The Complementary Role of Cardiopulmonary Exercise Testing in Coronary Artery Disease: From Early Diagnosis to Tailored Management
Abstract
1. Background
2. Methods
3. Discussion
3.1. Pathophysiological Mechanisms of Ischemia and Its Interaction with Gas Exchange
3.2. Limitations of Traditional Ergometric Testing and the Advantages of Cardiopulmonary Exercise Testing
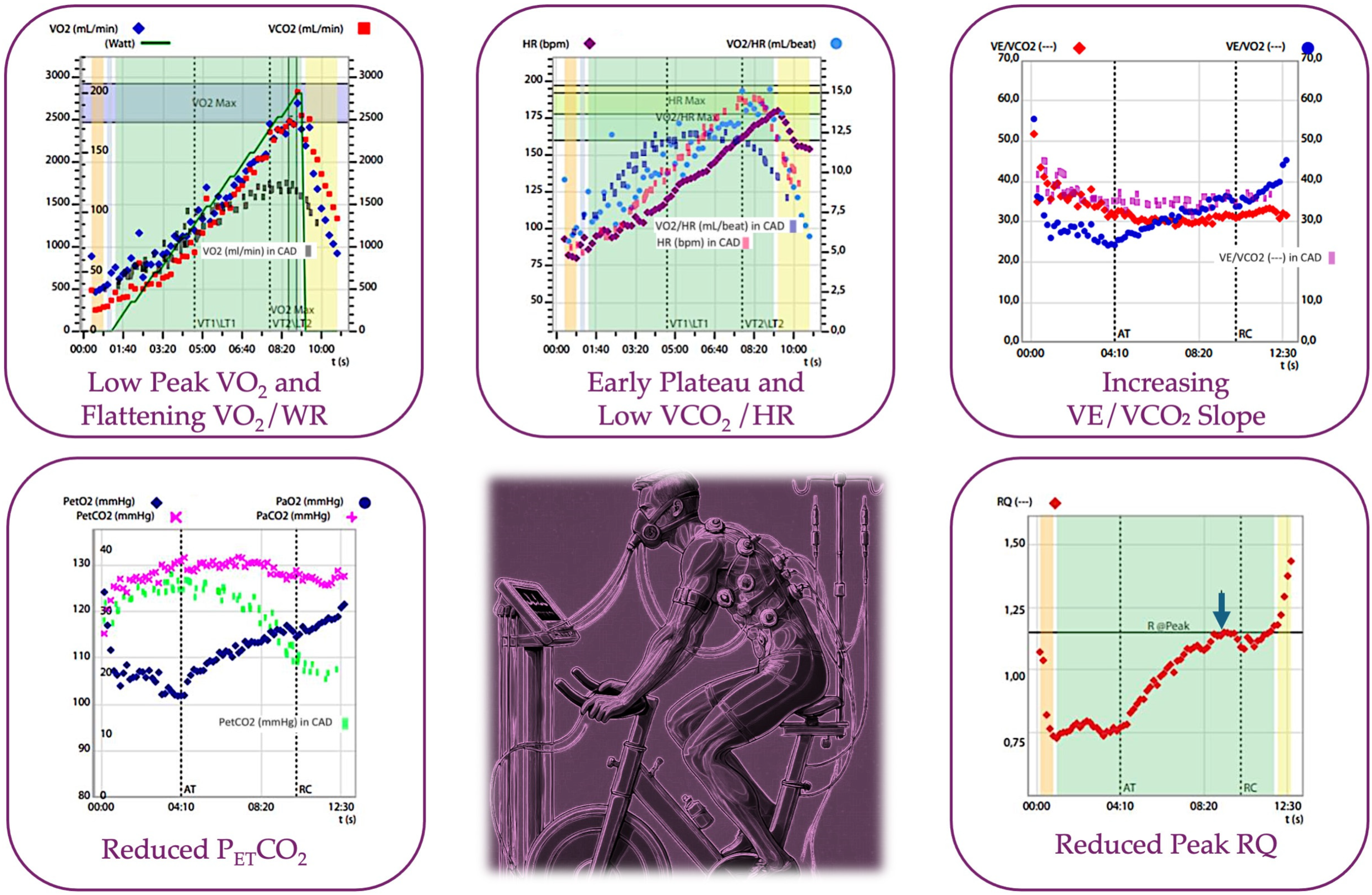
3.3. The Evolution of CPET in the Assessment of Myocardial Ischemia: Not Only VO2
3.4. CPET Parameters in the Assessment of CAD: A Practical Approach
| Value | Reference Value | Explanation |
|---|---|---|
| VO2 max [5] | >85% of the predicted | Reduced in ischemia due to impaired oxygen delivery from reduced cardiac output, limiting maximal aerobic capacity. |
| VE/VCO2 slope [54] | <35 L/min/L | Elevated in ischemia as inefficient ventilation and gas exchange occur due to poor perfusion. |
| Oxygen Pulse [54] | >80% of the predicted | Flattened or declining during exercise may indicate reduced stroke volume, often seen in myocardial ischemia. |
| PETCO2 [72] | 36–42 mmHg | Decreased in ischemia due to impaired perfusion, affecting CO2 clearance from the lungs. |
| Heart Rate Recovery [55] | <15 bpm | Delayed heart rate recovery post-exercise indicates autonomic dysfunction, which is often observed in myocardial ischemia. |
| Exercise Time [5] | 8–12 min | Shorter exercise duration is common in ischemia due to early onset of symptoms like angina and reduced cardiac output limiting exercise. |
3.5. Prognostic Value of CPET and Cardiorespiratory Fitness Versus Ergometric Test
3.6. Special Populations of Interest
3.7. Tailoring Treatment and Integration of CPET in the Current Management of CAD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Duggan, J.P.; Peters, A.S.; Trachiotis, G.D.; Antevil, J.L. Epidemiology of Coronary Artery Disease. Surg. Clin. N. Am. 2022, 102, 499–516. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Belardinelli, R.; Lacalaprice, F.; Carle, F.; Minnucci, A.; Cianci, G.; Perna, G.; D’Eusanio, G. Exercise-induced myocardial ischaemia detected by cardiopulmonary exercise testing. Eur. Heart J. 2003, 24, 1304–1313. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.; Ren, Y.; Cheng, J. Diagnostic value of the cardiopulmonary exercise test in coronary artery disease. J. Thorac. Dis. 2022, 14, 607–613. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.; Liu, T.; Xie, Y.; He, X.; Zuo, H.; Zeng, H. The Value of Cardiopulmonary Exercise Testing in Predicting the Severity of Coronary Artery Disease. J. Clin. Med. 2022, 11, 4170. [Google Scholar] [CrossRef]
- Belardinelli, R.; Lacalaprice, F.; Tiano, L.; Muçai, A.; Perna, G.P. Cardiopulmonary exercise testing is more accurate than ECG-stress testing in diagnosing myocardial ischemia in subjects with chest pain. Int. J. Cardiol. 2014, 174, 337–342. [Google Scholar] [CrossRef]
- Chaudhry, S.; Kumar, N.; Arena, R.; Verma, S. The evolving role of cardiopulmonary exercise testing in ischemic heart disease—State of the art review. Curr. Opin. Cardiol. 2023, 38, 552–572. [Google Scholar] [CrossRef]
- Taegtmeyer, H. Energy metabolism of the heart: From basic concepts to clinical applications applications. Curr. Probl. Cardiol. 1994, 19, 61–86. [Google Scholar] [CrossRef]
- Wasserman, K. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications, 5th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; ISBN 9781609138998. [Google Scholar]
- Chaudhry, S.; Arena, R.; Wasserman, K.; Hansen, J.E.; Lewis, G.D.; Myers, J.; Chronos, N.; Boden, W.E. Exercise-induced Myocardial Ischemia Detected by Cardiopulmonary Exercise Testing. Am. J. Cardiol. 2009, 103, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s Guide to Cardiopulmonary Exercise Testing in Adults: A Scientific Statement From the American Heart Association. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [PubMed]
- Popovic, D.; Martic, D.; Djordjevic, T.; Pesic, V.; Guazzi, M.; Myers, J.; Mohebi, R.; Arena, R. Oxygen consumption and carbon-dioxide recovery kinetics in the prediction of coronary artery disease severity and outcome. Int. J. Cardiol. 2017, 248, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Garner, K.K.; Pomeroy, W.; Arnold, J.J. Exercise Stress Testing: Indications and Common Questions. Am. Fam. Physician 2017, 96, 293–299. [Google Scholar] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Mark, D.B.; Hlatky, M.A.; Harrell, F.E.; Lee, K.L.; Califf, R.M.; Pryor, D.B. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann. Intern. Med. 1987, 106, 793–800. [Google Scholar] [CrossRef]
- Ellestad, M.H.; Cooke, B.M.; Greenberg, P.S. Stress testing: Clinical application and predictive capacity. Prog. Cardiovasc. Dis. 1979, 21, 431–460. [Google Scholar] [CrossRef]
- Parasuraman, S.; Schwarz, K.; Singh, S.; Abraham, D.; Garg, D.; Frenneaux, M.P. Cardiopulmonary exercise test in myocardial ischemia detection. Future Cardiol. 2020, 16, 113–121. [Google Scholar] [CrossRef]
- Chaudhry, S.; Kumar, N.; Behbahani, H.; Bagai, A.; Singh, B.K.; Menasco, N.; Lewis, G.D.; Sperling, L.; Myers, J. Abnormal heart-rate response during cardiopulmonary exercise testing identifies cardiac dysfunction in symptomatic patients with non-obstructive coronary artery disease. Int. J. Cardiol. 2017, 228, 114–121. [Google Scholar] [CrossRef]
- Herdy, A.H.; Ritt, L.E.; Stein, R.; Araujo, C.G.; Milani, M.; Meneghelo, R.S.; Ferraz, A.S.; Hossri, C.; Almeida, A.E.; Fernandes-Silva, M.M.; et al. Cardiopulmonary Exercise Test: Background, Applicability and Interpretation. Arq. Bras. Cardiol. 2016, 107, 467–481. [Google Scholar] [CrossRef]
- Pritchard, A.; Burns, P.; Correia, J.; Jamieson, P.; Moxon, P.; Purvis, J.; Thomas, M.; Tighe, H.; Sylvester, K.P. ARTP statement on cardiopulmonary exercise testing 2021. BMJ Open Respir. Res. 2021, 8, e001121. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.M. ATS/ACCP statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 1451. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Apostolo, A.; Cattadori, G.; Salvioni, E.; Berna, G.; Antonioli, L.; Vignati, C.; Schina, M.; Sciomer, S.; Bussotti, M.; et al. Effects of β-blockers on ventilation efficiency in heart failure. Am. Heart J. 2010, 159, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Segreti, A.; Verolino, G.; Crispino, S.P.; Agostoni, P. Listing Criteria for Heart Transplant: Role of Cardiopulmonary Exercise Test and of Prognostic Scores. Heart Fail. Clin. 2021, 17, 635–646. [Google Scholar] [CrossRef]
- Agostoni, P.; Corrà, U.; Cattadori, G.; Veglia, F.; Battaia, E.; La Gioia, R.; Scardovi, A.B.; Emdin, M.; Metra, M.; Sinagra, G.; et al. Prognostic value of indeterminable anaerobic threshold in heart failure. Circ. Heart Fail. 2013, 6, 977–987. [Google Scholar] [CrossRef]
- Metra, M.; Cas, L.D.; Panina, G.; Visioli, O. Exercise hyperventilation chronic congestive heart failure, and its relation to functional capacity and hemodynamics. Am. J. Cardiol. 1992, 70, 622–628. [Google Scholar] [CrossRef]
- Wasserman, K.; Zhang, Y.-Y.; Gitt, A.; Belardinelli, R.; Koike, A.; Lubarsky, L.; Agostoni, P.G. Lung Function and Exercise Gas Exchange in Chronic Heart Failure. Circulation 1997, 96, 2221–2227. [Google Scholar] [CrossRef]
- Segreti, A.; Picarelli, F.; DI Gioia, G.; Coletti, F.; Crispino, S.P.; Fanale, V.; Fossati, C.; Antonelli Incalzi, R.; Pigozzi, F.; Grigioni, F. Athlete’s heart or heart disease in the athlete? Evaluation by cardiopulmonary exercise testing. J. Sports Med. Phys. Fit. 2023, 63, 873–890. [Google Scholar] [CrossRef]
- Bussotti, M.; Apostolo, A.; Andreini, D.; Palermo, P.; Contini, M.; Agostoni, P. Cardiopulmonary evidence of exercise-induced silent ischaemia. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 249–253. [Google Scholar] [CrossRef]
- Li, S.; Yuan, Y.; Zhao, L.; Lv, T.; She, F.; Liu, F.; Xue, Y.; Zhou, B.; Xie, Y.; Geng, Y.; et al. Coronary stenosis is a risk marker for impaired cardiac function on cardiopulmonary exercise test. BMC Cardiovasc. Disord. 2022, 22, 486. [Google Scholar] [CrossRef]
- Yoshida, S.; Adachi, H.; Murata, M.; Tomono, J.; Oshima, S.; Kurabayashi, M. Importance of compensatory heart rate increase during myocardial ischemia to preserve appropriate oxygen kinetics. J. Cardiol. 2017, 70, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, E.C.; Hollanda, R.; Vargas, J.P.; Silveira, C.W.; Lemos, A.L.; Hollanda, R.M.K.; Ribeiro, J.P. Flattening of Oxygen Pulse during Exercise May Detect Extensive Myocardial Ischemia. Med. Sci. Sports Exerc. 2007, 39, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, R.; Shakerian, F.; Vasheghani-Farahani, A.; Halabchi, F.; Mirshahi, M.; Mansournia, M.A. The usefulness of cardiopulmonary exercise testing in assessment of patients with suspected coronary artery disease. Postgrad. Med. J. 2016, 92, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Petek, B.J.; Churchill, T.W.; Sawalla Guseh, J.; Loomer, G.; Gustus, S.K.; Lewis, G.D.; Weiner, R.B.; Baggish, A.L.; Wasfy, M.M. Utility of the oxygen pulse in the diagnosis of obstructive coronary artery disease in physically fit patients. Physiol. Rep. 2021, 9, e15105. [Google Scholar] [CrossRef]
- Ganesananthan, S.; Rajkumar, C.A.; Foley, M.; Thompson, D.; Nowbar, A.N.; Seligman, H.; Petraco, R.; Sen, S.; Nijjer, S.; Thom, S.A.; et al. Cardiopulmonary exercise testing and efficacy of percutaneous coronary intervention: A substudy of the ORBITA trial. Eur. Heart J. 2022, 43, 3132–3145. [Google Scholar] [CrossRef]
- Tajima, A.; Itoh, H.; Osada, N.; Omiya, K.; Maeda, T.; Ohkoshi, N.; Kawara, T.; Aizawa, T.; Wasserman, K. Oxygen Uptake Kinetics During and After Exercise are Useful Markers of Coronary Artery Disease in Patients with Exercise Electrocardiography Suggesting Myocardial Ischemia. Circ. J. 2009, 73, 1864–1870. [Google Scholar] [CrossRef]
- Uliari, S.; Myers, J.; Bernardi, E.; Chiaranda, G.; Conconi, F.; Terranova, F.; Mazzoni, G.; Grazzi, G. Oxygen Uptake Attenuation at Ventilatory Threshold in Men with Coronary Artery Disease. J. Cardiopulm. Rehabil. Prev. 2016, 36, 258–262. [Google Scholar] [CrossRef]
- Van De Sande, D.A.J.P.; Schoots, T.; Hoogsteen, J.; Doevendans, P.A.; Kemps, H.M.C. O2 Pulse Patterns in Male Master Athletes with Normal and Abnormal Exercise Tests. Med. Sci. Sports Exerc. 2019, 51, 12–18. [Google Scholar] [CrossRef]
- Bechsgaard, D.F.; Hove, J.D.; Suhrs, H.E.; Bové, K.B.; Shahriari, P.; Gustafsson, I.; Prescott, E. Women with coronary microvascular dysfunction and no obstructive coronary artery disease have reduced exercise capacity. Int. J. Cardiol. 2019, 293, 1–9. [Google Scholar] [CrossRef]
- Niu, S.; Wang, F.; Yang, S.; Jin, Z.; Han, X.; Zou, S.; Guo, D.; Guo, C. Predictive value of cardiopulmonary fitness parameters in the prognosis of patients with acute coronary syndrome after percutaneous coronary intervention. J. Int. Med. Res. 2020, 48, 300060520949081. [Google Scholar] [CrossRef]
- Smarz, K.; Jaxa-Chamiec, T.; Zaborska, B.; Tysarowski, M.; Budaj, A. Combined use of stress echocardiography and cardiopulmonary exercise testing to assess exercise intolerance in patients treated for acute myocardial infarction. PLoS ONE 2021, 16, e0255682. [Google Scholar] [CrossRef] [PubMed]
- Blankstein, R.; Cannon, C.; Udelson, J. Update on pharmacological cardiac stress testing: Efficacy, risk stratification and patient selection. Am. J. Med. 2014, 127, e16–e17. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F. The emergence of the coronary vasomotor dysfunction era. Int. J. Cardiol. 2018, 254, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Skalski, J.; Allison, T.G.; Miller, T.D. The safety of cardiopulmonary exercise testing in a population with high-risk cardiovascular diseases. Circulation 2012, 126, 2465–2472. [Google Scholar] [CrossRef]
- Khan, H.; Jaffar, N.; Rauramaa, R.; Kurl, S.; Savonen, K.; Laukkanen, J.A. Cardiorespiratory fitness and nonfatalcardiovascular events: A population-based follow-up study. Am. Heart J. 2017, 184, 55–61. [Google Scholar] [CrossRef]
- Kavanagh, T.; Mertens, D.J.; Hamm, L.F.; Beyene, J.; Kennedy, J.; Corey, P.; Shephard, R.J. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation 2002, 106, 666–671. [Google Scholar] [CrossRef]
- Kavanagh, T.; Mertens, D.J.; Hamm, L.F.; Beyene, J.; Kennedy, J.; Corey, P.; Shephard, R.J. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J. Am. Coll. Cardiol. 2003, 42, 2139–2143. [Google Scholar] [CrossRef]
- Fujimoto, W.; Oishi, S.; Kawai, H. The Prognostic Significance of Cardiopulmonary Exercise Testing at Discharge for the Patients with Acute Myocardial Infarction. J. Card. Fail. 2016, 22, S174. [Google Scholar] [CrossRef]
- Imboden, M.T.; Harber, M.P.; Whaley, M.H.; Finch, W.H.; Bishop, D.L.; Kaminsky, L.A. Cardiorespiratory Fitness and Mortality in Healthy Men and Women. J. Am. Coll. Cardiol. 2018, 72, 2283–2292. [Google Scholar] [CrossRef]
- Chaudhry, S.; Arena, R.; Bhatt, D.L.; Verma, S.; Kumar, N. A practical clinical approach to utilize cardiopulmonary exercise testing in the evaluation and management of coronary artery disease: A primer for cardiologists. Curr. Opin. Cardiol. 2018, 33, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Thirapatarapong, W.; Armstrong, H.F.; Bartels, M.N. Comparison of cardiopulmonary exercise testing variables in COPD patients with and without coronary artery disease. Heart Lung 2014, 43, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Myers, J.; Abella, J.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Guazzi, M. Development of a ventilatory classification system in patients with heart failure. Circulation 2007, 115, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Shetler, K.; Marcus, R.; Froelicher, V.F.; Vora, S.; Kalisetti, D.; Prakash, M.; Do, D.; Myers, J. Heart rate recovery: Validation and methodologic issues. J. Am. Coll. Cardiol. 2001, 38, 1980–1987. [Google Scholar] [CrossRef]
- Chorbajian, T. Normographic approach for the estimation of heart rate recovery time after exercise. J. Appl. Physiol. 1971, 31, 962–964. [Google Scholar] [CrossRef]
- Lipinski, M.J.; Vetrovec, G.W.; Froelicher, V.F. Importance of the first two minutes of heart rate recovery after exercise treadmill testing in predicting mortality and the presence of coronary artery disease in men. Am. J. Cardiol. 2004, 93, 445–449. [Google Scholar] [CrossRef]
- Dewar, A.; Kass, L.; Stephens, R.C.M.; Tetlow, N.; Desai, T. Heart Rate Recovery Assessed by Cardiopulmonary Exercise Testing in Patients with Cardiovascular Disease: Relationship with Prognosis. Int. J. Environ. Res. Public Health 2023, 20, 4678. [Google Scholar] [CrossRef]
- Li, H.; Wei, M.; Zhang, L.; Huang, L.; Wang, Y.; Wang, J.; Zhuang, S.; Wu, X.; Wu, J. Factors contributing to exercise tolerance in patients with coronary artery disease undergoing percutaneous coronary intervention. BMC Sports Sci. Med. Rehabil. 2023, 15, 35. [Google Scholar] [CrossRef]
- Forman, D.E.; Arena, R.; Boxer, R.; Dolansky, M.A.; Eng, J.J.; Fleg, J.L.; Haykowsky, M.; Jahangir, A.; Kaminsky, L.A.; Kitzman, D.W.; et al. Prioritizing Functional Capacity as a Principal End Point for Therapies Oriented to Older Adults with Cardiovascular Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2017, 135, e894–e918. [Google Scholar] [CrossRef]
- Lavie, C.J.; Menezes, A.R.; De Schutter, A.; Milani, R.V.; Blumenthal, J.A. Impact of Cardiac Rehabilitation and Exercise Training on Psychological Risk Factors and Subsequent Prognosis in Patients with Cardiovascular Disease. Can. J. Cardiol. 2016, 32, S365–S373. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, X.; Chen, B.; Liu, H. Retrospective analysis of exercise capacity in patients with coronary artery disease after percutaneous coronary intervention or coronary artery bypass graft. Int. J. Nurs. Sci. 2021, 8, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Cassar, A.; Holmes, D.R.; Rihal, C.S.; Gersh, B.J. Chronic coronary artery disease: Diagnosis and management. Mayo Clin. Proc. 2009, 84, 1130–1146. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; Balady, G.J.; Bricker, J.T.; Chaitman, B.R.; Fletcher, G.F.; Froelicher, V.F.; Mark, D.B.; McCallister, B.D.; Mooss, A.N.; O’Reilly, M.G.; et al. ACC/AHA 2002 guideline update for exercise testing: Summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J. Am. Coll. Cardiol. 2002, 40, 1531–1540. [Google Scholar] [CrossRef]
- Salokari, E.; Laukkanen, J.A.; Lehtimaki, T.; Kurl, S.; Kunutsor, S.; Zaccardi, F.; Viik, J.; Lehtinen, R.; Nikus, K.; Kööbi, T.; et al. The Duke treadmill score with bicycle ergometer: Exercise capacity is the most important predictor of cardiovascular mortality. Eur. J. Prev. Cardiol. 2019, 26, 199–207. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Knight, J.D.; Higginbotham, M.B.; Cobb, F.R. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation 1989, 80, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Kinasewitz, G.T.; Janicki, J.S.; Fishman, A.P. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation 1982, 65, 1213–1223. [Google Scholar] [CrossRef]
- Koike, A.; Hiroe, M.; Taniguchi, K.; Marumo, F. Respiratory Control during Exercise in Patients with Cardiovascular Disease. Am. Rev. Respir. Dis. 1993, 147, 425–429. [Google Scholar] [CrossRef]
- Brown, C.C.; Fry, D.L.; Ebert, R.V. The mechanics of pulmonary ventilation in patients with heart diseases. Am. J. Med. 1954, 17, 438–446. [Google Scholar] [CrossRef]
- Matsumoto, A.; Itoh, H.; Eto, Y.; Kobayashi, T.; Kato, M.; Omata, M.; Watanabe, H.; Kato, K.; Momomura, S. End-tidal CO2 pressure decreases during exercise in cardiac patients. J. Am. Coll. Cardiol. 2000, 36, 242–249. [Google Scholar] [CrossRef]
- Sun, X.G.; Hansen, J.E.; Oudiz, R.J.; Wasserman, K. Gas Exchange Detection of Exercise-Induced Right-to-Left Shunt in Patients with Primary Pulmonary Hypertension. Circ. 2002, 105, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.D.; Askew, J.W.; Herrmann, J. Assessing clinical impact of myocardial perfusion studies: Ischemia or other prognostic indicators? Curr. Cardiol. Rep. 2014, 16, 465. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xian, H.; Chandiramani, P.; Bainter, E.; Wan, L.; Martin, W.H. A prognostic scoring system for arm exercise stress testing. Open Heart 2016, 3, e000333. [Google Scholar] [CrossRef][Green Version]
- Lloyd-Jones, D.M.; Leip, E.P.; Larson, M.G.; D’Agostino, R.B.; Beiser, A.; Wilson, P.W.F.; Wolf, P.A.; Levy, D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006, 113, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Ezzatvar, Y.; Izquierdo, M.; Núñez, J.; Calatayud, J.; Ramírez-Vélez, R.; García-Hermoso, A. Cardiorespiratory fitness measured with cardiopulmonary exercise testing and mortality in patients with cardiovascular disease: A systematic review and meta-analysis. J. Sport. Health Sci. 2021, 10, 609–619. [Google Scholar] [CrossRef]
- De Assumpção, C.R.A.; Do Prado, D.M.L.; Jordão, C.P.; Dourado, L.O.C.; Vieira, M.L.C.; Montenegro, C.G.D.S.P.; Negrão, C.E.; Gowdak, L.H.W.; De Matos, L.D.N.J. Cardiopulmonary exercise test in patients with refractory angina: Functional and ischemic evaluation. Clinics 2022, 77, 100003. [Google Scholar] [CrossRef]
- Mygind, N.D.; Michelsen, M.M.; Pena, A.; Frestad, D.; Dose, N.; Aziz, A.; Faber, R.; Høst, N.; Gustafsson, I.; Hansen, P.R.; et al. Coronary Microvascular Function and Cardiovascular Risk Factors in Women with Angina Pectoris and No Obstructive Coronary Artery Disease: The iPOWER Study. JAHA 2016, 5, e003064. [Google Scholar] [CrossRef]
- Bove, K.B.; Michelsen, M.M.; Schroder, J.; Suhrs, H.E.; Bechsgaard, D.F.; Mygind, N.D.; Aziz, A.; Kastrup, J.; Gustafsson, I.; Prescott, E. Impaired coronary flow velocity reserve is associated with cardiovascular risk factors but not with angina symptoms. Open Heart 2021, 8, e001486. [Google Scholar] [CrossRef]
- Segreti, A.; Grigioni, F.; Campodonico, J.; Magini, A.; Zaffalon, D.; Sinagra, G.; Sciascio, G.D.; Swenson, E.R.; Agostoni, P. Chemoreceptor hyperactivity in heart failure: Is lactate the culprit? Eur. J. Prev. Cardiol. 2021, 28, e8–e10. [Google Scholar] [CrossRef]
- O’Neill, J.O.; Young, J.B.; Pothier, C.E.; Lauer, M.S. Peak Oxygen Consumption as a Predictor of Death in Patients with Heart Failure Receiving β-Blockers. Circulation 2005, 111, 2313–2318. [Google Scholar] [CrossRef]
- Mordi, I.; Stanton, T.; Carrick, D.; McClure, J.; Oldroyd, K.; Berry, C.; Tzemos, N. Comprehensive Dobutamine Stress CMR Versus Echocardiography in LBBB and Suspected Coronary Artery Disease. JACC Cardiovasc. Imaging 2014, 7, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 Focused Update: Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circ. 2016, 133, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Faselis, C.; Kokkinos, P.; Tsimploulis, A.; Pittaras, A.; Myers, J.; Lavie, C.J.; Kyritsi, F.; Lovic, D.; Karasik, P.; Moore, H. Exercise Capacity and Atrial Fibrillation Risk in Veterans. Mayo Clin. Proc. 2016, 91, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cai, F.; Geng, C.; Wang, Z.; Tang, X. Diagnostic Performance of CMR, SPECT, and PET Imaging for the Identification of Coronary Artery Disease: A Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 621389. [Google Scholar] [CrossRef] [PubMed]
- Gulsin, G.S.; Henson, J.; Brady, E.M.; Sargeant, J.A.; Wilmot, E.G.; Athithan, L.; Htike, Z.Z.; Marsh, A.-M.; Biglands, J.D.; Kellman, P.; et al. Cardiovascular Determinants of Aerobic Exercise Capacity in Adults with Type 2 Diabetes. Diabetes Care 2020, 43, 2248–2256. [Google Scholar] [CrossRef]
- Almeida, V.R.D.; Ostolin, T.L.V.D.P.; Gonze, B.D.B.; De Almeida, F.R.; Romiti, M.; Arantes, R.L.; Dourado, V.Z. Early flattening of the oxygen pulse during the cardiopulmonary exercise test in asymptomatic adults and its association with cardiovascular risk factors. Int. J. Cardiol. 2022, 367, 65–73. [Google Scholar] [CrossRef]
- Smith, D.L.; Graham, E.L.; Douglas, J.A.; Jack, K.; Conner, M.J.; Arena, R.; Chaudhry, S. Subclinical Cardiac Dysfunction is Associated with Reduced Cardiorespiratory Fitness and Cardiometabolic Risk Factors in Firefighters. Am. J. Med. 2022, 135, 752–760.e3. [Google Scholar] [CrossRef]
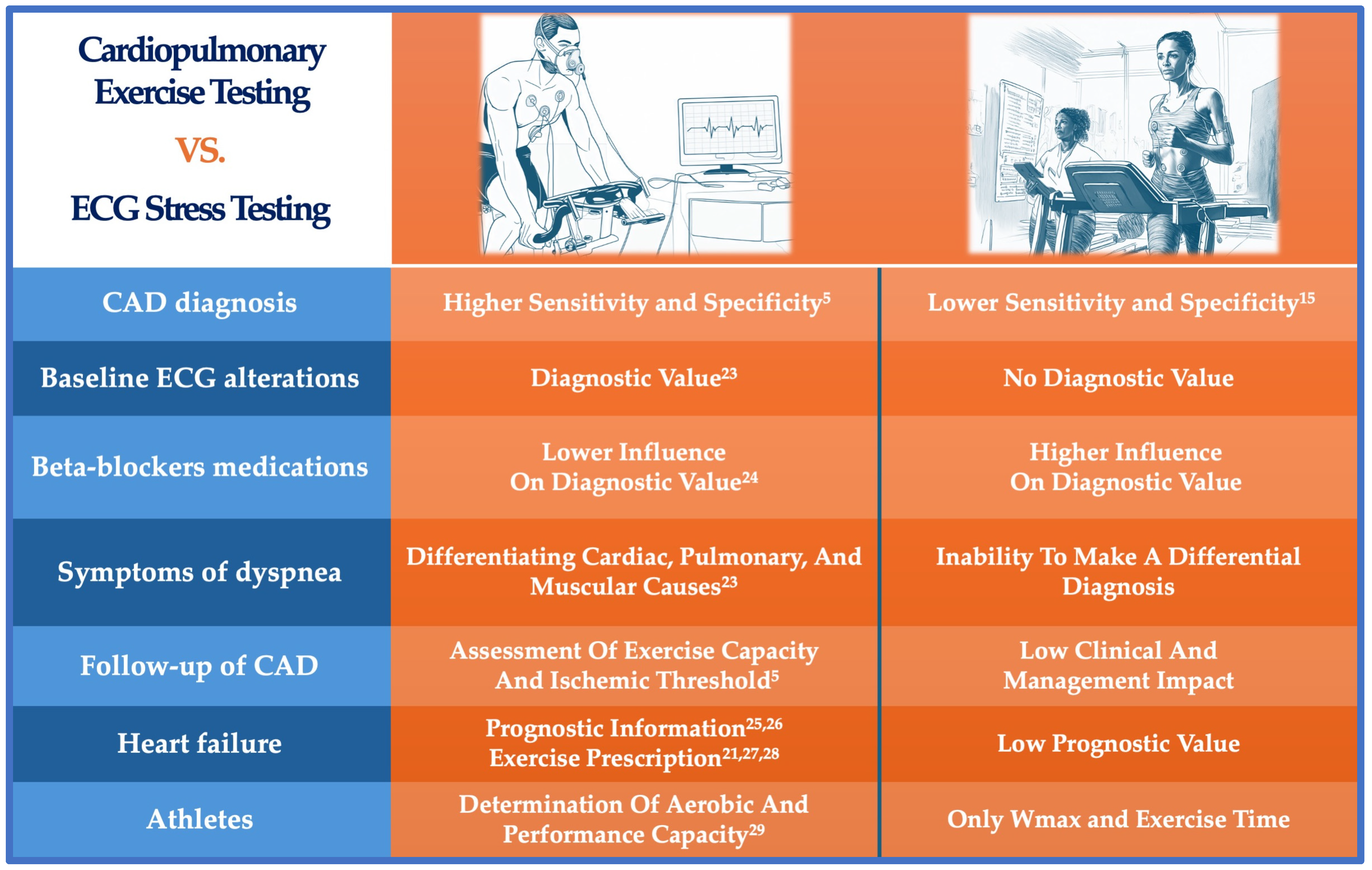
| Aspect | CPET [5] | Traditional Exercise Test | Imaging-Based Functional Tests: CMR, SPECT, PET [85] |
|---|---|---|---|
| Sensitivity | 86–87% [1,6] | 45–70% [15,19] | 86% (CMR), 83% (SPECT), and 85% (PET) [85] |
| Specificity | 74–98% [1,6] | 70–80% [15] | 83% (CMR), 77% (SPECT), and 86% (PET) [85] |
| Influence of medications | Low | High (e.g., beta-blockers) | Variable |
| Prognostic value | Strong (e.g., VE/VCO2, VO2 max) | Moderate (Duke Treadmill Score) | Strong (perfusion defects, wall motion) |
| Special population suitability | High (athletes, women, heart failure) | Low | Moderate (depending on imaging modality) |
| Exercise capacity assessment | Detailed | Basic | Limited to imaging stress capability |
| Risk stratification | Comprehensive | Basic (based on exercise tolerance) | Advanced (perfusion, wall motion, viability) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crispino, S.P.; Segreti, A.; Ciancio, M.; Polito, D.; Guerra, E.; Di Gioia, G.; Ussia, G.P.; Grigioni, F. The Complementary Role of Cardiopulmonary Exercise Testing in Coronary Artery Disease: From Early Diagnosis to Tailored Management. J. Cardiovasc. Dev. Dis. 2024, 11, 357. https://doi.org/10.3390/jcdd11110357
Crispino SP, Segreti A, Ciancio M, Polito D, Guerra E, Di Gioia G, Ussia GP, Grigioni F. The Complementary Role of Cardiopulmonary Exercise Testing in Coronary Artery Disease: From Early Diagnosis to Tailored Management. Journal of Cardiovascular Development and Disease. 2024; 11(11):357. https://doi.org/10.3390/jcdd11110357
Chicago/Turabian StyleCrispino, Simone Pasquale, Andrea Segreti, Martina Ciancio, Dajana Polito, Emiliano Guerra, Giuseppe Di Gioia, Gian Paolo Ussia, and Francesco Grigioni. 2024. "The Complementary Role of Cardiopulmonary Exercise Testing in Coronary Artery Disease: From Early Diagnosis to Tailored Management" Journal of Cardiovascular Development and Disease 11, no. 11: 357. https://doi.org/10.3390/jcdd11110357
APA StyleCrispino, S. P., Segreti, A., Ciancio, M., Polito, D., Guerra, E., Di Gioia, G., Ussia, G. P., & Grigioni, F. (2024). The Complementary Role of Cardiopulmonary Exercise Testing in Coronary Artery Disease: From Early Diagnosis to Tailored Management. Journal of Cardiovascular Development and Disease, 11(11), 357. https://doi.org/10.3390/jcdd11110357










