Endo-Aortic Clamp for Minimally Invasive Redo Mitral Valve Surgery: Early Outcome
Abstract
:1. Introduction
2. Materials and Methods
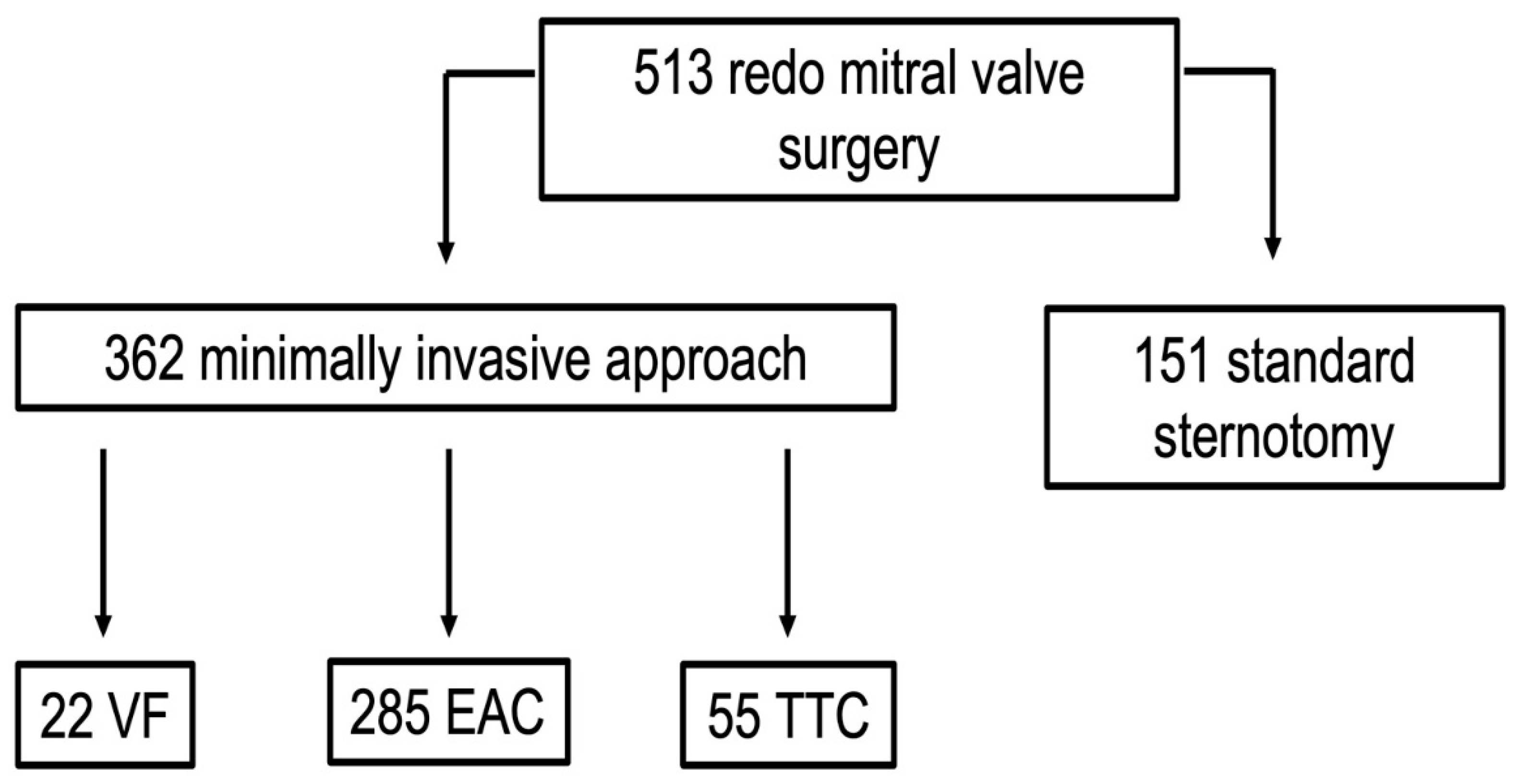
2.1. Study Design
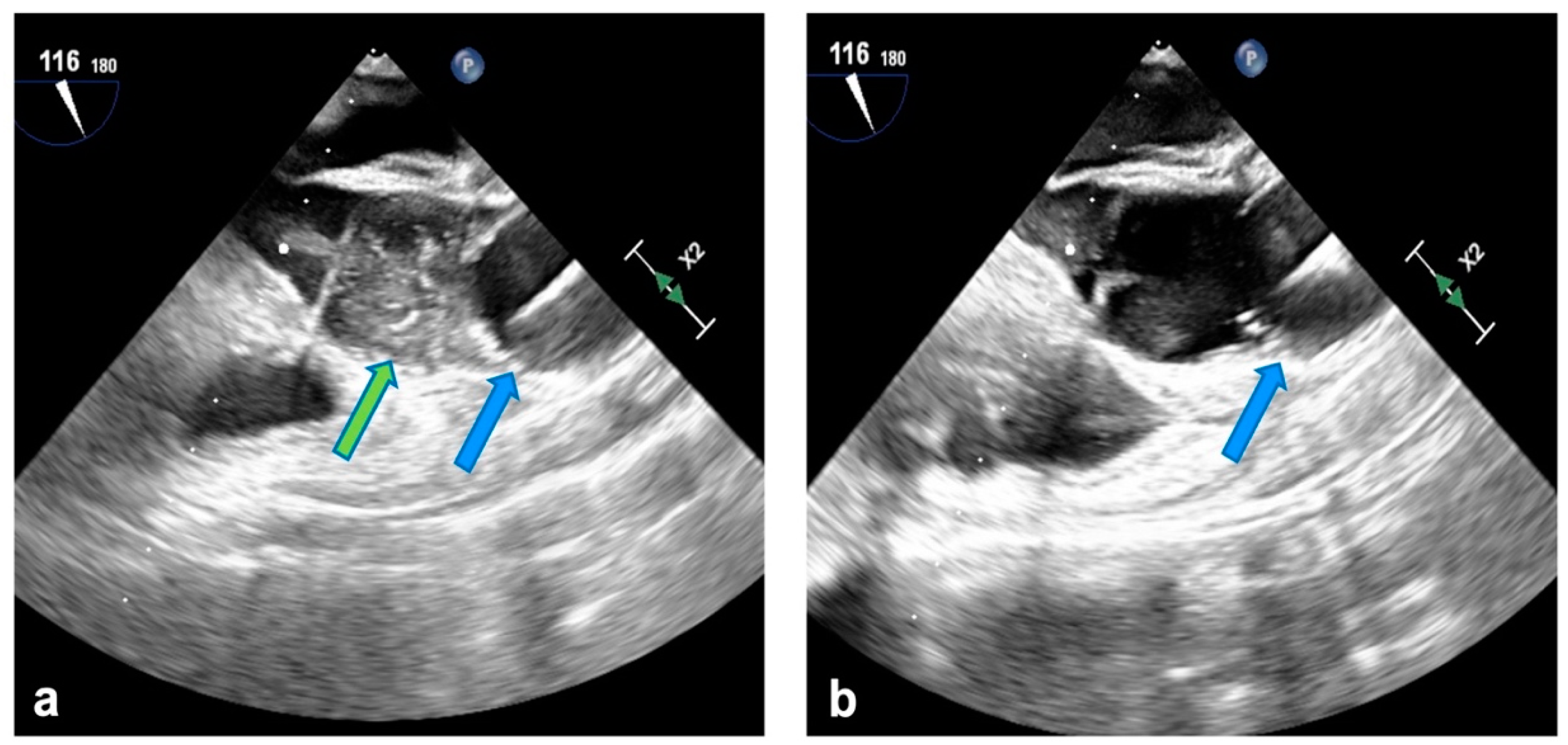
2.2. Surgical Technique
2.3. Data Presentation and Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehaffey, H.J.; Hawkins, R.B.; Schubert, S.; Fonner, C.; Yarboro, L.T.; Quader, M.; Speir, A.; Rich, J.; Kron, I.L.; Ailawadi, G. Contemporary outcomes in reoperative mitral valve surgery. Heart 2018, 104, 652–656. [Google Scholar] [CrossRef]
- Kilic, A.; Acker, M.A.; Gleason, T.G.; Sultan, I.; Vemulapalli, S.; Thibault, D.; Ailawadi, G.; Badhwar, V.; Thourani, V.; Kilic, A. Clinical outcomes of mitral valve reoperations in the United States: An analysis of the society of thoracic surgeons national database. Ann. Thorac. Surg. 2019, 107, 754–759. [Google Scholar] [CrossRef]
- Onorati, F.; Perrotti, A.; Reichart, D.; Mariscalco, G.; Della Ratta, E.; Santarpino, G.; Salsano, A.; Rubino, A.; Biancari, F.; Gatti, G.; et al. Surgical factors and complications affecting hospital outcome in redo mitral surgery: Insights from a multicentre experience. Eur. J. Cardiothorac. Surg. 2016, 49, 127–133. [Google Scholar] [CrossRef]
- Akay, T.H.; Gultekin, B.; Ozkan, S.; Aslim, E.; Uguz, E.; Sezgin, A.; Aslamaci, S. Mitral valve replacements in redo patients with previous mitral valve procedures: Mid-term results and risk factors for survival. J. Card. Surg. 2008, 23, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Suri, R.M.; Burkhart, H.M.; Greason, K.L.; Dearani, J.A.; Schaff, H.V.; Sundt, T.M. Identifying patients at particular risk of injury during repeat sternotomy: Analysis of 2555 cardiac reoperations. J. Thorac. Cardiovasc. Surg. 2010, 140, 1028–1035. [Google Scholar] [CrossRef]
- Barbero, C.; Marchetto, G.; Ricci, D.; El Qarra, S.; Attisani, M.; Filippini, C.; Boffini, M.; Rinaldi, M. Minimal access mitral valve surgery: Impact of tailored strategies on early outcome. Ann. Thorac. Surg. 2016, 102, 1989–1994. [Google Scholar] [CrossRef]
- Barbero, C.; Pocar, M.; Marchetto, G.; Stura, E.C.; Calia, C.; Boffini, M.; Rinaldi, M.; Ricci, D. Antegrade perfusion for mini-thoracotomy mitral valve surgery in patients with atherosclerotic burden. Heart Lung Circ. 2022, 31, 415–419. [Google Scholar] [CrossRef]
- Barbero, C.; Pocar, M.; Marchetto, G.; Cura Stura, E.; Calia, C.; Dalbesio, B.; Filippini, C.; Salizzoni, S.; Boffini, M.; Rinaldi, M.; et al. Single-dose St. Thomas versus Custodiol® cardioplegia for right mini-thoracotomy mitral valve surgery. J. Cardiovasc. Transl. Res. 2022, 16, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Barbero, C.; Spitaleri, A.; Pocar, M.; Parrella, B.; Santonocito, A.; Bozzo, E.; Depaoli, A.; Faletti, R.; Rinaldi, M. Handling extensive mitral annular calcification via a minimally invasive right mini-thoracotomy approach. Appl. Sci. 2023, 13, 2563. [Google Scholar] [CrossRef]
- Ghoreishi, M.; Dawood, M.; Hobbs, G.; Pasrija, C.; Riley, P.; Petrose, L.; Griffith, B.P.; Gammie, J.S. Repeat sternotomy: No longer a risk factor in mitral valve surgical procedures. Ann. Thorac. Surg. 2013, 96, 1358–1365. [Google Scholar] [CrossRef]
- Seeburger, J.; Borger, M.A.; Falk, V.; Passage, J.; Walther, T.; Doll, N.; Mohr, F.W. Minimally invasive mitral valve surgery after previous sternotomy: Experience in 181 patients. Ann. Thorac. Surg. 2009, 87, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Onorati, F.; Mariscalco, G.; Reichart, D.; Perrotti, A.; Gatti, G.; De Feo, M.; Rubino, A.; Santarpino, G.; Biancari, F.; Detter, C.; et al. Hospital outcome and risk indices of mortality after redo-mitral valve surgery in potential candidates for transcatheter procedures: Results from a european registry. J. Cardiothorac. Vasc. Anesth. 2018, 32, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Chacko, J.; Uzzaman, M.; Hamid, O.; Butt, S.; Zakai, S.B.; Khan, H. Minimally invasive (mini-thoracotomy) versus median sternotomy in redo mitral valve surgery: A meta-analysis of observational studies. Asian Cardiovasc. Thorac. Ann. 2021, 29, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Daemen, J.H.T.; Heuts, S.; Olsthoorn, J.R.; Maessen, J.G.; Sardary Nia, P. Right minithoracotomy versus median sternotomy for reoperative mitral valve surgery: A systematic review and metaanalysis of observational studies. Eur. J. Cardiothorac. Surg. 2018, 54, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Woo, Y.J. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N. Engl. J. Med. 2017, 377, 1847–1857. [Google Scholar] [CrossRef]
- Albeyoglu, S.C.; Filizcan, U.; Sargin, M.; Cakmak, M.; Goksel, O.; Bayserke, O.; Cınar, B.; Eren, E. Determinants of hospital mortality after repeat mitral valve surgery for rheumatic mitral valve disease. Thorac. Cardiovasc. Surg. 2006, 54, 244–249. [Google Scholar] [CrossRef]
- Vohra, H.A.; Whistance, R.N.; Roubelakis, A.; Burton, A.; Barlow, C.W.; Tsang, G.M.; Livesey, S.A.; Ohri, S.K. Outcome after redo-mitral valve replacement in adult patients: A 10-year single-centre experience. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 575–579. [Google Scholar] [CrossRef]
- Toker, M.E.; Eren, E.; Guler, M.; Kirali, K.; Yanartas, M.; Balkanay, M.; Yakut, C. Second and third cardiac valve reoperations: Factors influencing death and long-term survival. Tex. Heart Inst. J. 2009, 36, 557–562. [Google Scholar]
- Expósito, V.; García-Camarero, T.; Bernal, J.M.; Arnáiz, E.; Sarralde, A.; García, I.; Berrazueta, J.R.; Revuelta, J.M. Repeat mitral valve replacement: 30-years’ experience. Rev. Esp. Cardiol. 2009, 62, 929–932. [Google Scholar] [CrossRef]
- Casselman, F.; Aramendi, J.; Bentala, M.; Candolfi, P.; Coppoolse, R.; Gersak, B.; Greco, E.; Herijgers, P.; Hunter, S.; Krakor, R.; et al. Endoaortic clamping does not increase the risk of stroke in minimal access mitral valve surgery: A multicenter experience. Ann. Thorac. Surg. 2015, 100, 1334–1339. [Google Scholar] [CrossRef]
- Barbero, C.; Krakor, R.; Bentala, M.; Casselman, F.; Candolfi, P.; Goldstein, J.; Rinaldi, M. Comparison of endoaortic and transthoracic aortic clamping in less-invasive mitral valve surgery. Ann. Thorac. Surg. 2018, 105, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Barbero, C.; Rinaldi, M.; Pocar, M.; Stura, E.C.; Calia, C.; Sebastiano, V.; Marchetto, G.; Filippini, C.; Boffini, M.; Ricci, D. Endo-aortic vs. trans-thoracic clamping in right mini-thoracotomy mitral valve surgery: Outcome on myocardial protection. Front. Cardiovasc. Med. 2021, 8, 719687. [Google Scholar] [CrossRef] [PubMed]
- Marullo, A.G.M.; Irace, F.G.; Vitulli, P.; Peruzzi, M.; Rose, D.; D’Ascoli, R.; Iaccarino, A.; Pisani, A.; De Carlo, C.; Mazzesi, G.; et al. Recent developments in minimally invasive cardiac surgery: Evolution or revolution? BioMed Res. Int. 2015, 2015, 483025. [Google Scholar] [CrossRef]
| n = 285 | |
|---|---|
| Female | 158 (55.4) |
| Age, years | 63.8 ± 13.3 |
| BMI, kg/m2 | 24.6 ± 4.8 |
| Log EuroSCORE | 16.5 ± 14.4 |
| NYHA class ≥ 3 | 173 (60.7) |
| Atrial fibrillation | 153 (53.7) |
| Chronic kidney disease | 55 (19.3) |
| Hypertension | 177 (62.1) |
| COPD | 31 (10.9) |
| Cirrhosis | 4 (1.4) |
| Diabetes | 48 (16.8) |
| Mild peripheral vasculopathy | 15 (5.3) |
| Coronary artery disease | 20 (7) |
| Two or more previous cardiac operations | 75 (26.3) |
| Previous CABG | 36 (12.6) |
| Previous MV repair | 100 (35.1) |
| Previous MV replacement | 92 (32.3) |
| Previous tricuspid valve repair | 3 (1.1) |
| Previous aortic valve surgery | 39 (13.7) |
| LVEDD, mm | 53.4 ± 13.1 |
| LVEF, % | 53.1 ± 11.4 |
| sPAP, mmHg | 47.7 ± 16.1 |
| n = 285 | |
|---|---|
| Isolated MV surgery | 205 (71.9) |
| MV repair | 42 (14.7) |
| MV replacement | 143 (50.2) |
| Mitral prosthesis replacement | 100 (35.1) |
| TV surgery | 65 (22.8) |
| ASD closure | 10 (2.8) |
| AF ablation | 13 (4.6) |
| CPB time, min | 141.6 ± 45.3 |
| Cross-clamp time, min | 93.9 ± 28.6 |
| Acute aortic dissection | – |
| Conversion to sternotomy | 1 (0.3) |
| Mechanical ventilation > 72 h | 29 (10.2) |
| Re-intubation | 21 (7.4) |
| Acute kidney injury | 18 (6.3) |
| Stroke | 6 (2.1) |
| Re-exploration for bleeding | 20 (7) |
| Thoracic wound dehiscence | 5 (1.8) |
| Permanent pacemaker | 18 (6.3) |
| ICU length of stay, days | 1 [1–3] |
| In-hospital length of stay, days | 8 [7–13] |
| 30-day mortality | 11 (3.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbero, C.; Pocar, M.; Costamagna, A.; Capozza, C.; Aloi, V.; Cura Stura, E.; Salizzoni, S.; Rinaldi, M. Endo-Aortic Clamp for Minimally Invasive Redo Mitral Valve Surgery: Early Outcome. J. Cardiovasc. Dev. Dis. 2024, 11, 358. https://doi.org/10.3390/jcdd11110358
Barbero C, Pocar M, Costamagna A, Capozza C, Aloi V, Cura Stura E, Salizzoni S, Rinaldi M. Endo-Aortic Clamp for Minimally Invasive Redo Mitral Valve Surgery: Early Outcome. Journal of Cardiovascular Development and Disease. 2024; 11(11):358. https://doi.org/10.3390/jcdd11110358
Chicago/Turabian StyleBarbero, Cristina, Marco Pocar, Andrea Costamagna, Cecilia Capozza, Valentina Aloi, Erik Cura Stura, Stefano Salizzoni, and Mauro Rinaldi. 2024. "Endo-Aortic Clamp for Minimally Invasive Redo Mitral Valve Surgery: Early Outcome" Journal of Cardiovascular Development and Disease 11, no. 11: 358. https://doi.org/10.3390/jcdd11110358
APA StyleBarbero, C., Pocar, M., Costamagna, A., Capozza, C., Aloi, V., Cura Stura, E., Salizzoni, S., & Rinaldi, M. (2024). Endo-Aortic Clamp for Minimally Invasive Redo Mitral Valve Surgery: Early Outcome. Journal of Cardiovascular Development and Disease, 11(11), 358. https://doi.org/10.3390/jcdd11110358







