Plaque Rupture in a Hodgkin Lymphoma Survivor without Cardiovascular Risk Factors 20 Years after Thoracic Radiotherapy: A Case Report
Abstract
1. Introduction
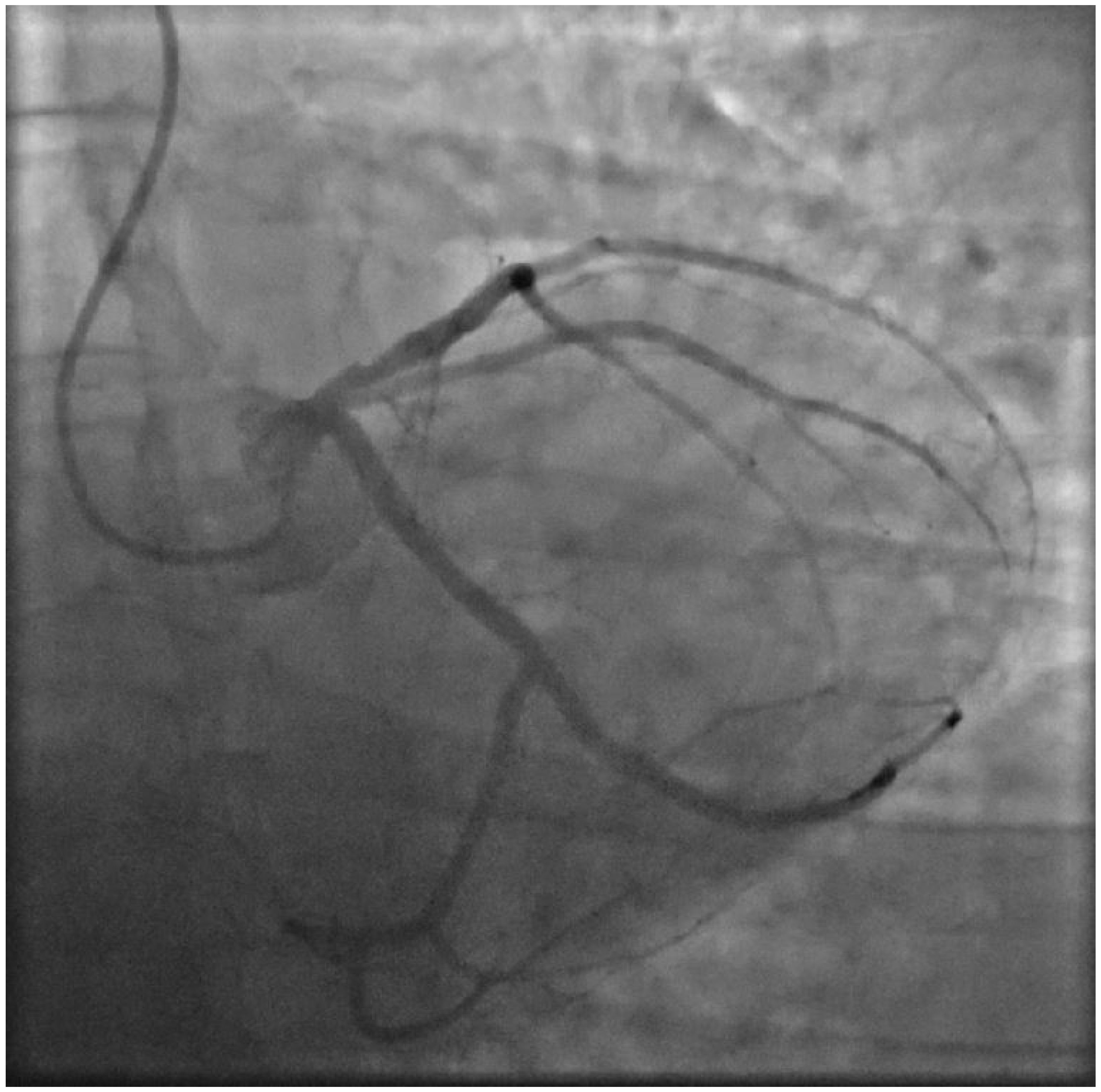
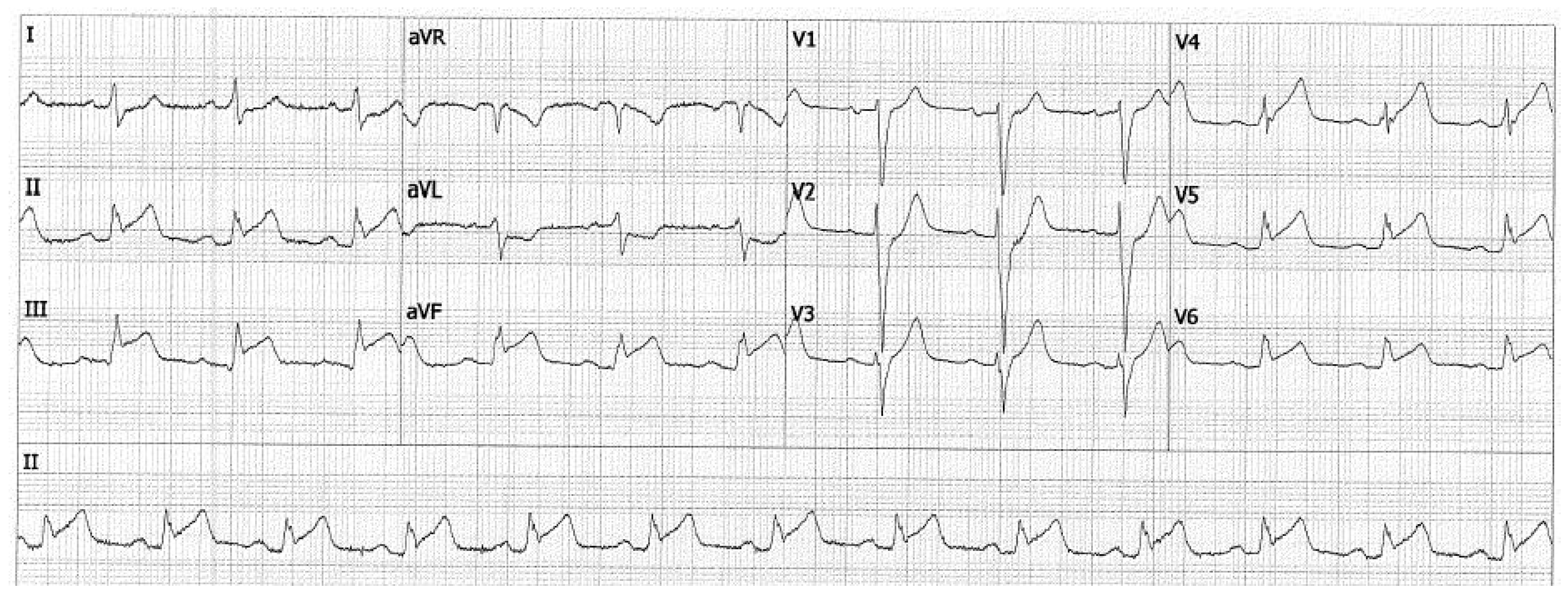
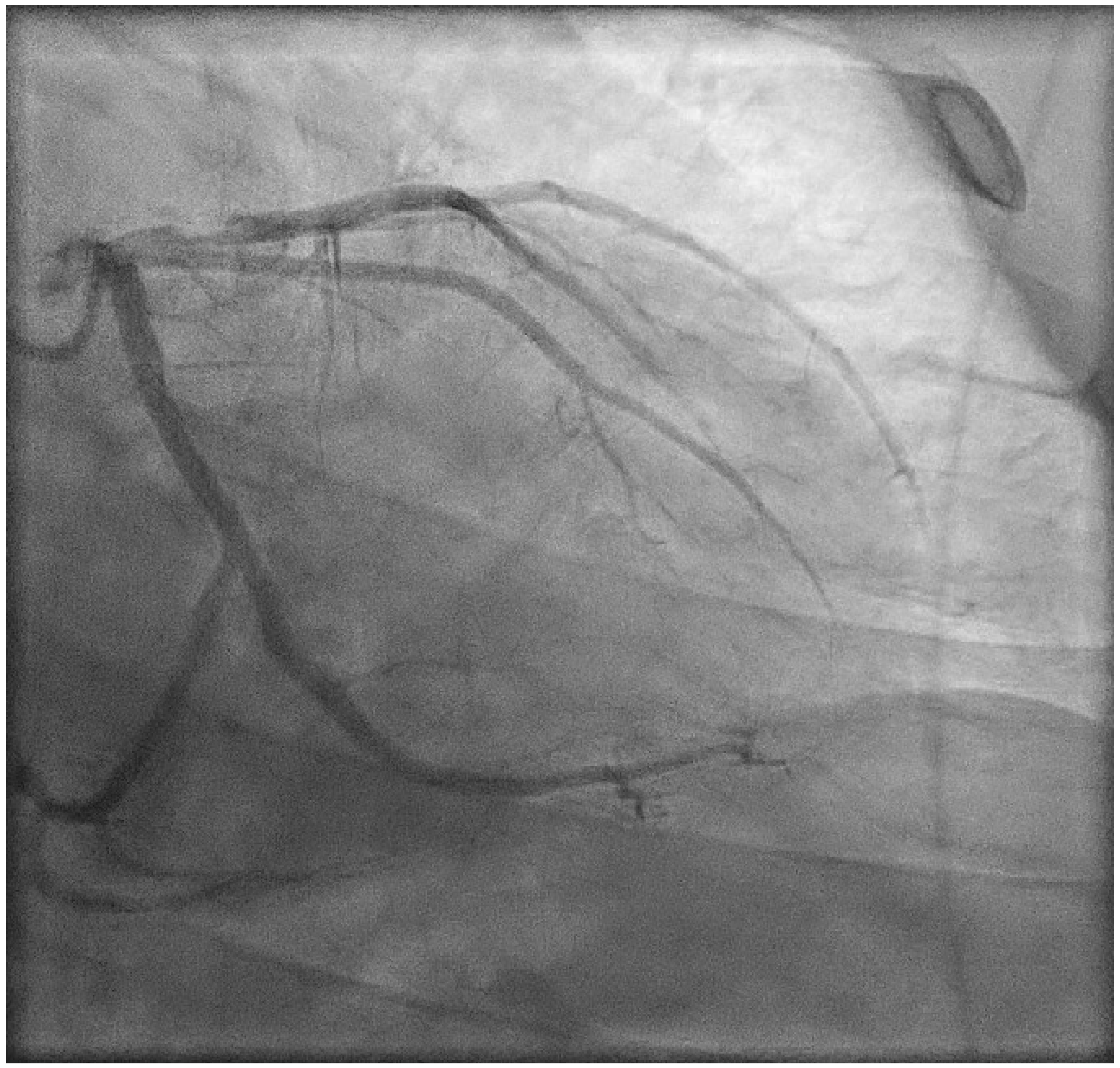
2. Case Presentation
3. Discussion
3.1. Concomitant Anthracycline Chemotherapy
3.2. Non-Invasive Modalities for Detecting CAD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Nkomo, V.T.; Badano, L.P.; Bergler-Klein, J.; Bogaert, J.; Davin, L.; Cosyns, B.; Coucke, P.; Dulgheru, R.; Edvardsen, T.; et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: A report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography (vol 26, pg 1013, 2013). Eur. Heart J.-Cardiovasc. Imaging 2013, 14, 1217. [Google Scholar] [CrossRef]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J.-Cardiovasc. Imaging 2022, e333–e465. [Google Scholar] [CrossRef]
- Cuomo, J.R.; Javaheri, S.P.; Sharma, G.K.; Kapoor, D.; Berman, A.E.; Weintraub, N.L. How to prevent and manage radiation-induced coronary artery disease. Heart 2018, 104, 1647–1653. [Google Scholar] [CrossRef]
- van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.M.; Krol, A.D.G.; Petersen, E.J.; Raemaekers, J.M.M.; Kok, W.E.M.; Aleman, B.M.P.; van Leeuwen, F.E. Cardiovascular Disease After Hodgkin Lymphoma Treatment 40-Year Disease Risk. JAMA Intern. Med. 2015, 175, 1007–1017. [Google Scholar] [CrossRef]
- van Rosendael, A.R.; Daniels, L.A.; Dimitriu-Leen, A.C.; Smit, J.M.; van Rosendael, P.J.; Schalij, M.J.; Bax, J.J.; Scholte, A. Different manifestation of irradiation induced coronary artery disease detected with coronary computed tomography compared with matched non-irradiated controls. Radiother. Oncol. 2017, 125, 55–61. [Google Scholar] [CrossRef]
- Kloosterman, A.; van Dillen, T.; Bijwaard, H.; Heeneman, S.; Hoving, S.; Stewart, F.A.; Dekkers, F. How radiation influences atherosclerotic plaque development: A biophysical approach in ApoE(−/−) mice. Radiat. Environ. Biophys. 2017, 56, 423–431. [Google Scholar] [CrossRef]
- Kupeli, S.; Hazirolan, T.; Varan, A.; Akata, D.; Alehan, D.; Hayran, M.; Besim, A.; Buyukpamukcu, M. Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin’s lymphoma. J. Clin. Oncol. 2010, 28, 1025–1030. [Google Scholar] [CrossRef]
- Tanimura, K.; Otake, H.; Kawamori, H.; Toba, T.; Nagasawa, A.; Nakano, S.; Takahashi, Y.; Fukuyama, Y.; Kozuki, A.; Shite, J.; et al. Morphological Plaque Characteristics and Clinical Outcomes in Patients with Acute Coronary Syndrome and a Cancer History. J. Am. Heart Assoc. 2021, 10, e020243. [Google Scholar] [CrossRef]
- O’Rourke, R.A.; Brundage, B.H.; Froelicher, V.F.; Greenland, P.; Grundy, S.M.; Hachamovitch, R.; Pohost, G.M.; Shaw, L.J.; Weintraub, W.S.; Winters, W.L.; et al. American College of Cardiology/American Heart Association expert consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. J. Am. Coll. Cardiol. 2000, 36, 326–340. [Google Scholar] [CrossRef]
- Lai, Y.H.; Chen, H.H.W.; Tsai, Y.S. Accelerated coronary calcium burden in breast cancer patients after radiotherapy: A comparison with age and race matched healthy women. Radiat. Oncol. 2021, 16, 210. [Google Scholar] [CrossRef]
- Engbers, E.M.; Mouden, M.; Jager, P.L.; Timmer, J.R. Zero coronary calcium in the presence of three-vessel and left main coronary artery disease in a Hodgkin lymphoma survivor. Neth. Heart J. 2015, 23, 395–398. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Yeazel, M.W.; Kawashima, T.; Mertens, A.C.; Mitby, P.; Stovall, M.; Donaldson, S.S.; Green, D.M.; Sklar, C.A.; Robison, L.L.; et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ-Br. Med. J. 2009, 339, b4606. [Google Scholar] [CrossRef]
- Tukenova, M. Role of Cancer Treatment in Long-Term Overall and Cardiovascular Mortality After Childhood Cancer (vol 28, pg 1308, 2010). J. Clin. Oncol. 2010, 28, 3205. [Google Scholar] [CrossRef]
- Hancock, S.L.; Donaldson, S.S.; Hoppe, R.T. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J. Clin. Oncol. 1993, 11, 1208–1215. [Google Scholar] [CrossRef]
- Haddy, N.; Diallo, S.; El-Fayech, C.; Schwartz, B.; Pein, F.; Hawkins, M.; Veres, C.; Oberlin, O.; Guibout, C.; Pacquement, H.; et al. Cardiac Diseases Following Childhood Cancer Treatment: Cohort Study. Circulation 2016, 133, 31–38. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Schnittger, I.; Strauss, H.W.; Vagelos, R.H.; Lee, B.K.; Mariscal, C.S.; Tate, D.J.; Horning, S.J.; Hoppe, R.T.; Hancock, S.L. Screening for coronary artery disease after mediastinal irradiation for Hodgkin’s disease. J. Clin. Oncol. 2007, 25, 43–49. [Google Scholar] [CrossRef]
- de Geus-Oei, L.-F.; Mavinkurve-Groothuis, A.M.C.; Bellersen, L.; Gotthardt, M.; Oyen, W.J.G.; Kapusta, L.; van Laarhoven, H.W.M. Scintigraphic Techniques for Early Detection of Cancer Treatment–Induced Cardiotoxicity. J. Nucl. Med. 2011, 52, 560–571. [Google Scholar] [CrossRef]
- Correa, C.R.; Litt, H.I.; Hwang, W.T.; Ferrari, V.A.; Solin, L.J.; Harris, E.E. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J. Clin. Oncol. 2007, 25, 3031–3037. [Google Scholar] [CrossRef]
- Hardenbergh, P.H.; Munley, M.T.; Bentel, G.C.; Kedem, R.; Borges-Neto, S.; Hollis, D.; Prosnitz, L.R.; Marks, L.B. Cardiac perfusion changes in patients treated for breast cancer with radiation therapy and doxorubicin: Preliminary results. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1023–1028. [Google Scholar] [CrossRef]
- Seddon, B.; Cook, A.; Gothard, L.; Salmon, E.; Latus, K.; Underwood, S.R.; Yarnold, J. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiother. Oncol. 2002, 64, 53–63. [Google Scholar] [CrossRef]
- Gyenes, G.; Fornander, T.; Carlens, P.; Glas, U.; Rutqvist, L.E. Myocardial damage in breast cancer patients treated with adjuvant radiotherapy: A prospective study. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 899–905. [Google Scholar] [CrossRef]
- Gyenes, G.; Fornander, T.; Carlens, P.; Glas, U.; Rutqvist, L.E. Detection of radiation-induced myocardial damage by technetium-99m sestamibi scintigraphy. Eur. J. Nucl. Med. 1997, 24, 286–292. [Google Scholar] [CrossRef]
- Marks, L.B.; Yu, X.; Prosnitz, R.G.; Zhou, S.M.; Hardenbergh, P.H.; Blazing, M.; Hollis, D.; Lind, P.; Tisch, A.; Wong, T.Z.; et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 214–223. [Google Scholar] [CrossRef]
- Prosnitz, R.G.; Hubbs, J.L.; Evans, E.S.; Zhou, S.M.; Yu, X.; Blazing, M.A.; Hollis, D.R.; Tisch, A.; Wong, T.Z.; Borges-Neto, S.; et al. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: Analysis of data 3 to 6 years after treatment. Cancer 2007, 110, 1840–1850. [Google Scholar] [CrossRef]
- Chung, E.; Corbett, J.R.; Moran, J.M.; Griffith, K.A.; Marsh, R.B.; Feng, M.; Jagsi, R.; Kessler, M.L.; Ficaro, E.C.; Pierce, L.J. Is There a Dose-Response Relationship for Heart Disease With Low-Dose Radiation Therapy? Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 959–964. [Google Scholar] [CrossRef][Green Version]
- Zagar, T.M.; Kaidar-Person, O.; Tang, X.; Jones, E.E.; Matney, J.; Das, S.K.; Green, R.L.; Sheikh, A.; Khandani, A.H.; McCartney, W.H.; et al. Utility of Deep Inspiration Breath Hold for Left-Sided Breast Radiation Therapy in Preventing Early Cardiac Perfusion Defects: A Prospective Study. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 903–909. [Google Scholar] [CrossRef]
- Sciagra, R.; Lubberink, M.; Hyafil, F.; Saraste, A.; Slart, R.; Agostini, D.; Nappi, C.; Georgoulias, P.; Bucerius, J.; Rischpler, C.; et al. EANM procedural guidelines for PET/CT quantitative myocardial perfusion imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1040–1069. [Google Scholar] [CrossRef]
- Gould, K.L.; Johnson, N.P.; Bateman, T.M.; Beanlands, R.S.; Bengel, F.M.; Bober, R.; Camici, P.G.; Cerqueira, M.D.; Chow, B.J.W.; Di Carli, M.F.; et al. Anatomic versus assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J. Am. Coll. Cardiol. 2013, 62, 1639–1653. [Google Scholar] [CrossRef]
- Danad, I.; Raijmakers, P.G.; Driessen, R.S.; Leipsic, J.; Raju, R.; Naoum, C.; Knuuti, J.; Maki, M.; Underwood, R.S.; Min, J.K.; et al. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiol. 2017, 2, 1100–1107. [Google Scholar] [CrossRef]
- Juneau, D.; Erthal, F.; Ohira, H.; Mc Ardle, B.; Hessian, R.; deKemp, R.A.; Beanlands, R.S. Clinical PET Myocardial Perfusion Imaging and Flow Quantification. Cardiol. Clin. 2016, 34, 69–85. [Google Scholar] [CrossRef]
- Mc Ardle, B.A.; Dowsley, T.F.; deKemp, R.A.; Wells, G.A.; Beanlands, R.S. Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease?: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2012, 60, 1828–1837. [Google Scholar] [CrossRef]
- Parker, M.W.; Iskandar, A.; Limone, B.; Perugini, A.; Kim, H.; Jones, C.; Calamari, B.; Coleman, C.I.; Heller, G.V. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: A bivariate meta-analysis. Circ. Cardiovasc. Imaging 2012, 5, 700–707. [Google Scholar] [CrossRef]
- Ziadi, M.C.; Dekemp, R.A.; Williams, K.; Guo, A.; Renaud, J.M.; Chow, B.J.; Klein, R.; Ruddy, T.D.; Aung, M.; Garrard, L.; et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J. Nucl. Cardiol. 2012, 19, 670–680. [Google Scholar] [CrossRef]
- Hamirani, Y.S.; Kramer, C.M. Cardiac MRI assessment of myocardial perfusion. Future Cardiol. 2014, 10, 349–358. [Google Scholar] [CrossRef]
- de Jong, M.C.; Genders, T.S.S.; van Geuns, R.J.; Moelker, A.; Hunink, M.G.M. Diagnostic performance of stress myocardial perfusion imaging for coronary artery disease: A systematic review and meta-analysis. Eur. Radiol. 2012, 22, 1881–1895. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, T.; Tian, L.; Yang, Y.; Xue, C.; Sheng, W.; Wang, C. Early detection and serial monitoring during chemotherapy-radiation therapy: Using T1 and T2 mapping cardiac magnetic resonance imaging. Front. Cardiovasc. Med. 2023, 10, 1085737. [Google Scholar] [CrossRef]
- Lopez-Mattei, J.; Yang, E.H.; Baldassarre, L.A.; Agha, A.; Blankstein, R.; Choi, A.D.; Chen, M.Y.; Meyersohn, N.; Daly, R.; Slim, A.; et al. Cardiac Computed Tomographic Imaging in Cardio-Oncology: An Expert Consensus Statement from the Society of Cardiovascular Computed Tomography, Endorsed by the International Cardio-Oncology Society (ICOS). J. Cardiovasc. Comput. Tomogr. 2022. [Google Scholar] [CrossRef]
- Li, J.; Montarello, N.J.; Hoogendoorn, A.; Verjans, J.W.; Bursill, C.A.; Peter, K.; Nicholls, S.J.; McLaughlin, R.A.; Psaltis, P.J. Multimodality Intravascular Imaging of High-Risk Coronary Plaque. JACC Cardiovasc. Imaging 2022, 15, 145–159. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polomski, E.A.S.; de Graaf, M.A.; Jukema, J.W.; Antoni, M.L. Plaque Rupture in a Hodgkin Lymphoma Survivor without Cardiovascular Risk Factors 20 Years after Thoracic Radiotherapy: A Case Report. J. Cardiovasc. Dev. Dis. 2023, 10, 324. https://doi.org/10.3390/jcdd10080324
Polomski EAS, de Graaf MA, Jukema JW, Antoni ML. Plaque Rupture in a Hodgkin Lymphoma Survivor without Cardiovascular Risk Factors 20 Years after Thoracic Radiotherapy: A Case Report. Journal of Cardiovascular Development and Disease. 2023; 10(8):324. https://doi.org/10.3390/jcdd10080324
Chicago/Turabian StylePolomski, Elissa A. S., Michiel A. de Graaf, J. Wouter Jukema, and M. Louisa Antoni. 2023. "Plaque Rupture in a Hodgkin Lymphoma Survivor without Cardiovascular Risk Factors 20 Years after Thoracic Radiotherapy: A Case Report" Journal of Cardiovascular Development and Disease 10, no. 8: 324. https://doi.org/10.3390/jcdd10080324
APA StylePolomski, E. A. S., de Graaf, M. A., Jukema, J. W., & Antoni, M. L. (2023). Plaque Rupture in a Hodgkin Lymphoma Survivor without Cardiovascular Risk Factors 20 Years after Thoracic Radiotherapy: A Case Report. Journal of Cardiovascular Development and Disease, 10(8), 324. https://doi.org/10.3390/jcdd10080324







