Early versus Delayed Surgery in Patients with Left-Sided Infective Endocarditis and Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patients
2.3. Endocarditis Diagnosis and Surgery
2.4. Neurological Complications
2.5. Postoperative Neurological Outcome
2.6. Statistics
3. Results
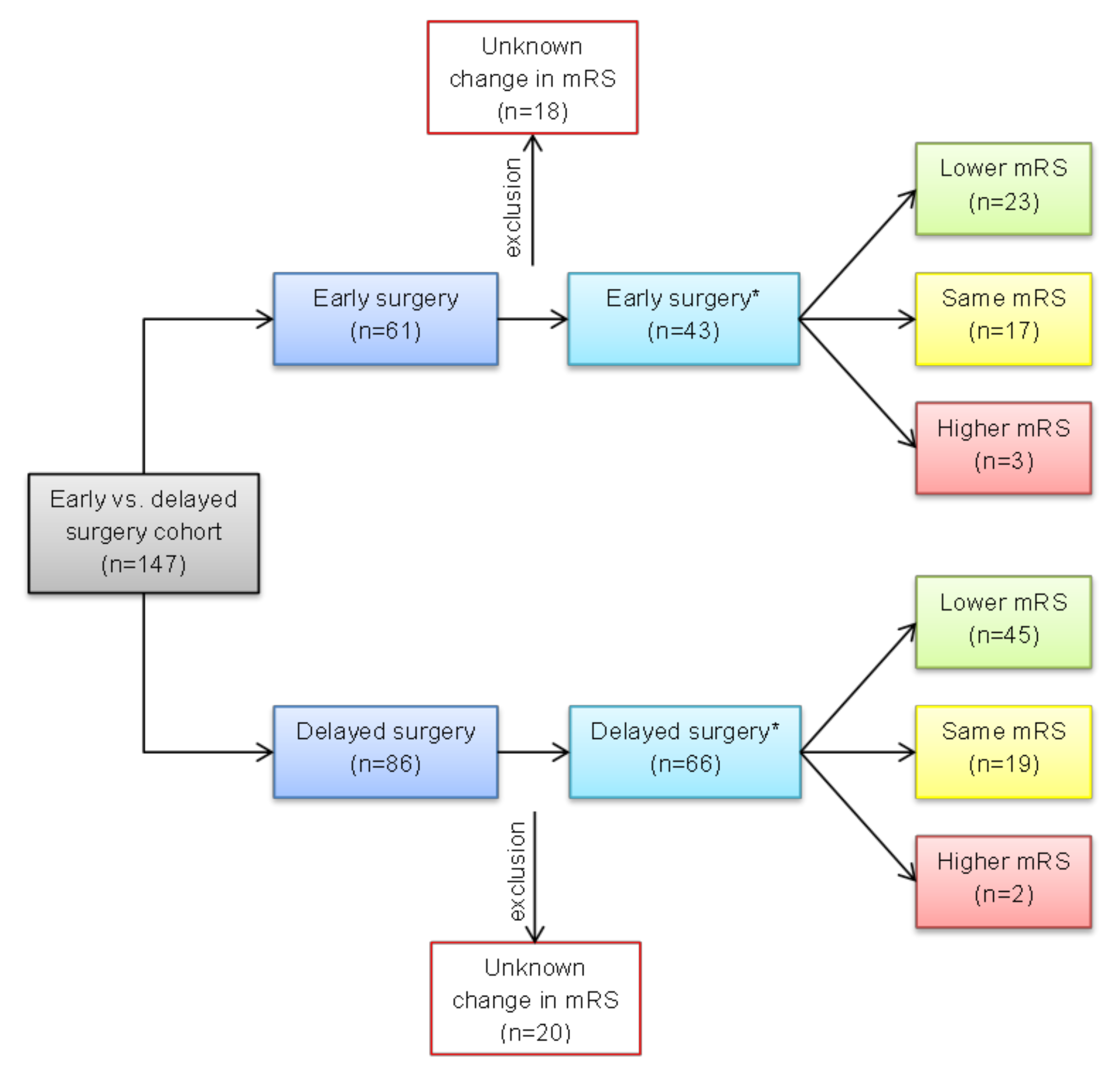
3.1. Patient Selection
3.2. Pathology of Endocarditis
3.3. Early vs. Delayed Surgery after Preoperative Stroke
3.3.1. Neurological Status
3.3.2. Perioperative and Postoperative Outcome
4. Discussion
4.1. Infective Valvular Endocarditis
4.2. Timing of Surgery
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.Q.; Cho, S.-M.; Rice, C.J.; Khoury, J.; Marquardt, R.J.; Buletko, A.B.; Hardman, J.; Wisco, D.; Uchino, K. Valve surgery for infective endocarditis complicated by stroke: Surgical timing and perioperative neurological complications. Eur. J. Neurol. 2020, 27, 2430–2438. [Google Scholar] [CrossRef] [PubMed]
- Heiro, M.; Helenius, H.; Hurme, S.; Savunen, T.; Engblom, E.; Nikoskelainen, J.; Kotilainen, P. Short-term and one-year outcome of infective endocarditis in adult patients treated in a Finnish teaching hospital during 1980–2004. BMC Infect. Dis. 2007, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Avierinos, J.-F.; Tribouilloy, C.; Giorgi, R.; Casalta, J.-P.; Milandre, L.; Brahim, A.; Nadji, G.; Riberi, A.; Collart, F.; et al. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: A prospective multicentre study. Eur. Heart J. 2007, 28, 1155–1161. [Google Scholar] [CrossRef]
- García-Cabrera, E.; Fernández-Hidalgo, N.; Almirante, B.; Ivanova-Georgieva, R.; Noureddine, M.; Plata, A.; Lomas, J.M.; Gálvez-Acebal, J.; Hidalgo-Tenorio, C.; Ruíz-Morales, J.; et al. Neurological complications of infective endocarditis: Risk factors, outcome, and impact of cardiac surgery: A multicenter observational study. Circulation 2013, 127, 2272–2284. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Ruttmann, E.; Abfalterer, H.; Wagner, J.; Grimm, M.; Müller, L.; Bates, K.; Ulmer, H.; Bonaros, N. Endocarditis-related stroke is not a contraindication for early cardiac surgery: An investigation among 440 patients with left-sided endocarditis. Eur. J. Cardiothorac. Surg. 2020, 58, 1161–1167. [Google Scholar] [CrossRef]
- Khatri, R.; McKinney, A.M.; Swenson, B.; Janardhan, V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 2012, 79, S52–S57. [Google Scholar] [CrossRef]
- Eishi, K.; Kawazoe, K.; Kuriyama, Y.; Kitoh, Y.; Kawashima, Y.; Omae, T. Surgical management of infective endocarditis associated with cerebral complications. J. Thorac. Cardiovasc. Surg. 1995, 110, 1745–1755. [Google Scholar] [CrossRef]
- Satriano, U.M.; Nenna, A.; Spadaccio, C.; Pollari, F.; Fischlein, T.; Chello, M.; Nappi, F. Guidelines on prosthetic heart valve management in infective endocarditis: A narrative review comparing American Heart Association/American College of Cardiology and European Society of Cardiology guidelines. Ann. Transl. Med. 2020, 8, 1625. [Google Scholar] [CrossRef]
- Yanagawa, B.; Pettersson, G.B.; Habib, G.; Ruel, M.; Saposnik, G.; Latter, D.A.; Verma, S. Surgical Management of Infective Endocarditis Complicated by Embolic Stroke: Practical Recommendations for Clinicians. Circulation 2016, 134, 1280–1292. [Google Scholar] [CrossRef]
- Ruttmann, E.; Willeit, J.; Ulmer, H.; Chevtchik, O.; Höfer, D.; Poewe, W.; Laufer, G.; Müller, L.C. Neurological outcome of septic cardioembolic stroke after infective endocarditis. Stroke 2006, 37, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Piper, C.; Wiemer, M.; Schulte, H.D.; Horstkotte, D. Stroke is not a contraindication for urgent valve replacement in acute infective endocarditis. J. Heart Valve Dis. 2001, 10, 703–711. [Google Scholar] [PubMed]
- Yoshioka, D.; Sakaguchi, T.; Yamauchi, T.; Okazaki, S.; Miyagawa, S.; Nishi, H.; Yoshikawa, Y.; Fukushima, S.; Saito, S.; Sawa, Y. Impact of early surgical treatment on postoperative neurologic outcome for active infective endocarditis complicated by cerebral infarction. Ann. Thorac. Surg. 2012, 94, 489–495, discussion 496. [Google Scholar] [CrossRef] [PubMed]
- Barsic, B.; Dickerman, S.; Krajinovic, V.; Pappas, P.; Altclas, J.; Carosi, G.; Casabé, J.H.; Chu, V.H.; Delahaye, F.; Edathodu, J.; et al. Influence of the timing of cardiac surgery on the outcome of patients with infective endocarditis and stroke. Clin. Infect. Dis. 2013, 56, 209–217. [Google Scholar] [CrossRef]
- Tam, D.Y.; Yanagawa, B.; Verma, S.; Ruel, M.; Fremes, S.E.; Mazine, A.; Adams, S.; Friedrich, J.O. Early vs Late Surgery for Patients with Endocarditis and Neurological Injury: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2018, 34, 1185–1199. [Google Scholar] [CrossRef]
- Dashkevich, A.; Bratkov, G.; Li, Y.; Joskowiak, D.; Peterss, S.; Juchem, G.; Hagl, C.; Luehr, M. Impact of Operative Timing in Infective Endocarditis with Cerebral Embolism-The Risk of Intermediate Deterioration. J. Clin. Med. 2021, 10, 2136. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Olmos, C.; Vilacosta, I.; Habib, G.; Maroto, L.; Fernández, C.; López, J.; Sarriá, C.; Salaun, E.; Di Stefano, S.; Carnero, M.; et al. Risk score for cardiac surgery in active left-sided infective endocarditis. Heart 2017, 103, 1435–1442. [Google Scholar] [CrossRef]
- Kang, D.-H.; Kim, Y.-J.; Kim, S.-H.; Sun, B.J.; Kim, D.-H.; Yun, S.-C.; Song, J.-M.; Choo, S.J.; Chung, C.-H.; Song, J.-K.; et al. Early surgery versus conventional treatment for infective endocarditis. New Engl. J. Med. 2012, 366, 2466–2473. [Google Scholar] [CrossRef]
- Tornos, P.; Iung, B.; Permanyer-Miralda, G.; Baron, G.; Delahaye, F.; Gohlke-Bärwolf, C.; Butchart, E.G.; Ravaud, P.; Vahanian, A. Infective endocarditis in Europe: Lessons from the Euro heart survey. Heart 2005, 91, 571–575. [Google Scholar] [CrossRef]
- Olmos, C.; Vilacosta, I.; Fernández, C.; Sarriá, C.; López, J.; Del Trigo, M.; Ferrera, C.; Vivas, D.; Maroto, L.; Hernández, M.; et al. Comparison of clinical features of left-sided infective endocarditis involving previously normal versus previously abnormal valves. Am. J. Cardiol. 2014, 114, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Nadji, G.; Rusinaru, D.; Rémadi, J.-P.; Jeu, A.; Sorel, C.; Tribouilloy, C. Heart failure in left-sided native valve infective endocarditis: Characteristics, prognosis, and results of surgical treatment. Eur. J. Heart Fail. 2009, 11, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Croft, C.H.; Woodward, W.; Elliott, A.; Commerford, P.J.; Barnard, C.N.; Beck, W. Analysis of surgical versus medical therapy in active complicated native valve infective endocarditis. Am. J. Cardiol. 1983, 51, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.V.; Karp, R.B.; Kirklin, J.W.; Dismukes, W.E. Treatment of infective endocarditis: A 10-year comparative analysis. Circulation 1978, 58, 589–597. [Google Scholar] [CrossRef]
- Hasbun, R.; Vikram, H.R.; Barakat, L.A.; Buenconsejo, J.; Quagliarello, V.J. Complicated left-sided native valve endocarditis in adults: Risk classification for mortality. JAMA 2003, 289, 1933–1940. [Google Scholar] [CrossRef]
- Leone, S.; Ravasio, V.; Durante-Mangoni, E.; Crapis, M.; Carosi, G.; Scotton, P.G.; Barzaghi, N.; Falcone, M.; Chinello, P.; Pasticci, M.B.; et al. Epidemiology, characteristics, and outcome of infective endocarditis in Italy: The Italian Study on Endocarditis. Infection 2012, 40, 527–535. [Google Scholar] [CrossRef]
- Arregle, F.; Martel, H.; Philip, M.; Gouriet, F.; Casalta, J.P.; Riberi, A.; Torras, O.; Casalta, A.-C.; Camoin-Jau, L.; Lavagna, F.; et al. Infective endocarditis with neurological complications: Delaying cardiac surgery is associated with worse outcome. Arch. Cardiovasc. Dis. 2021, 114, 527–536. [Google Scholar] [CrossRef]
- Diab, M.; Musleh, R.; Lehmann, T.; Sponholz, C.; Pletz, M.W.; Franz, M.; Schulze, P.C.; Witte, O.W.; Kirchhof, K.; Doenst, T.; et al. Risk of postoperative neurological exacerbation in patients with infective endocarditis and intracranial haemorrhage. Eur. J. Cardiothorac. Surg. 2020, 59, 426–433. [Google Scholar] [CrossRef]
- Pericàs, J.M.; Hernández-Meneses, M.; Muñoz, P.; Álvarez-Uría, A.; Pinilla-Llorente, B.; de Alarcón, A.; Reviejo, K.; Fariñas, M.C.; Falces, C.; Goikoetxea-Agirre, J.; et al. Outcomes and Risk Factors of Septic Shock in Patients with Infective Endocarditis: A Prospective Cohort Study. Open Forum Infect. Dis. 2021, 8, ofab119. [Google Scholar] [CrossRef]
- Botelho-Nevers, E.; Thuny, F.; Casalta, J.P.; Richet, H.; Gouriet, F.; Collart, F.; Riberi, A.; Habib, G.; Raoult, D. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch. Intern. Med. 2009, 169, 1290–1298. [Google Scholar] [CrossRef]

| Variable | Early Surgery (n = 61) | Delayed Surgery (n = 86) | p-Value |
|---|---|---|---|
| Previous IE | 1 (1.6) | 9 (10.5) | 0.046 |
| Affected valve | |||
| Aortic | 33 (54.1) | 51 (59.3) | 0.53 |
| Mitral | 42 (68.9) | 50 (58.1) | 0.19 |
| Double valve | 14 (23.0) | 15 (17.4) | 0.41 |
| Prosthetic valve endocarditis | 6 (9.8) | 20 (23.3) | 0.04 |
| Sepsis | 24 (39.3) | 42 (48.8) | 0.25 |
| RISK-E score | 26 (15–36) | 24 (14–35) | 0.39 |
| Congestive heart failure | 19 (31.1) | 24 (27.9) | 0.67 |
| Embolic events | 61 (100) | 78 (90.7) | 0.02 |
| Brain | 61 (100) | 78 (90.7) | 0.02 |
| Peripheral emboli | 19 (31.1) | 17 (19.8) | 0.11 |
| Laboratory values | |||
| C-reactive protein (mg/dL) | 8.0 (4.2–18.9) | 6.1 (2.5–9.9) | 0.01 |
| WBC (x109/L) | 9.5 (7.8–16.0) | 10.0 (7.6–13.1) | 0.49 |
| Creatinine (mg/dL) | 1.1 (0.8–1.9) | 1.1 (0.7–1.7) | 0.26 |
| Bilirubin (mg/dL) | 0.8 (0.5–3.0) | 0.6 (0.3–0.9) | 0.02 |
| Causative microorganism | |||
| Staphylococcus spp. | 27 (44.3) | 30 (34.9) | 0.25 |
| Staphylococcus aureus | 22 (36.1) | 26 (30.2) | 0.46 |
| MRSA | 1 (1.6) | 2 (2.3) | >0.99 |
| Coagulase-negative staphylococci | 5 (8.2) | 4 (4.7) | 0.49 |
| Streptococcus spp. | 15 (24.6) | 21 (24.4) | 0.98 |
| Viridans group streptococci | 8 (13.1) | 9 (10.5) | 0.62 |
| Streptococcus bovis | 4 (6.6) | 9 (10.5) | 0.41 |
| Enterococcus spp. | 3 (4.9) | 6 (7.0) | 0.74 |
| Fungi | 1 (1.6) | 0 | 0.42 |
| Other | 5 (8.2) | 4 (4.7) | 0.49 |
| Annular abscess | 29 (47.5) | 30 (34.9) | 0.02 |
| Vegetation | 53 (86.9) | 71 (82.6) | 0.09 |
| Vegetation size (mm) | 20 (15–30) | 16 (11–20) | 0.006 |
| Variable | Early Surgery (n = 61) | Delayed Surgery (n = 86) | p-Value |
|---|---|---|---|
| Neurological symptoms | 41 (67.2) | 66 (76.7) | 0.33 |
| Focal | 30 (49.2) | 53 (61.6) | |
| Non-focal | 11 (18.0) | 13 (15.1) | |
| Modified Rankin Scale | 1 (1–2) | 2 (1-3) | 0.03 |
| Type of stroke | 0.003 | ||
| Ischemic stroke | 59 (96.7) | 69 (80.2) | |
| Haemorrhage | 2 (3.3) | 17 (19.8) | |
| Affected brain areas | 0.64 | ||
| Frontoparietal | 3 (4.9) | 7 (8.1) | |
| Middle cerebral artery | 10 (16.4) | 17 (19.8) | |
| Parietal | 2 (3.3) | 1 (1.2) | |
| Thalamus | 1 (1.6) | 2 (2.3) | |
| Occipital | 2 (3.3) | 0 | |
| Brain stem | 0 | 2 (2.3) | |
| Cerebellum | 2 (3.3) | 4 (4.7) | |
| Multiple sites | 38 (62.3) | 51 (59.3) | |
| Unknown | 3 (4.9) | 2 (2.3) | |
| Other neurological complications | |||
| TIA | 0 | 0 | |
| Septic encephalopathy/meningitis | 4 (6.6) | 8 (9.3) | 0.76 |
| Brain abscess | 2 (3.3) | 1 (1.2) | 0.57 |
| Variable | Early Surgery (n = 61) | Delayed Surgery (n = 86) | p-Value |
|---|---|---|---|
| Acute kidney failure | 5 (8.2) | 9 (10.5) | 0.82 |
| Need for dialysis | 4 (6.6) | 5 (5.8) | >0.99 |
| Need for postoperative pacemaker | 5 (8.2) | 12 (14.0) | 0.28 |
| Sepsis | 30 (49.2) | 23 (26.7) | 0.005 |
| Re-thoracotomy for bleeding | 3 (4.9) | 3 (3.5) | 0.69 |
| Postoperative CVA | 5 (8.2) | 2 (2.3) | 0.13 |
| Stroke | 2 (3.3) | 1 (1.2) | 0.57 |
| TIA | 1 (1.6) | 0 | 0.42 |
| Seizure | 2 (3.3) | 1 (1.2) | 0.57 |
| Haemorrhagic transformation of preoperative stroke | 1 (1.6) | 0 | 0.42 |
| Postoperative modified Rankin Scale | 0 (0–1) | 0 (0–2) | 0.80 |
| ICU stay (days) | 3 (1–5) | 3 (1–5) | 0.48 |
| Hospital stay (days) | 15 (8–27) | 16 (8-31) | 0.63 |
| In-hospital mortality | 7 (11.5) | 7 (8.1) | 0.50 |
| 30-day mortality | 13 (21.3) | 13 (15.1) | 0.37 |
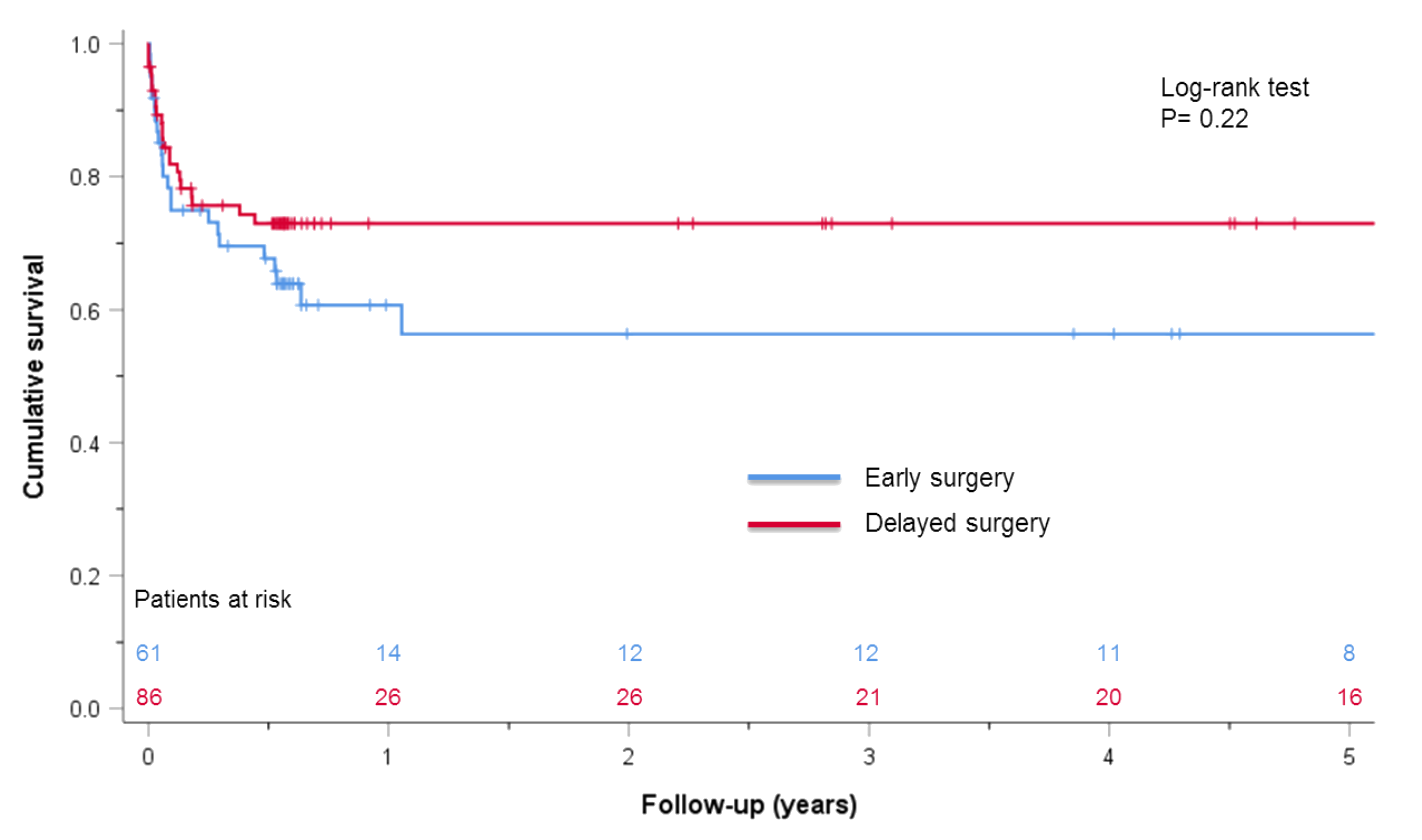
| Overall mortality | 23 (37.7) | 23 (26.7) | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kremer, J.; Jahn, J.; Klein, S.; Farag, M.; Borst, T.; Karck, M. Early versus Delayed Surgery in Patients with Left-Sided Infective Endocarditis and Stroke. J. Cardiovasc. Dev. Dis. 2023, 10, 356. https://doi.org/10.3390/jcdd10080356
Kremer J, Jahn J, Klein S, Farag M, Borst T, Karck M. Early versus Delayed Surgery in Patients with Left-Sided Infective Endocarditis and Stroke. Journal of Cardiovascular Development and Disease. 2023; 10(8):356. https://doi.org/10.3390/jcdd10080356
Chicago/Turabian StyleKremer, Jamila, Joshua Jahn, Sabrina Klein, Mina Farag, Tobias Borst, and Matthias Karck. 2023. "Early versus Delayed Surgery in Patients with Left-Sided Infective Endocarditis and Stroke" Journal of Cardiovascular Development and Disease 10, no. 8: 356. https://doi.org/10.3390/jcdd10080356
APA StyleKremer, J., Jahn, J., Klein, S., Farag, M., Borst, T., & Karck, M. (2023). Early versus Delayed Surgery in Patients with Left-Sided Infective Endocarditis and Stroke. Journal of Cardiovascular Development and Disease, 10(8), 356. https://doi.org/10.3390/jcdd10080356






