Interventricular Septal Hematoma Complicating Left Bundle Branch Area Pacing: A Case Report—The Devil Is Not So Black as He Is Painted
Abstract
1. Introduction
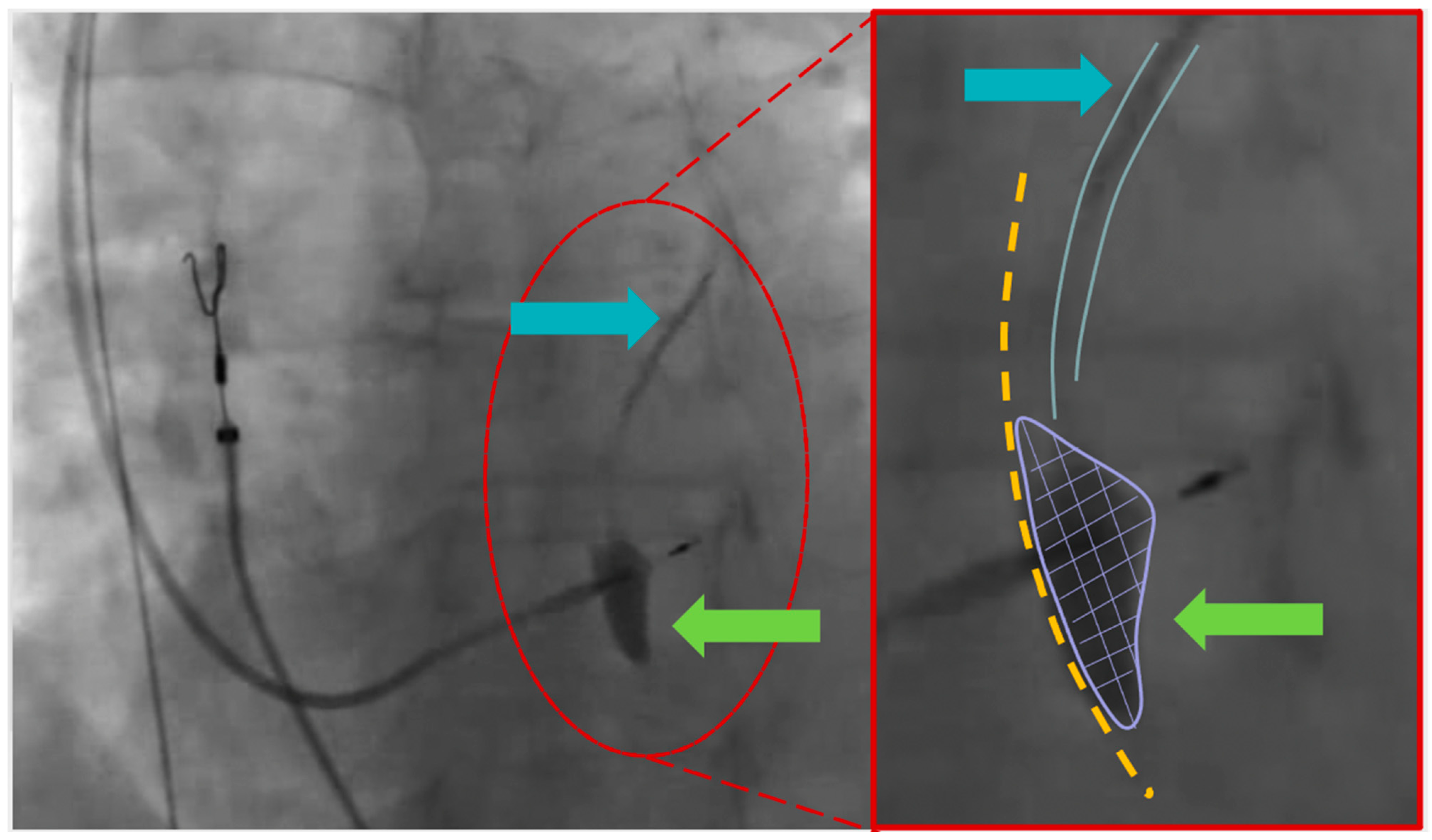
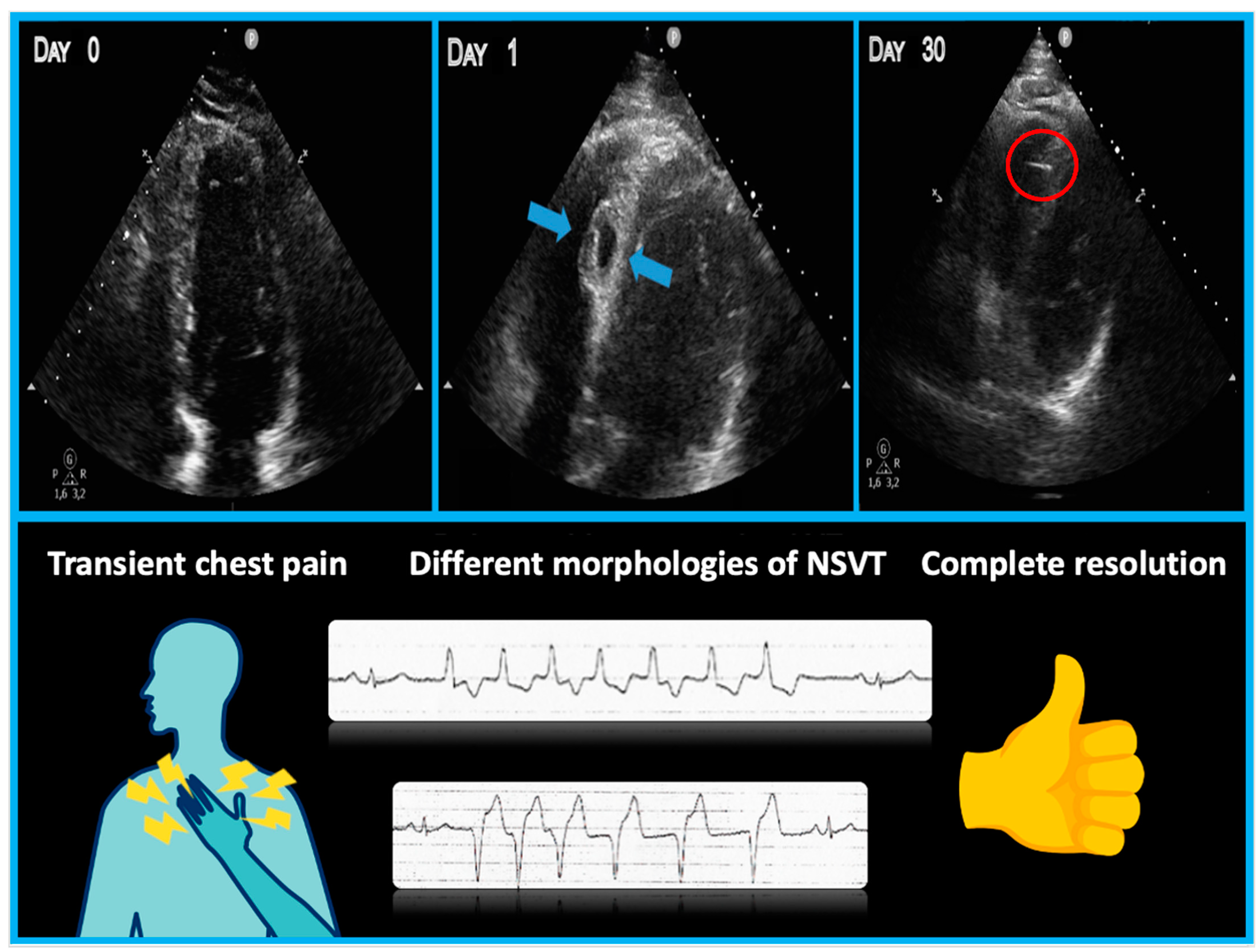
2. Detailed Case Description
3. Discussion
4. Key Take-Home Messages:
- -
- Minimize the number of attempts during lead positioning within the septum to reduce the risk of septal perforator branch injury;
- -
- Patients with interventricular septal hematoma may experience chest pain or dyspnea immediately after transseptal perforation;
- -
- Electrocardiographic abnormalities such as ventricular ectopic beats or NSVT of different morphologies can serve as markers of septal injury;
- -
- Bedside echocardiograms are crucial for early diagnosis and should be repeated the day after the procedure, especially when ventricular arrhythmias are present;
- -
- Troponin elevation, although commonly observed after LBBAP, may not be useful for discriminating this specific complication.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECG | electrocardiogram |
| LBBAP | left bundle branch area pacing |
| IVS | interventricular septum |
| VA | ventricular arrhythmias |
| VEB | ventricular ectopic beats |
| NSVT | non-sustained ventricular tachycardia |
| RV | right ventricle |
| RBBB | right bundle branch block |
| HBP | His bundle pacing |
References
- Jastrzębski, M.; Kiełbasa, G.; Cano, O.; Curila, K.; Heckman, L.; De Pooter, J.; Chovanec, M.; Rademakers, L.; Huybrechts, W.; Grieco, D.; et al. Left bundle branch area pacing outcomes: The multicentre European MELOS study. Eur. Heart J. 2022, 43, 4161–4173. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Wu, S.; Wang, S.; Su, L.; Ellenbogen, K.A.; Huang, W. Case Report: Interventricular Septal Hematoma Complicating Left Bundle Branch Pacing Lead Implantation. Front. Cardiovasc. Med. 2021, 8, 744079. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, H.; Xu, L.; Chen, H.; Xu, Y.; Xu, S.; Wang, Q.; Qian, J.; Ge, J. Interventricular Septal Hematoma with Pericardial Effusion after Left Bundle Branch Pacing Implantation. JACC Clin. Electrophysiol. 2023, 9, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Rattigan, E.; Bauch, T.D.; Mascarenhas, V.; Ahmad, T.; Subzposh, F.A.; Vijayaraman, P. Giant Interventricular Septal Hematoma Complicating Left Bundle Branch Pacing: A Cautionary Tale. JACC Case Rep. 2023, 16, 101887. [Google Scholar] [CrossRef]
- Del Monte, A.; Chierchia, G.B.; de Asmundis, C.; Sorgente, A. When Good Goes Bad: Interventricular Septal Hematoma Complicating Left Bundle Branch Area Pacing. JACC Case Rep. 2023, 16, 101889. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastori, P.; De Rosa, F.; Vitali, F.; Fasulo, A.; Tortorella, G.; Pastore, M.; Malagù, M.; Bertini, M. Interventricular Septal Hematoma Complicating Left Bundle Branch Area Pacing: A Case Report—The Devil Is Not So Black as He Is Painted. J. Cardiovasc. Dev. Dis. 2024, 11, 52. https://doi.org/10.3390/jcdd11020052
Pastori P, De Rosa F, Vitali F, Fasulo A, Tortorella G, Pastore M, Malagù M, Bertini M. Interventricular Septal Hematoma Complicating Left Bundle Branch Area Pacing: A Case Report—The Devil Is Not So Black as He Is Painted. Journal of Cardiovascular Development and Disease. 2024; 11(2):52. https://doi.org/10.3390/jcdd11020052
Chicago/Turabian StylePastori, Paolo, Fabrizio De Rosa, Francesco Vitali, Andrea Fasulo, Giovanni Tortorella, Monica Pastore, Michele Malagù, and Matteo Bertini. 2024. "Interventricular Septal Hematoma Complicating Left Bundle Branch Area Pacing: A Case Report—The Devil Is Not So Black as He Is Painted" Journal of Cardiovascular Development and Disease 11, no. 2: 52. https://doi.org/10.3390/jcdd11020052
APA StylePastori, P., De Rosa, F., Vitali, F., Fasulo, A., Tortorella, G., Pastore, M., Malagù, M., & Bertini, M. (2024). Interventricular Septal Hematoma Complicating Left Bundle Branch Area Pacing: A Case Report—The Devil Is Not So Black as He Is Painted. Journal of Cardiovascular Development and Disease, 11(2), 52. https://doi.org/10.3390/jcdd11020052







