic Changes with Age in Japanese Patients with Noonan Syndrome. Journal of Cardiovascular Development and Disease. 2024; 11(1):10. https://doi.org/10.3390/jcdd11010010
Chicago/Turabian StyleIchikawa, Yasuhiro, Hiroyuki Kuroda, Takeshi Ikegawa, Shun Kawai, Shin Ono, Ki-Sung Kim, Sadamitsu Yanagi, Kenji Kurosawa, Yoko Aoki, Mari Iwamoto, and et al. 2024. "Electrocardiographic Changes with Age in Japanese Patients with Noonan Syndrome" Journal of Cardiovascular Development and Disease 11, no. 1: 10. https://doi.org/10.3390/jcdd11010010
APA StyleIchikawa, Y., Kuroda, H., Ikegawa, T., Kawai, S., Ono, S., Kim, K.-S., Yanagi, S., Kurosawa, K., Aoki, Y., Iwamoto, M., & Ueda, H. (2024). Electrocardiographic Changes with Age in Japanese Patients with Noonan Syndrome. Journal of Cardiovascular Development and Disease, 11(1), 10. https://doi.org/10.3390/jcdd11010010






