Detection of Early Myocardial Dysfunction by Imaging Biomarkers in Cancer Patients Undergoing Photon Beam vs. Proton Beam Radiotherapy: A Prospective Study
Abstract
:1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. 2D Transthoracic Echocardiography
2.3. 2D Speckle Tracking Echocardiography
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Conventional Echocardiography
3.3. 2D Speckle Tracking Echocardiography
3.4. Intraobserver and Interobserver Variability
4. Discussion
4.1. Conventional Echocardiography
4.2. 2D Speckle Tracking Echocardiography
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | 2-dimensional |
| 2D-STE | 2-dimensional speckle tracking echocardiography |
| A | late diastolic mitral inflow velocity |
| E | early diastolic mitral inflow velocity |
| e′ | early diastolic tissue Doppler velocity |
| E/A | ratio of early to late diastolic mitral inflow velocities |
| GCS | global circumferential strain |
| GCSRe | global circumferential early diastolic strain rate |
| GLS | global longitudinal strain |
| GLSRe | global longitudinal early diastolic strain rate |
| GLSRs | global longitudinal systolic strain rate |
| GRS | global radial strain |
| GRSRe | global radial early diastolic strain rate |
| LV | left ventricular |
| LVEF | left ventricular ejection fraction |
| PBT | proton beam therapy |
| PhT | photon beam therapy |
| RT | radiotherapy |
| RV | right ventricular |
| S′ | systolic annular tissue velocity |
| SRe | early diastolic strain rate |
| SRs | systolic strain rate |
| TAPSE | tricuspid annular plane systolic excursion |
| TTE | transthoracic echocardiography |
References
- Lancellotti, P.; Nkomo, V.T.; Badano, L.P.; Bergler-Klein, J.; Bogaert, J.; Davin, L.; Cosyns, B.; Coucke, P.; Dulgheru, R.; Edvardsen, T.; et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: A report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2013, 26, 1013–1032. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Cutter, D.J.; Boerma, M.; Constine, L.S.; Fajardo, L.F.; Kodama, K.; Mabuchi, K.; Marks, L.B.; Mettler, F.A.; Pierce, L.J.; et al. Radiation-related heart disease: Current knowledge and future prospects. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 656–665. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Bronnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaard, V.A.; Ta, B.D.; van der Schaaf, A.; Bouma, A.B.; Middag, A.M.; Bantema-Joppe, E.J.; van Dijk, L.V.; van Dijk-Peters, F.B.; Marteijn, L.A.; de Bock, G.H.; et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients With Breast Cancer Treated With Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J. Clin. Oncol. 2017, 35, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Citrin, D.E. Recent Developments in Radiotherapy. N. Engl. J. Med. 2017, 377, 2200–2201. [Google Scholar] [CrossRef]
- Bradley, J.A.; Dagan, R.; Ho, M.W.; Rutenberg, M.; Morris, C.G.; Li, Z.; Mendenhall, N.P. Initial Report of a Prospective Dosimetric and Clinical Feasibility Trial Demonstrates the Potential of Protons to Increase the Therapeutic Ratio in Breast Cancer Compared With Photons. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 411–421. [Google Scholar] [CrossRef]
- Chang, J.Y.; Jabbour, S.K.; De Ruysscher, D.; Schild, S.E.; Simone, C.B., 2nd; Rengan, R.; Feigenberg, S.; Khan, A.J.; Choi, N.C.; Bradley, J.D.; et al. Consensus Statement on Proton Therapy in Early-Stage and Locally Advanced Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 505–516. [Google Scholar] [CrossRef]
- Mitin, T.; Zietman, A.L. Promise and pitfalls of heavy-particle therapy. J. Clin. Oncol. 2014, 32, 2855–2863. [Google Scholar] [CrossRef]
- Lin, L.L.; Vennarini, S.; Dimofte, A.; Ravanelli, D.; Shillington, K.; Batra, S.; Tochner, Z.; Both, S.; Freedman, G. Proton beam versus photon beam dose to the heart and left anterior descending artery for left-sided breast cancer. Acta Oncol. 2015, 54, 1032–1039. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Ishizu, T.; Seo, Y.; Enomoto, Y.; Sugimori, H.; Yamamoto, M.; Machino, T.; Kawamura, R.; Aonuma, K. Experimental validation of left ventricular transmural strain gradient with echocardiographic two-dimensional speckle tracking imaging. Eur. J. Echocardiogr. 2010, 11, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Firstenberg, M.S.; Greenberg, N.L.; Garcia, M.J.; Thomas, J.D. Relationship Between Ventricular Contractility and Early Diastolic Intraventricular Pressure Gradients: A Diastolic Link to Systolic Function. J. Am. Soc. Echocardiogr. 2008, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, R.A.; Hoffmann, S.; Brainin, P.; Hagemann, C.A.; Fritz-Hansen, T.; Olsen, F.J.; Møgelvang, R.; Biering-Sørensen, T. Early diastolic strain rate by two-dimensional speckle tracking echocardiography is a predictor of coronary artery disease and cardiovascular events in stable angina pectoris. Int. J. Cardiovasc. Imaging 2020, 36, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.S.; Barros-Gomes, S.; Videbæk, L.; Poulsen, M.K.; Issa, I.F.; Carter-Storch, R.; Christensen, N.L.; Kumme, A.; Pellikka, P.A.; Møller, J.E. Early Diastolic Strain Rate in Relation to Systolic and Diastolic Function and Prognosis in Aortic Stenosis. JACC Cardiovasc. Imaging 2016, 9, 519–528. [Google Scholar] [CrossRef]
- Ersbøll, M.; Andersen, M.J.; Valeur, N.; Mogensen, U.M.; Fakhri, Y.; Thune, J.J.; Møller, J.E.; Hassager, C.; Søgaard, P.; Køber, L. Early diastolic strain rate in relation to systolic and diastolic function and prognosis in acute myocardial infarction: A two-dimensional speckle-tracking study. Eur. Heart J. 2014, 35, 648–656. [Google Scholar] [CrossRef]
- Stoodley, P.W.; Richards, D.A.; Boyd, A.; Hui, R.; Harnett, P.R.; Meikle, S.R.; Clarke, J.L.; Thomas, L. Altered left ventricular longitudinal diastolic function correlates with reduced systolic function immediately after anthracycline chemotherapy. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 228–234. [Google Scholar] [CrossRef]
- Mutter, R.W.; Giri, S.; Fruth, B.F.; Remmes, N.B.; Boughey, J.C.; Harless, C.A.; Ruddy, K.J.; McGee, L.A.; Afzal, A.; Gao, R.W.; et al. Conventional versus hypofractionated postmastectomy proton radiotherapy in the USA (MC1631): A randomised phase 2 trial. Lancet Oncol. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Paganetti, H.; Niemierko, A.; Ancukiewicz, M.; Gerweck, L.E.; Goitein, M.; Loeffler, J.S.; Suit, H.D. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 407–421. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1063–1093. [Google Scholar] [CrossRef]
- Gao, R.W.; Mullikin, T.C.; Aziz, K.A.; Afzal, A.; Smith, N.L.; Routman, D.M.; Gergelis, K.R.; Harmsen, W.S.; Remmes, N.B.; Tseung, H.; et al. Postmastectomy Intensity Modulated Proton Therapy: 5-Year Oncologic and Patient-Reported Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Mutter, R.W.; Remmes, N.B.; Kahila, M.M.; Hoeft, K.A.; Pafundi, D.H.; Zhang, Y.; Corbin, K.S.; Park, S.S.; Yan, E.S.; Lemaine, V.; et al. Initial clinical experience of postmastectomy intensity modulated proton therapy in patients with breast expanders with metallic ports. Pract. Radiat. Oncol. 2017, 7, e243–e252. [Google Scholar] [CrossRef]
- Ikaheimo, M.J.; Niemela, K.O.; Linnaluoto, M.M.; Jakobsson, M.J.; Takkunen, J.T.; Taskinen, P.J. Early cardiac changes related to radiation therapy. Am. J. Cardiol. 1985, 56, 943–946. [Google Scholar] [CrossRef]
- Erven, K.; Florian, A.; Slagmolen, P.; Sweldens, C.; Jurcut, R.; Wildiers, H.; Voigt, J.U.; Weltens, C. Subclinical cardiotoxicity detected by strain rate imaging up to 14 months after breast radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.J.; Tang, S.; Byth, K.; Stefani, L.; Lo, Q.; Otton, J.; Jameson, M.; Tran, D.; Batumalai, V.; Holloway, L.; et al. Segmental Cardiac Radiation Dose Determines Magnitude of Regional Cardiac Dysfunction. J. Am. Heart Assoc. 2021, 10, e019476. [Google Scholar] [CrossRef] [PubMed]
- Erven, K.; Jurcut, R.; Weltens, C.; Giusca, S.; Ector, J.; Wildiers, H.; Van den Bogaert, W.; Voigt, J.U. Acute radiation effects on cardiac function detected by strain rate imaging in breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Clasen, S.C.; Shou, H.; Freedman, G.; Plastaras, J.P.; Taunk, N.K.; Kevin Teo, B.K.; Smith, A.M.; Demissei, B.G.; Ky, B. Early Cardiac Effects of Contemporary Radiation Therapy in Patients With Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1301–1310. [Google Scholar] [CrossRef]
- Lo, Q.; Hee, L.; Batumalai, V.; Allman, C.; MacDonald, P.; Delaney, G.P.; Lonergan, D.; Thomas, L. Subclinical cardiac dysfunction detected by strain imaging during breast irradiation with persistent changes 6 weeks after treatment. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 268–276. [Google Scholar] [CrossRef]
- Morris, D.A.; Takeuchi, M.; Nakatani, S.; Otsuji, Y.; Belyavskiy, E.; Aravind Kumar, R.; Frydas, A.; Kropf, M.; Kraft, R.; Marquez, E.; et al. Lower limit of normality and clinical relevance of left ventricular early diastolic strain rate for the detection of left ventricular diastolic dysfunction. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 905–915. [Google Scholar] [CrossRef]
- Boda-Heggemann, J.; Knopf, A.C.; Simeonova-Chergou, A.; Wertz, H.; Stieler, F.; Jahnke, A.; Jahnke, L.; Fleckenstein, J.; Vogel, L.; Arns, A.; et al. Deep Inspiration Breath Hold-Based Radiation Therapy: A Clinical Review. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 478–492. [Google Scholar] [CrossRef]
- Remouchamps, V.M.; Vicini, F.A.; Sharpe, M.B.; Kestin, L.L.; Martinez, A.A.; Wong, J.W. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Correa, C.; Duane, F.K.; Aznar, M.C.; Anderson, S.J.; Bergh, J.; Dodwell, D.; Ewertz, M.; Gray, R.; Jagsi, R.; et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence From Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J. Clin. Oncol. 2017, 35, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Mutter, R.W.; Jethwa, K.R.; Wan Chan Tseung, H.S.; Wick, S.M.; Kahila, M.M.H.; Viehman, J.K.; Shumway, D.A.; Corbin, K.S.; Park, S.S.; Remmes, N.B.; et al. Incorporation of Biologic Response Variance Modeling Into the Clinic: Limiting Risk of Brachial Plexopathy and Other Late Effects of Breast Cancer Proton Beam Therapy. Pract. Radiat. Oncol. 2020, 10, e71–e81. [Google Scholar] [CrossRef] [PubMed]
- Underwood, T.S.A.; Grassberger, C.; Bass, R.; MacDonald, S.M.; Meyersohn, N.M.; Yeap, B.Y.; Jimenez, R.B.; Paganetti, H. Asymptomatic Late-phase Radiographic Changes Among Chest-Wall Patients Are Associated With a Proton RBE Exceeding 1.1. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 809–819. [Google Scholar] [CrossRef]
- Mutter, R.W.; Choi, J.I.; Jimenez, R.B.; Kirova, Y.M.; Fagundes, M.; Haffty, B.G.; Amos, R.A.; Bradley, J.A.; Chen, P.Y.; Ding, X.; et al. Proton Therapy for Breast Cancer: A Consensus Statement From the Particle Therapy Cooperative Group Breast Cancer Subcommittee. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 337–359. [Google Scholar] [CrossRef]
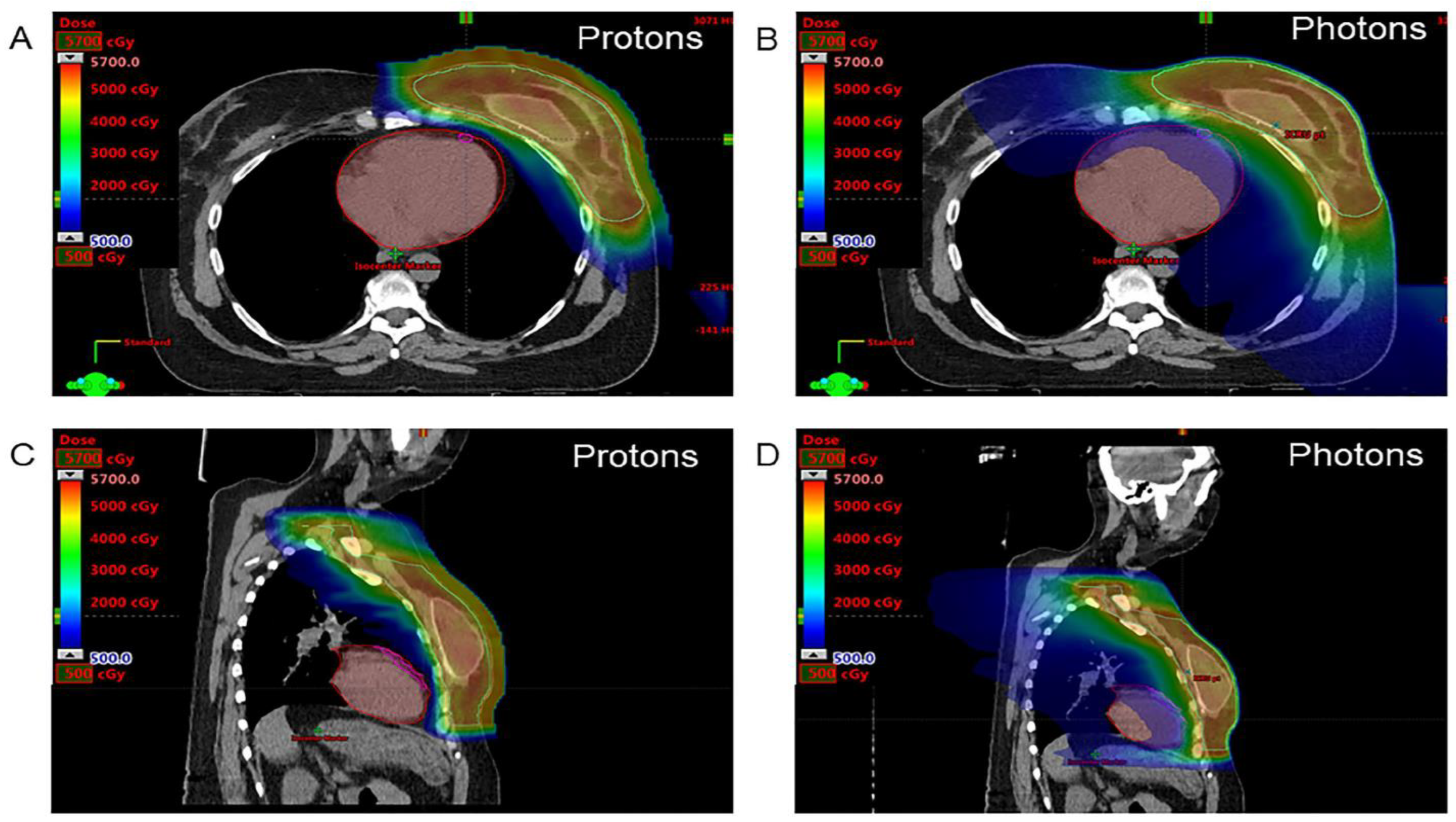
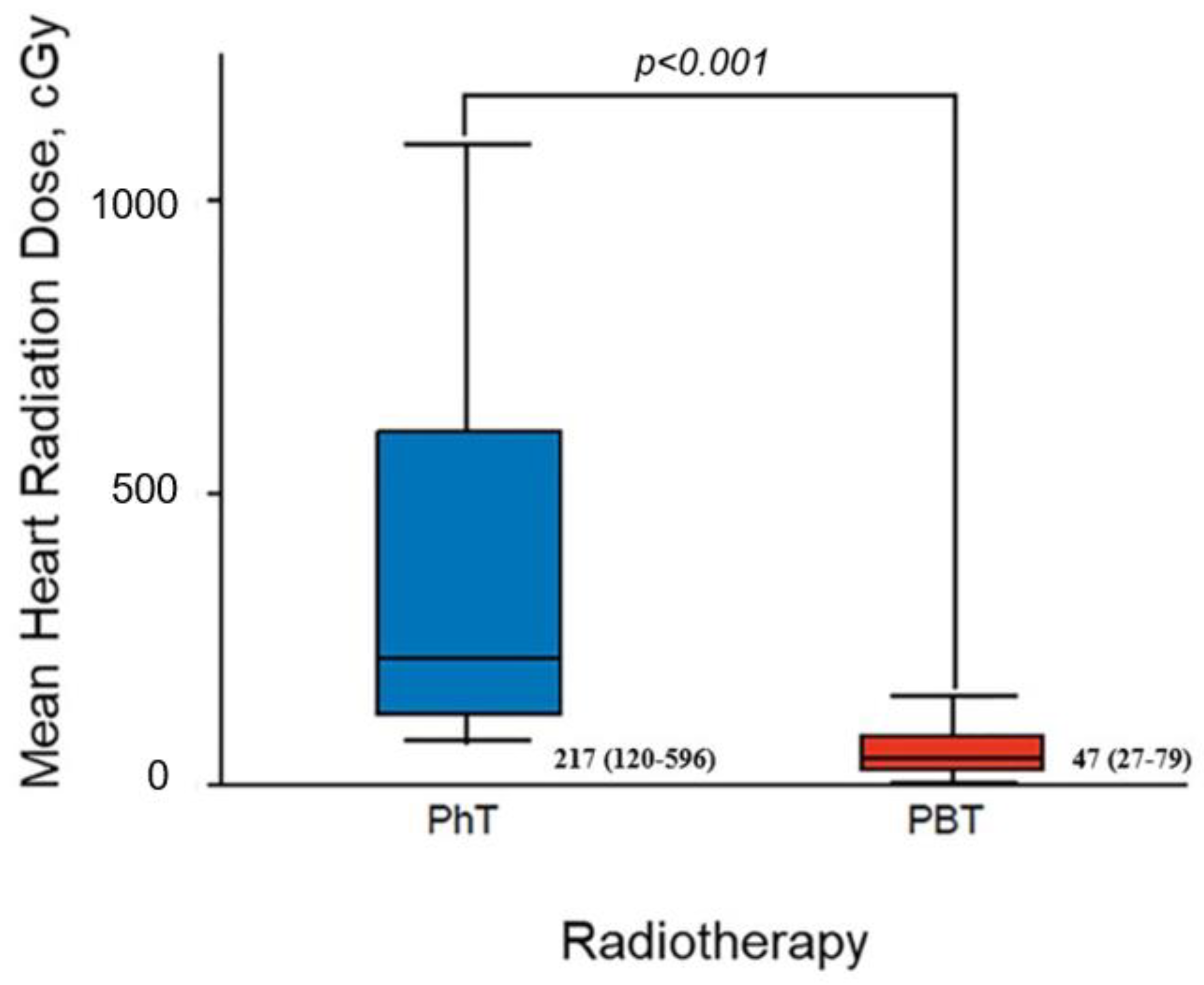
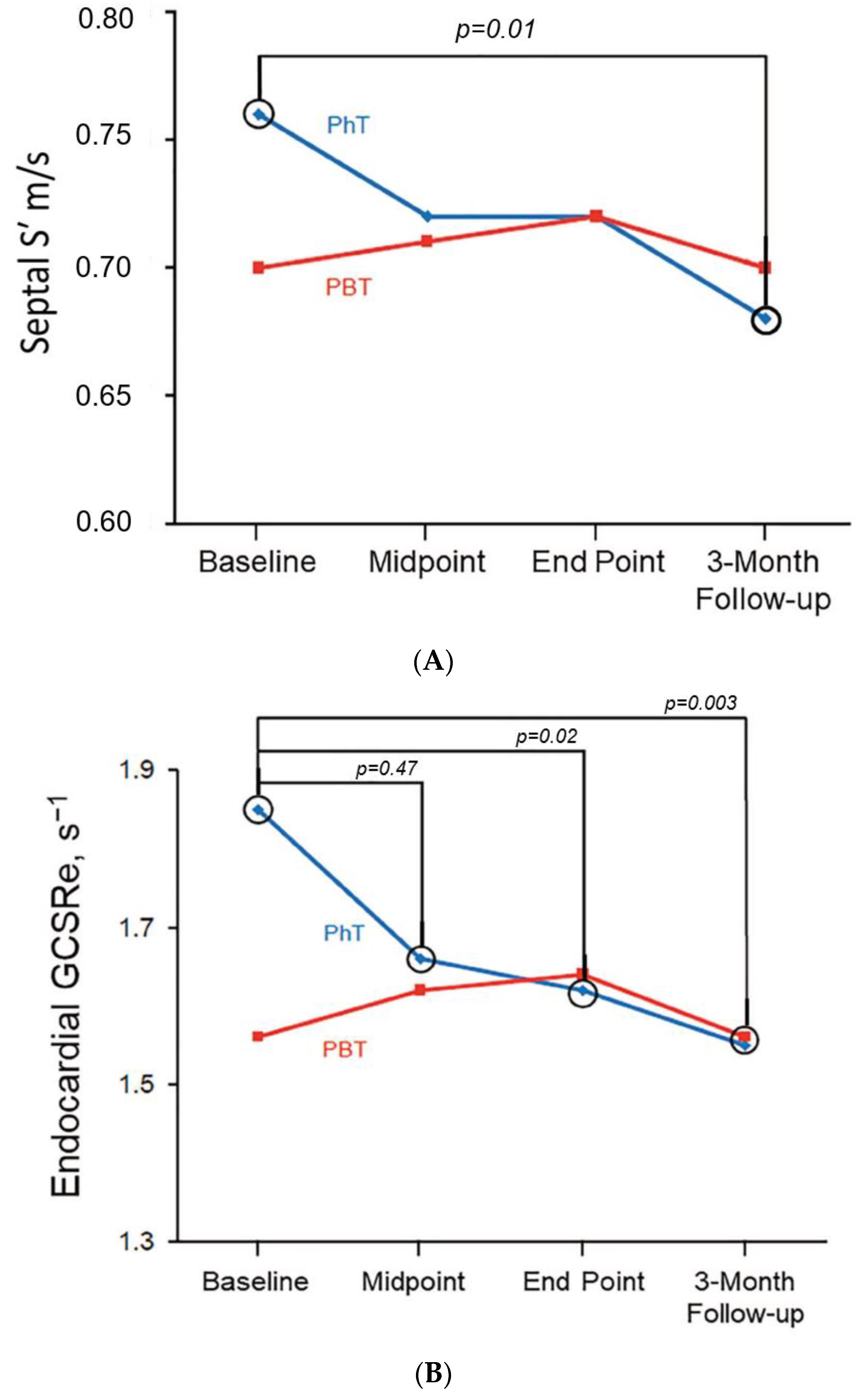
| Characteristic | Overall Cohort (n = 70) | Conventional Photon Therapy (n = 26) | Proton Beam Therapy (n = 44) | p Value |
|---|---|---|---|---|
| Age, y | 55.1 ± 11.5 | 54.7 ± 11.0 | 55.3 ± 11.9 | 0.85 |
| Women (%) | 65 (93) | 22 (85) | 43 (98) | 0.06 |
| BMI, kg/m2 | 27.9 ± 5.4 | 28.4 ± 5.5 | 27.6 ± 5.4 | 0.54 |
| BSA, m2 | 1.84 ± 0.23 | 1.88 ± 0.25 | 1.82 ± 0.22 | 0.27 |
| Active smoking (%) | 8 (11) | 5 (19) | 3 (7) | 0.24 |
| Obesity (%) | 24 (34) | 9 (35) | 15 (34) | 1.0 |
| Hypertension (%) | 11 (16) | 4 (15) | 7 (16) | 1.0 |
| Diabetes mellitus (%) | 6 (9) | 2 (8) | 4 (9) | 1.0 |
| Dyslipidemia (%) | 16 (23) | 5 (19) | 11 (25) | 0.77 |
| Breast/lung/esophageal cancer (%) | 62/6/2 (88/9/3) | 19/6/1 (73/23/4) | 43/0/1 (98/0/2) | 0.003 |
| Right/left/bilateral tumor (%) | 28/35/7 (40/50/10) | 12/13/1 (46/50/4) | 16/22/6 (36/50/14) | 0.38 |
| Previous systemic therapy (%) | 55 (79) | 20 (77) | 35 (80) | 1.0 |
| Concurrent systemic therapy (%) | 26 (37) | 11 (42) | 15 (34) | 0.61 |
| Trastuzumab/anthracyclines/taxanes (%) | 11/0/5 (16/0/7) | 4/0/3 (15/0/12) | 7/0/2 (16/0/5) | 1.0 |
| Parameter | Overall Cohort (n = 70) | PhT (n = 26) | PBT (n = 44) | p Value |
|---|---|---|---|---|
| LV mass index, g/m2 | 77.8 ± 16.5 | 76.8 ± 16.1 | 78.3 ± 17.0 | 0.71 |
| RWT | 0.41 ± 0.07 | 0.41 ± 0.07 | 0.40 ± 0.07 | 0.45 |
| LVEF, % | 61 ± 4 | 61 ± 4 | 61 ± 4 | 0.92 |
| LAVI, mL/m2 | 23.7 ± 7.1 | 23.2 ± 5.5 | 24.0 ± 7.9 | 0.62 |
| E/A | 1.08 ± 0.39 | 1.09 ± 0.46 | 1.07 ± 0.35 | 0.80 |
| Septal e′, m/s | 0.080 ± 0.023 | 0.076 ± 0.022 | 0.082 ± 0.023 | 0.23 |
| Lateral e′, m/s | 0.093 ± 0.029 | 0.089 ± 0.024 | 0.095 ± 0.031 | 0.35 |
| E/e′ | 8.38 ± 2.99 | 8.55 ± 3.04 | 8.29 ± 2.99 | 0.74 |
| Septal S′, m/s | 0.072 ± 0.014 | 0.076 ± 0.014 | 0.070 ± 0.012 | 0.11 |
| Lateral S′, m/s | 0.078 ± 0.017 | 0.077 ± 0.018 | 0.078 ± 0.017 | 0.81 |
| Transthoracic Echocardiography | ||||
|---|---|---|---|---|
| Parameter | Baseline | Midpoint | End Point | 3-Month Follow-Up |
| LVEF, % | ||||
| PhT | 61 ± 4 | 60 ± 4 | 61 ± 4 | 62 ± 3 |
| PBT | 61 ± 4 | 61 ± 4 | 61 ± 4 | 61 ± 4 |
| Septal S′, m/s | ||||
| PhT | 0.076 ± 0.014 | 0.072 ± 0.015 | 0.072 ± 0.013 | 0.068 ± 0.011 b |
| PBT | 0.070 ± 0.012 | 0.071 ± 0.017 | 0.072 ± 0.012 | 0.070 ± 0.015 |
| Lateral S′, m/s | ||||
| PhT | 0.077 ± 0.018 | 0.074 ± 0.021 | 0.080 ± 0.023 | 0.069 ± 0.016 b |
| PBT | 0.078 ± 0.017 | 0.079 ± 0.020 | 0.080 ± 0.022 | 0.085 ± 0.030 |
| LAVI, mL/m2 | ||||
| PhT | 23.2 ± 5.5 | 22.0 ± 6.1 | 22.5 ± 6.3 | 24.2 ± 6.9 |
| PBT | 24.0 ± 7.9 | 25.9 ± 7.8 | 25.1 ± 9.0 | 26.6 ± 7.2 |
| E/A | ||||
| PhT | 1.09 ± 0.46 | 1.10 ± 0.41 | 1.06 ± 0.52 | 1.10 ± 0.43 |
| PBT | 1.07 ± 0.35 | 1.10 ± 0.37 | 1.08 ± 0.38 | 1.08 ± 0.35 |
| E/e′ | ||||
| PhT | 8.40 ± 2.95 | 8.65 ± 2.25 | 8.40 ± 2.22 | 8.56 ± 2.43 |
| PBT | 8.29 ± 2.99 | 7.73 ± 2.99 | 7.76 ± 2.24 | 7.85 ± 2.41 |
| Septal e′, m/s | ||||
| PhT | 0.077 ± 0.021 | 0.069 ± 0.019 b | 0.074 ± 0.020 | 0.079 ± 0.024 |
| PBT | 0.082 ± 0.023 | 0.087 ± 0.025 | 0.088 ± 0.028 | 0.090 ± 0.032 |
| Lateral e′, m/s | ||||
| PhT | 0.089 ± 0.024 | 0.092 ± 0.031 | 0.094 ± 0.028 | 0.097 ± 0.030 |
| PBT | 0.095 ± 0.031 | 0.102 ± 0.032 | 0.100 ± 0.033 | 0.099 ± 0.034 |
| Parameter | Overall Cohort (n = 70) | PhT (n = 26) | PBT (n = 44) | p Value |
|---|---|---|---|---|
| GLS, % | −18.82 ± 2.53 | −18.03 ± 2.78 | −19.26 ± 2.28 | 0.07 |
| GCS, % | −24.19 ± 4.36 | −24.99 ± 4.58 | −23.70 ± 4.20 | 0.25 |
| GRS, % | 56.93 ± 25.42 | 55.67 ± 14.49 | 57.70 ± 30.32 | 0.71 |
| RV S, % | −20.98 ± 3.52 | −20.78 ± 2.84 | −21.09 ± 3.88 | 0.71 |
| RV free wall S, % | −22.86 ± 4.84 | −22.32 ± 4.49 | −23.16 ± 5.05 | 0.49 |
| GLSRs, s−1 | −1.09 ± 0.21 | −1.10 ± 0.27 | −1.09 ± 0.17 | 0.88 |
| GCSRs, s−1 | −1.61 ± 0.40 | −1.69 ± 0.44 | −1.55 ± 0.37 | 0.18 |
| GRSRs, s−1 | 2.70 ± 0.80 | 2.73 ± 0.75 | 2.67 ± 0.84 | 0.78 |
| GLSRe, s−1 | 1.14 ± 0.25 | 1.17 ± 0.25 | 1.12 ± 0.25 | 0.43 |
| GCSRe, s−1 | 1.67 ± 0.45 | 1.85 ± 0.39 | 1.56 ± 0.45 | 0.006 |
| GRSRe, s−1 | −2.50 ± 0.86 | −2.78 ± 0.96 | −2.33 ± 0.75 | 0.05 |
| Transthoracic Echocardiography | ||||
|---|---|---|---|---|
| Parameter | Baseline | Midpoint | End Point | 3-Month Follow-Up |
| GLS, % | ||||
| PhT | −18.03 ± 2.78 | −18.13 ± 1.99 | −17.84 ± 2.60 | −19.07 ± 2.32 |
| PBT | −19.26 ± 2.28 | −19.33 ± 2.74 | −19.41 ± 2.50 | −18.95 ± 2.25 |
| GCS, % | ||||
| PhT | −24.99 ± 4.58 | −25.08 ± 3.26 | −23.41 ± 5.14 | −24.06 ± 3.92 |
| PBT | −23.70 ± 4.20 | −23.60 ± 4.30 | −24.45 ± 3.72 | −23.83 ± 3.64 |
| GRS, % | ||||
| PhT | 55.67 ± 14.49 | 49.04 ± 16.78 | 54.67 ± 22.91 | 61.01 ± 20.38 |
| PBT | 57.70 ± 30.32 | 56.18 ± 22.79 | 61.58 ± 23.20 | 63.24 ± 17.10 |
| GLSRs, s−1 | ||||
| PhT | −1.10 ± 0.27 | −1.11 ± 0.16 | −1.09 ± 0.18 | −1.11 ± 0.17 |
| PBT | −1.09 ± 0.17 | −1.12 ± 0.19 | −1.09 ± 0.18 | −1.10 ± 0.16 |
| GCSRs, s−1 | ||||
| PhT | −1.69 ± 0.44 | −1.66 ± 0.29 | −1.56 ± 0.28 | −1.61 ± 0.34 |
| PBT | −1.55 ± 0.37 | −1.56 ± 0.33 | −1.58 ± 0.31 | −1.60 ± 0.33 |
| GRSRs, s−1 | ||||
| PhT | 2.73 ± 0.75 | 2.57 ± 0.63 | 2.57 ± 0.63 | 2.79 ± 0.79 |
| PBT | 2.67 ± 0.84 | 2.60 ± 0.83 | 2.74 ± 0.68 | 2.92 ± 0.70 |
| GLSRe, s−1 | ||||
| PhT | 1.17 ± 0.25 | 1.07 ± 0.22 b | 1.06 ± 0.26 b | 1.10 ± 0.23 |
| PBT | 1.12 ± 0.25 | 1.06 ± 0.29 | 1.10 ± 0.27 | 1.13 ± 0.24 |
| GCSRe, s−1 | ||||
| PhT | 1.85 ± 0.39 | 1.66 ± 0.29 b | 1.62 ± 0.31 b | 1.55 ± 0.36 b |
| PBT | 1.56 ± 0.45 | 1.62 ± 0.57 | 1.64 ± 0.44 | 1.56 ± 0.29 |
| GRSRe, s−1 | ||||
| PhT | −2.78 ± 0.96 | −2.35 ± 0.74 b | −2.68 ± 0.90 | −2.23 ± 0.86 b |
| PBT | −2.33 ± 0.75 | −2.28 ± 0.94 | −2.38 ± 0.73 | −2.48 ± 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi, M.A.; Bruno, G.; Di Stefano, C.; Garcia Bello, L.; Laack, N.N.; Corbin, K.S.; Whitaker, T.J.; Pellikka, P.A.; Mutter, R.W.; Villarraga, H.R. Detection of Early Myocardial Dysfunction by Imaging Biomarkers in Cancer Patients Undergoing Photon Beam vs. Proton Beam Radiotherapy: A Prospective Study. J. Cardiovasc. Dev. Dis. 2023, 10, 418. https://doi.org/10.3390/jcdd10100418
Abbasi MA, Bruno G, Di Stefano C, Garcia Bello L, Laack NN, Corbin KS, Whitaker TJ, Pellikka PA, Mutter RW, Villarraga HR. Detection of Early Myocardial Dysfunction by Imaging Biomarkers in Cancer Patients Undergoing Photon Beam vs. Proton Beam Radiotherapy: A Prospective Study. Journal of Cardiovascular Development and Disease. 2023; 10(10):418. https://doi.org/10.3390/jcdd10100418
Chicago/Turabian StyleAbbasi, Muhannad Aboud, Giulia Bruno, Cristina Di Stefano, Laura Garcia Bello, Nadia N. Laack, Kimberly S. Corbin, Thomas J. Whitaker, Patricia A. Pellikka, Robert W. Mutter, and Hector R. Villarraga. 2023. "Detection of Early Myocardial Dysfunction by Imaging Biomarkers in Cancer Patients Undergoing Photon Beam vs. Proton Beam Radiotherapy: A Prospective Study" Journal of Cardiovascular Development and Disease 10, no. 10: 418. https://doi.org/10.3390/jcdd10100418





