Transcatheter Aortic Valve Implantation: Addressing the Subsequent Risk of Permanent Pacemaker Implantation
Abstract
1. Introduction
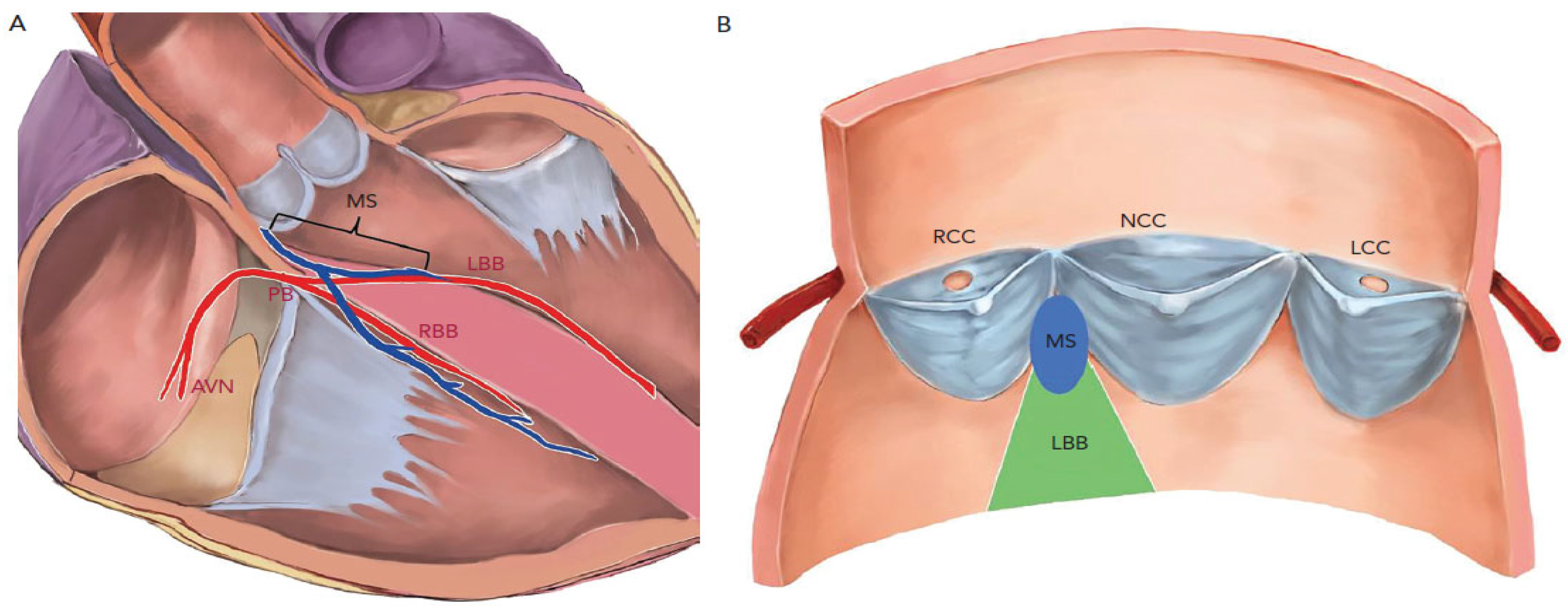
2. Cardiac Anatomy Lends Itself to Post-TAVI Conduction Blocks
3. Most Common Conduction Blocks Post-TAVI and Subsequent Need for PPI
3.1. Left Bundle Branch Block (LBBB)
3.2. Atrioventricular Block (AVB)
4. Possible Pre-Procedural Predictors for PPI
4.1. Demographic Predictors
4.1.1. Male Sex
4.1.2. Age
4.1.3. Other Factors?—Body Mass Index (BMI), Serum Creatinine
4.2. EKG and CT Predictors
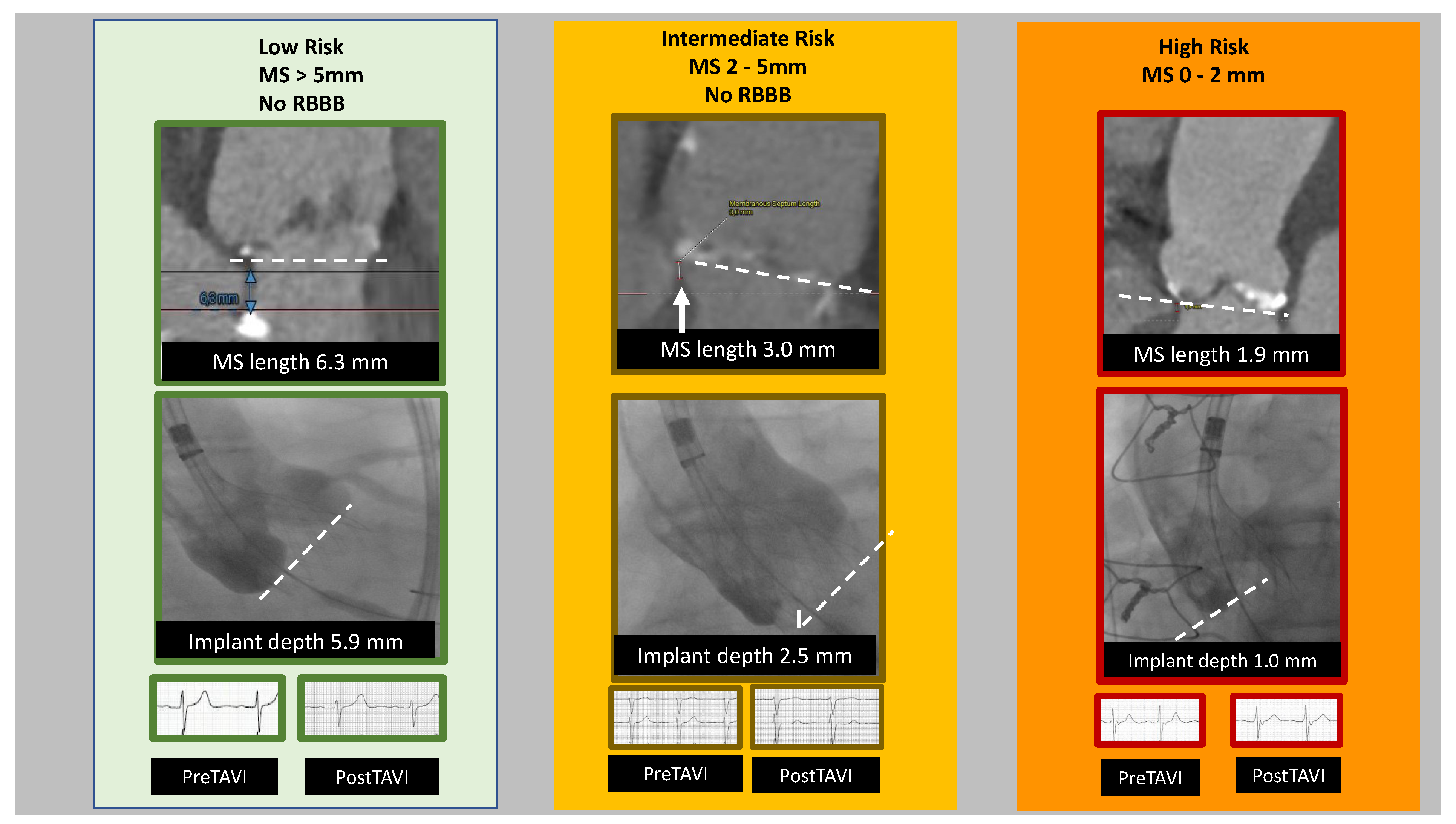
4.2.1. Short Membranous Septum
4.2.2. Distribution of Calcification and LVOT/Annulus Size and Shape
4.3. Pre-Intervention EKG Predictors
5. Procedural/Peri-Procedural Predictors
- Valve choice
- Implantation depth
- Post-dilatation
- EKG changes
6. Risk Score
7. Pre-Procedural Planning and Procedural Techniques to Optimize TAVI and Limit PPI
7.1. Valve Sizing
7.2. Coronary Ischemia and PPI
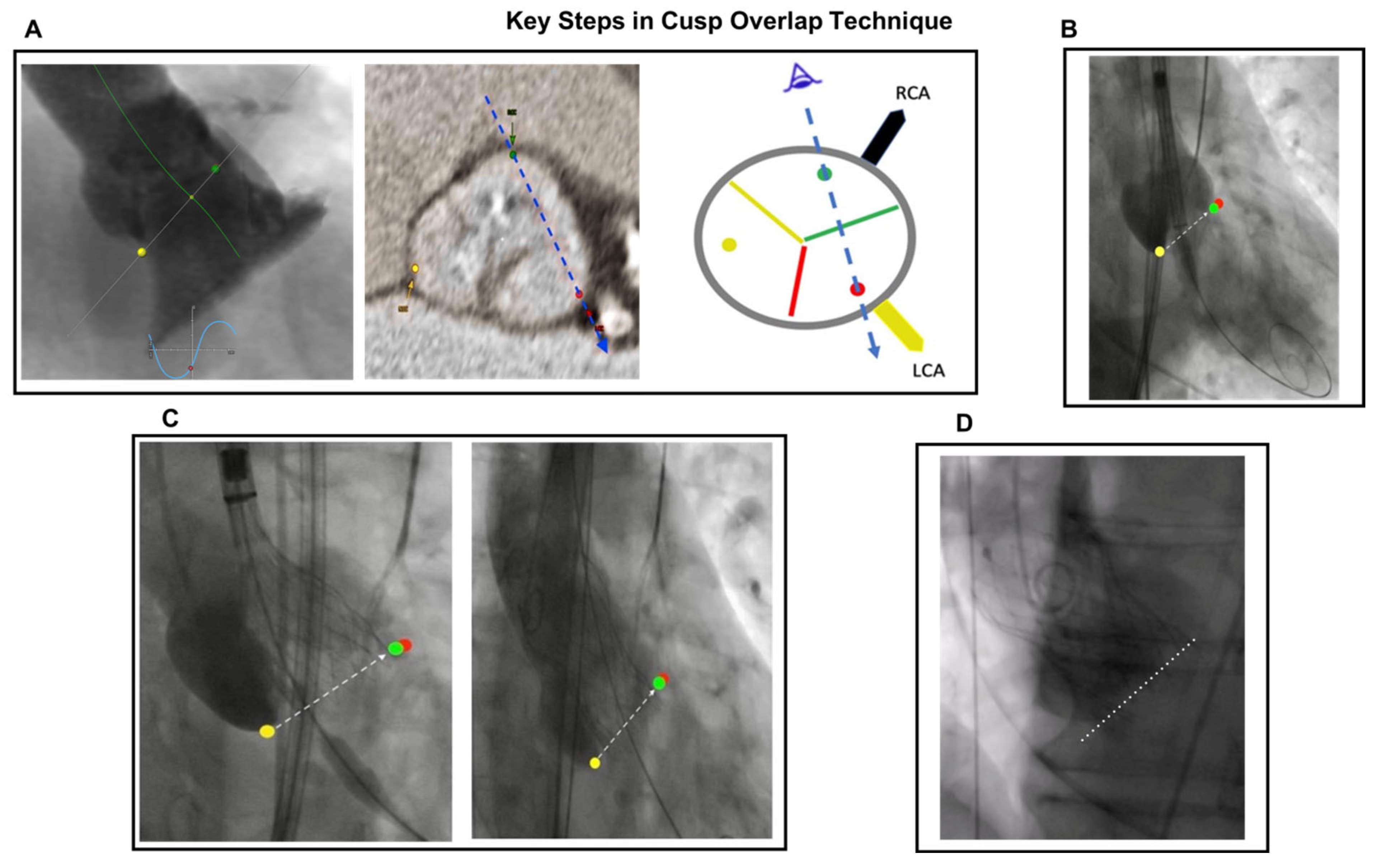
7.3. Implantation Height and MDCT Analysis, MIDAS Approach, Cusp-Overlap Technique, C-Arm Angulation
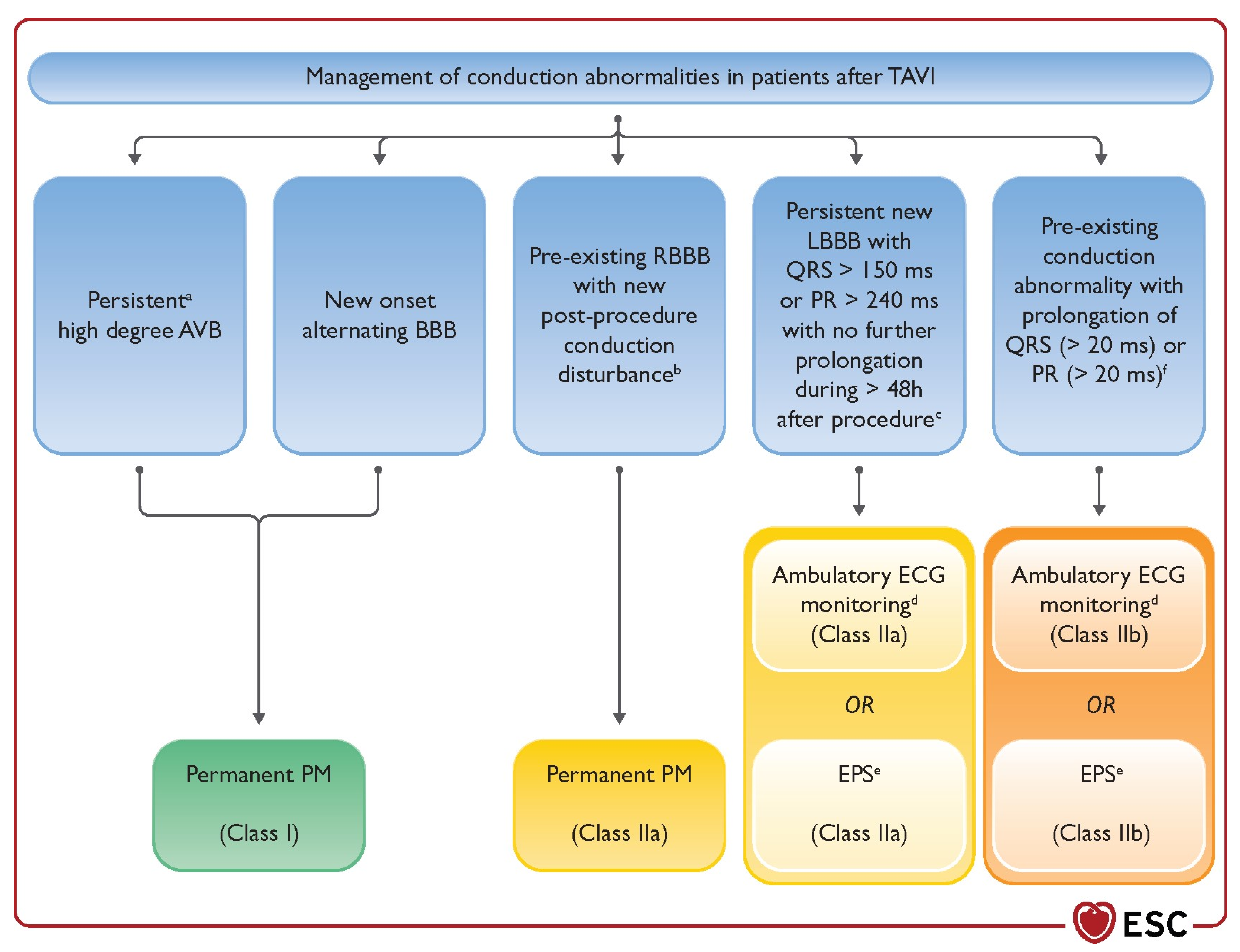
8. Post-Procedural Monitoring
9. Discussion
10. Conclusions
11. Future Directions
11.1. Lipoprotein (a) [Lp(a)] and Aortic Valve Stenosis
11.2. Risk Score for PPI after TAVI
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- De Torres-Alba, F.; Kaleschke, G.; Diller, G.P.; Vormbrock, J.; Orwat, S.; Radke, R.; Reinke, F.; Fischer, D.; Reinecke, H.; Baumgartner, H. Changes in the Pacemaker Rate after Transition from Edwards SAPIEN XT to SAPIEN 3 Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2016, 9, 805–813. [Google Scholar] [CrossRef]
- Van der Boon, R.M.A.; Houthuizen, P.; Urena, P.; Poels, T.T.; van Miegham, N.M.; Brueren, G.R.G.; Altintas, S.; Nuis, R.J.; Serruys, P.W.; van Garsse, L.A.F.M.; et al. Trends in occurrence of new conduction abnormalities after transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2015, 85, E144–E152. [Google Scholar] [CrossRef] [PubMed]
- Husser, O.; Pellegrini, C.; Kessler, T.; Burgdorf, C.; Thaller, H.; Mayr, N.P.; Kasel, A.M.; Kastrati, A.; Schunkert, H.; Hengstenberg, C. Predictors of Permanent Pacemaker Implantations and New-Onset Conduction Abnormalities with the SAPIEN3 Balloon-Expandable Transcatheter Heart Valve. JACC Cardiovasc. Interv. 2016, 9, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Lilly, S.M.; Deshmukh, A.J.; Epstein, A.E.; Ricciardi, M.J.; Shreenivas, S.; Velagapudi, P.; Wyman, J.F. 2020 ACC expert consensus decision pathway on management of conduction disturbances in patients undergoing transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2020, 76, 2391–2411. [Google Scholar] [CrossRef] [PubMed]
- Piazza, N.; de Jaegere, P.; Schultz, C.; Becker, A.E.; Serruys, P.W.; Anderson, R.H. Anatomy of the Aortic Valvar Complex and Its Implications for Transcatheter Implantation of the Aortic Valve. Circ. Cardiovasc. Interv. 2008, 1, 74–81. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. PARTNER 3 Investigators. Transcather aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Evolut Low Risk Trial Investigators Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Madhavan, M.V.; Chau, K.H.; Russo, M.; et al. PARTNER 3 Investigators, Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J. Am. Coll. Cardiol. 2021, 77, 1149–1161. [Google Scholar] [CrossRef]
- Elder, D.H.J.; Lang, C.C.; Choy, A. M Pacing-induced heart disease: Understanding the pathophysiology and improving outcomes. Expert Rev. Cardiovasc. Ther. 2011, 9, 877–886. [Google Scholar] [CrossRef]
- Akerström, F.; Arais, M.A.; Pachón, M.; Jiménez-López, J.; Puchol, A.; Juliá-Calvo, J. The importance of avoiding unnecessary right ventricular pacing in clinical practice. World J. Cardiol. 2013, 5, 410–419. [Google Scholar] [CrossRef]
- Curtis, A.B.; Worley, S.J.; Adamson, P.B.; Chung, E.S.; Niazi, I.; Sherfesee, L.; Shinn, T.; Sutton, M.S.J. Biventricular Pacing for Atrioventricular Block and Systolic Dysfunction. N. Engl. J. Med. 2013, 368, 1585–1593. [Google Scholar] [CrossRef]
- Rück, A.; Saleh, N.; Glaser, N. Outcomes Following Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. Interv. 2021, 14, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Bisson, A.; Bodin, A.; Herbert, J.; Lacour, T.; Saint Etienne, C.; Pierre, B.; Clementy, N.; Deharo, P.; Babuty, D.; Fauchier, L. Pacemaker Implantation after Balloon- or Self-Expandable Transcatheter Aortic Valve Replacement in Patients with Aortic Stenosis. J. Am. Heart Assoc. 2020, 9, e015896. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Mansour, M. Pacemaker Implantation after Transcatheter Aortic Valve Replacement: A Necessary Evil Perhaps but Are We Making Progress? J. Am. Heart Assoc. 2020, 9, e016700. [Google Scholar] [CrossRef] [PubMed]
- Nazif, T.M.; Dizon, J.M.; Hahn, R.T.; Xu, K.; Babaliaros, V.; Douglas, P.S.; El-Chami, M.F.; Herrmann, H.; Mack, M.; Makkar, R.R.; et al. Predictors and Clinical Outcomes of Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement The PARTNER (Placement of AoRtic TraNscathetER Valves) Trial and Registry. J. Am. Coll. Cardiol. Interv. 2015, 8, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Poels, T.T.; Engels, E.B.; Kats, S.; Veenstra, L.; van Ommen, V.; Vernooy, K.; Maessen, J.G.; Prinzen, F.W. Occurrence and Persistency of Conduction Disturbances during Transcatheter Aortic Valve Implantation. Medicina 2021, 57, 695. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, M.; Baan, J.; Spence, M.S.; Iacovelli, F.; Martinelli, G.L.; Saia, F.; Bortone, A.S.; Van der Kley, F.; Muir, D.F.; Densem, C.G.; et al. Feasibility and safety of early discharge after transfemoral transcatheter aortic valve implantation–rationale and design of the FAST-TAVI registry. BMC Cardiovasc. Disord. 2017, 17, 259. [Google Scholar] [CrossRef]
- Tian, Y.; Padmanabhan, D.; McLeod, C.J.; Zhang, P.; Xiao, P.; Sandhu, G.S.; Greason, K.L.; Gulati, R.; Nkomo, V.; Rihal, C.S.; et al. Utility of 30-Day Continuous Ambulatory Monitoring to Identify Patients with Delayed Occurrence of Atrioventricular Block after Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2019, 12, e007635. [Google Scholar] [CrossRef]
- Bagur, R.; Rodés-Cabau, J.; Gurvitch, R.; Dumont, É.; Velianou, J.L.; Manazzoni, J.; Toggweiler, S.; Cheung, A.; Ye, J.; Natarajan, M.K.; et al. Need for permanent pacemaker as a complication of transcatheter aortic valve implantation and surgical aortic valve replacement in elderly patients with severe aortic stenosis and similar baseline electrocardiographic findings. JACC Interv. 2012, 5, 540–551. [Google Scholar] [CrossRef]
- Roten, L.; Stortecky, S.; Scarcia, F.; Kadner, A.; Tanner, H.; Delacrétaz, E.; Meier, B.; Windecker, S.; Carrel, T.; Wenaweser, P. Atrioventricular Conduction after Transcatheter Aortic Valve Implantation and Surgical Aortic Valve Replacement. J. Cardiovasc. Electrophysiol. 2012, 23, 1115–1122. [Google Scholar] [CrossRef]
- Kanjanauthai, S.; Bhasin, K.; Pirelli, L.; Kliger, C. Conduction Abnormalities after Transcatheter Aortic Valve Replacement. US Cardiol. Rev. 2019, 13, 21–29. [Google Scholar] [CrossRef]
- Mangieri, A.; Montalto, C.; Pagnesi, M.; Lanzillo, G.; Demir, O.; Testa, L.; Colombo, A.; Latib, A. TAVI and Post Procedural Cardiac Conduction Abnormalities. Front. Cardiovasc. Med. 2018, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Sato, F. Visualizing anatomical evidences on atrioventricular conduction system for TAVI. Int. J. Cardiol. 2014, 174, 1–6. [Google Scholar] [CrossRef]
- Tretter, J.T.; Mori, S.; Anderson, R.H.; Taylor, M.D.; Ollberding, N.; Truong, V.; Choo, J.; Kereiakes, D.; Mazur, W. Anatomical predictors of conduction damage after transcatheter implantation of the aortic valve. Open Heart 2019, 6, e000972. [Google Scholar] [CrossRef]
- Lin, S.-I.; Miura, M.; Tagliari, A.P.; Lee, Y.-H.; Shirai, S.; Puri, R.; Maisano, F.; Taramasso, M. Intraventricular Conduction Disturbances After Transcatheter Aortic Valve Implantation. Interv. Cardiol. Rev. 2020, 15, e11. [Google Scholar] [CrossRef]
- Massoullié, G.; Bordachar, P.; Ellenbogen, K.A.; Souteyrand, G.; Jean, F.; Combaret, N.; Vorilhon, C.; Clerfond, G.; Farhat, M.; Ritter, P.; et al. New-onset left bundle branch block induced by transcutaneous aortic valve implantation. Am. J. Cardiol. 2016, 117, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Hamandi, M.; Tabachnick, D.; Lanfear, A.T.; Baxter, R.; Shin, K.; Zingler, B.; Mack, M.J.; DiMaio, J.M.; Kindsvater, S. Effect of new and persistent left bundle branch block after transcatheter aortic valve replacement on long-term need for pacemaker implantation. Proc. Baylor.Univ. Med. Cent. 2020, 33, 157–162. [Google Scholar] [CrossRef]
- Jørgensen, T.H.; De Backer, O.; Gerds, T.A.; Bieliauskas, G.; Svendsen, J.H.; Søndergaard, L.J. Immediate Post-Procedural 12-Lead Electrocardiography as Predictor of Late Conduction Defects after Transcatheter Aortic Valve Replacement. Am. Coll. Cardiol. Interv. 2018, 11, 1509–1518. [Google Scholar] [CrossRef]
- Houthuizen, P.; van der Boon, R.M.A.; Urena, M.; Van Mieghem, N.; Brueren, G.B.R.; Poels, T.T.; Van Garsse, L.A.F.M.; Rodés-Cabau, J.; Prinzen, F.W.; de Jaegere, P. Occurrence, fate and consequences of ventricular conduction abnormalities after transcatheter aortic valve implantation. EuroIntervention 2014, 9, 1142–1150. [Google Scholar] [CrossRef]
- Urena, M.; Mok, M.; Serra, V.; Dumont, E.; Nombela-Franco, L.; DeLarochellière, R.; Doyle, D.; Igual, A.; Larose, E.; Amat-Santos, I.; et al. Predictive Factors and Long-Term Clinical Consequences of Persistent Left Bundle Branch Block Following Transcatheter Aortic Valve Implantation with a Balloon-Expandable Valve. J. Am. Coll. Cardiol. 2012, 60, 1743–1752. [Google Scholar] [CrossRef]
- Nazif, T.M.; Chen, S.; George, I.; Dizon, J.M.; Hahn, R.T.; Crowley, A.; Alu, M.C.; Babaliaros, V.; Thourani, V.H.; Herrmann, H.C.; et al. New-onset left bundle branch block after transcatheter aortic valve replacement is associated with adverse long-term clinical outcomes in intermediate-risk patients: An analysis from the PARTNER II trial. Eur. Heart J. 2019, 40, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Urena, M.; Nombela-Franco, L.; Amat-Santos, I.; Kleiman, N.; Munoz-Garcia, A.; Atienza, F.; Serra, V.; Deyell, M.W.; Veiga-Fernandez, G.; et al. Arrhythmic Burden as Determined by Ambulatory Continuous Cardiac Monitoring in Patients with New-Onset Persistent Left Bundle Branch Block following Transcatheter Aortic Valve Replacement the MARE Study. J. Am. Coll. Cardiol. Interv. 2018, 11, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chau, K.T.; Nazif, T.M. The incidence and impact of cardiac conduction disturbances after transcatheter aortic valve replacement. Ann. Cardiothorac. Surg. 2020, 9, 452–467. [Google Scholar] [CrossRef]
- Kostopoulou, A.; Karyofillis, P.; Livanis, E.; Thomopoulou, S.; Stefopoulos, C.; Doudoumis, K.; Theodorakis, G.; Voudris, V. Permanent pacing after transcatheter aortic valve implantation of a CoreValve prosthesis as determined by electrocardiographic and electrophysiological predictors: A single-centre experience. Europace 2016, 18, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Siontis, G.C.M.; Jüni, P.; Pilgrim, T.; Stortecky, S.; Büllesfeld, L.; Meier, B.; Wenaweser, P.; Windecker, S. Predictors of Permanent Pacemaker Implantation in Patients with Severe Aortic Stenosis Undergoing TAVR: A Meta-Analysis. J. Am. Coll. Cardiol. 2014, 64, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Mazzella, A.J.; Sanders, M.; Yang, H.; Li, Q.; Vavalle, J.P.; Gehi, A. Predicting need for pacemaker implantation early and late after transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2020, 97, E588–E596. [Google Scholar] [CrossRef]
- Mazzella, A.J.; Arora, S.; Hendrickson, M.J.; Sanders, M.; Vavalle, J.P.; Gehi, A.K. Evaluation and Management of Heart Block after Transcatheter Aortic Valve Replacement. Card. Fail. Rev. 2021, 7, e12. [Google Scholar] [CrossRef]
- Urena, M.; Webb, J.G.; Tamburino, C.; Muñoz-García, A.J.; Cheema, A.; Dager, A.E.; Serra, V.; Amat-Santos, I.J.; Barbanti, M.; Immè, S.; et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: Impact on late clinical outcomes and left ventricular function. Circulation 2014, 129, 1233–1243. [Google Scholar] [CrossRef]
- Mazzella, A.J.; Hendrickson, M.J.; Arora, S.; Sanders, M.; Li, Q.; Vavalle, J.P.; Gehi, A.K. Shifting Trends in Timing of Pacemaker Implantation after Transcatheter Aortic Valve Replacement. JACC CV Interv. 2021, 14, 232–233. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Ellenbogen, K.A.; Krahn, A.D.; Latib, A.; Mack, M.; Mittal, S.; Muntané-Carol, G.; Nazif, T.M.; Sondergaard, L.; Urena, M.; et al. Management of Conduction Disturbances Associated with Transcatheter Aortic Valve Replacement JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 74, 1086–1106. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.S.; Redwood, S.R.; Allen, C.J.; Hurrell, H.; Chehab, O.; Rajani, R.; Prendergast, B.; Patterson, T. A 20-year journey in transcatheter aortic valve implantation: Evolution to current eminence. Front. Cardiovasc. Med. 2022, 9, 971762. [Google Scholar] [CrossRef] [PubMed]
- Toggweiler, S.; Stortecky, S.; Holy, E.; Zuk, K.; Cuculi, F.; Nietlispach, F.; Sabti, Z.; Suciu, R.; Maier, W.; Jamshidi, P.; et al. The Electrocardiogram after Transcatheter Aortic Valve Replacement Determines the Risk for Post-Procedural High-Degree AV Block and the Need for Telemetry Monitoring. J. Am. Coll. Cardiol. Interv. 2016, 9, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Zahid, S.; Zaidi, S.R.; Sarvepalli, D.; Haq, S.; Rommi, S.; Mukhtar, M.; Khan, M.A.; Gowda, S.N.; Ruggiero, N.; et al. Predictors of Permanent Pacemaker Implantation in Patients Undergoing Transcatheter Aortic Valve Replacement–A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e020906. [Google Scholar] [CrossRef] [PubMed]
- Elbaz-Greener, G.; Rahamim, E.; Ghosh, Z.A.; Carasso, S.; Yarkoni, M.; Radhakrishnan, S.; Wijeysundera, H.C.; Igor, T.; Planer, D.; Rozen, G.; et al. Sex difference and outcome trends following transcatheter aortic valve replacement. Front. Cardiovasc. Med. 2022, 9, 101373. [Google Scholar] [CrossRef]
- Ravaux, J.M.; Van Kuijk, S.M.J.; Di Mauro, M.; Vernooy, K.; Bidar, E.; Van’t Hof, A.W.; Veenstra, L.; Kats, S.; Houterman, S.; Maessen, J.G.; et al. Incidence and Predictors of Permanent Pacemaker Implantation after Transcatheter Aortic Valve Procedures: Data of The Netherlands Heart Registration (NHR). J. Clin. Med. 2022, 11, 560. [Google Scholar] [CrossRef]
- Attinger-Toller, A.; Ferrari, E.; Tueller, D.; Templin, C.; Muller, O.; Nietlispach, F.; Toggweiler, S.; Noble, S.; Roffi, M.; Jeger, R.; et al. Age-Related Outcomes after Transcatheter Aortic Valve Replacement Insights From the Swiss TAVI Registry. J. Am. Coll. Cardiol. Interv. 2021, 14, 952–960. [Google Scholar] [CrossRef]
- Giustino, G.; Van der Boon, R.M.A.; Molina-Martin de Nicolas, J.; Dumonteil, N.; Chieffo, A.; de Jaegere, P.P.T.; Tchetche, D.; Marcheix, B.; Millischer, D.; Cassagneau, R.; et al. Impact of permanent pacemaker on mortality after transcatheter aortic valve implantation: The PRAGMATIC (Pooled Rotterdam-Milan-Toulouse in Collaboration) Pacemaker substudy. EuroIntervention 2016, 12, 1185–1193. [Google Scholar] [CrossRef]
- Gama, F.; de Araújo Gonçalves, P.; Abecasis, J.; Ferreira, A.M.; Freitas, P.; Gonçalves, M.; Carvalho, S.; Oliveira, A.F.; Gabriel, H.M.; Brito, J.; et al. Predictors of pacemaker implantation after TAVI in a registry including self, balloon and mechanical expandable valves. Int. J. Cardiovasc. Imaging 2022, 38, 225–235. [Google Scholar] [CrossRef]
- Maeno, Y.; Abramowitz, Y.; Kawamori, H.; Kazuno, Y.; Kubo, S.; Takahashi, N.; Mangat, G.; Okuyama, K.; Kashif, M.; Chakravarty, T.; et al. A Highly Predictive Risk Model for Pacemaker Implantation after TAVR. J. Am. Coll. Cardiol. Imaging 2017, 10, 1139–1147. [Google Scholar] [CrossRef]
- Fujita, B.; Kütting, M.; Seiffert, M.; Scholtz, S.; Egron, S.; Prashovikj, E.; Börgermann, J.; Schäfer, T.; Scholtz, W.; Preuss, R.; et al. Calcium distribution patterns of the aortic valve as a risk factor for the need of permanent pacemaker implantation after transcatheter aortic valve implantation. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 1385–1393. [Google Scholar] [CrossRef]
- Mauri, V.; Reimann, A.; Stern, D.; Scherner, M.; Kuhn, E.; Rudolph, V.; Rosenkranz, S.; Eghbalzadeh, K.; Friedrichs, K.; Wahlers, T.; et al. Predictors of Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement with the SAPIEN 3. J. Am. Coll. Cardiol. Interv. 2016, 9, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Farhan, S.; Stachel, G.; Desch, S.; Kurz, T.; Feistritzer, H.-J.; Hartung, P.; Eitel, I.; Nef, H.; Doerr, O.; Lauten, A.; et al. Impact of moderate or severe left ventricular outflow tract calcification on clinical outcomes of patients with severe aortic stenosis undergoing transcatheter aortic valve implantation with self- and balloon-expandable valves: A post hoc analysis from the SOLVE-TAVI trial. EuroIntervention 2022, 18, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Veulemans, V.; Frank, D.; Seoudy, H.; Wundram, S.; Piayda, K.; Maier, O.; Jung, C.; Polzin, A.; Frey, N.; Kelm, M.; et al. New insights on potential permanent pacemaker predictors in TAVR using the largest self-expandable device. Cardiovasc. Diagn. Ther. 2020, 10, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; D’Ascenzo, F.; Vaira, M.P.; Elia, E.; Omedè, P.; Kodali, S.; Barbanti, M.; Rodés-Cabau, J.; Husser, O.; Sossalla, S.; et al. Predictors of pacemaker implantation after transcatheter aortic valve implantation according to kind of prosthesis and risk profile: A systematic review and contemporary meta-analysis. Eur. Heart J. 2021, 7, 143–153. [Google Scholar] [CrossRef]
- El-Sabawi, B.; Welle, G.A.; Cha, Y.-M.; Espinosa, R.E.; Gulati, R.; Sandhu, G.S.; Greason, K.L.; Crestanello, J.A.; Friedman, P.A.; Munger, T.M.; et al. Temporal Incidence and Predictors of High-Grade Atrioventricular Block after Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2021, 10, e020033. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/boston-scientific-announces-lotus-edgetm-aortic-valve-system-voluntary-recall-and-product#recall-announcement (accessed on 17 May 2023).
- Solomonica, A.; Choudhury, T.; Bagur, R. The mechanically expandable LOTUS Valve and LOTUS Edge transcatheter aortic valve systems. Expert Rev. Med. Devices 2018, 15, 763–769. [Google Scholar] [CrossRef]
- Medranda, G.A.; Rogers, T.; Case, B.C.; Shults, C.C.; Cohen, J.E.; Satler, L.F.; Ben-Dor, I. Waksman R Single-Center Experience with the LOTUS Edge Transcatheter Heart Valve. Cardiovasc. Revascularization Med. 2021, 29, 85–88. [Google Scholar] [CrossRef]
- Kim, W.-K.; Hengstenberg, C.; Hilker, M.; Kerber, S.; Schäfer, U.; Rudolph, T.; Linke, A.; Franz, N.; Kuntze, T.; Nef, H.; et al. The SAVI-TF Registry 1-Year Outcomes of the European Post-Market Registry Using the ACURATE neo Transcatheter Heart Valve Under Real-World Conditions in 1000 Patients. Am. Coll. Cardiol. Interv. 2018, 11, 1368–1374. [Google Scholar] [CrossRef]
- Pellegrini, C.; Garot, P.; Morice, M.-C.; Tamburino, C.; Bleiziffer, S.; Thiele, H.; Scholtz, S.; Schramm, R.; Cockburn, J.; Cunnington, M.; et al. Permanent pacemaker implantation and left bundle branch block with self-expanding valves—A SCOPE 2 subanalysis. EuroIntervention 2023, 18, e1077–e1087. [Google Scholar] [CrossRef]
- Hamdan, A.; Guetta, V.; Klempfner, R.; Konen, E.; Raanani, E.; Glikson, M.; Goitein, O.; Segev, A.; Barbash, I.; Fefer, P.; et al. Inverse Relationship Between Membranous Septal Length and the Risk of Atrioventricular Block in Patients Undergoing Transcatheter Aortic Valve Implantation. J. Am. Coll. Cardiol. Interv. 2015, 8, 1218–1228. [Google Scholar] [CrossRef]
- Jilaihawi, H.; Zhao, Z.; Du, R.; Staniloae, C.; Saric, M.; Neuburger, P.J.; Querijero, M.; Vainrib, A.; Hisamoto, K.; Ibrahim, H.; et al. Jilaihawi Minimizing Permanent Pacemaker Following Repositionable Self-Expanding Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. Interv. 2019, 12, 1796–1807. [Google Scholar] [CrossRef]
- Faroux, L.; Chen, S.; Muntané-Carol, G.; Regueiro, A.; Philippon, F.; Sondergaard, L.; Jørgensen, T.H.; Lopez-Aguilera, J.; Kodali, S.; Leon, M.; et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: A systematic review and meta-analysis. Eur. Heart J. 2020, 41, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Saadi, R.P.; Tagliari, A.P.; Saadi, E.K.; Miglioranza, M.H.; Polanczyck, C.A. Preoperative TAVR Planning: How to Do It. J. Clin Med. 2022, 11, 2582. [Google Scholar] [CrossRef] [PubMed]
- Blanke, P.; Euringer, W.; Baumann, T.; Reinöhl, J.; Schlensak, C.; Langer, M.; Pache, G. Combined Assessment of Aortic Root Anatomy and Aortoiliac Vasculature with Dual-Source CT as a Screening Tool in Patients Evaluated for Transcatheter Aortic Valve Implantation. Am. J. Roentgenol. 2010, 195, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Ludwig, S.; Kalbacher, D.; Spink, C.; Linder, M.; Bhandra, O.D.; Nikorowitsch, J.; Waldschmidt, L.; Demal, T.; Voigtländer, L.; et al. Prevention of coronary obstruction in patients at risk undergoing transcatheter aortic valve implantation: The Hamburg BASILICA experience. Clin. Res. Cardiol. 2021, 110, 1900–1911. [Google Scholar] [CrossRef]
- Arai, T.; Lefèvre, T.; Hovasse, T.; Garot, P.; Benamer, H.; Unterseeh, T.; Roy, A.K.; Romano, M.; Hayashida, K.; Watanabe, Y.; et al. Incidence and predictors of coronary obstruction following transcatheter aortic valve implantation in the real world. Catheter. Cardiovasc. Interv. 2017, 90, 1192–1197. [Google Scholar] [CrossRef]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Nørgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR) An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc. Imaging 2019, 12, 1–24. [Google Scholar] [CrossRef]
- Xenofontos, P.; Zamani, R.; Akrami, M. The application of 3D printing in preoperative planning for transcatheter aortic valve replacement: A systematic review. BioMed Eng. OnLine 2022, 21, 59. [Google Scholar] [CrossRef]
- Hokken, T.W.; van Wiechen, M.P.; Ooms, J.F.; El Azzouzi, I.; de Ronde, M.; Kardys, I.; Budde, R.; Daemen, J.; de Jaegere, P.P.; Van Mieghem, N.M. Impact of Interventricular membranous septum length on pacemaker need with different Transcatheter aortic valve implantation systems. Int. J. Cardiol. 2021, 333, 152–158. [Google Scholar] [CrossRef]
- Tang, G.H.L.; Zaid, S.; Michev, I.; Ahmad, H.; Kaple, R.; Undemir, C.; Cohen, M.; Lansman, S.L. “Cusp-Overlap” View Simplifies Fluoroscopy-Guided Implantation of Self-Expanding Valve in Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018, 11, 1663–1665. [Google Scholar] [CrossRef]
- Sengupta, A.; Alexis, S.L.; Lee, T.; Zaid, S.; Krishnamoorthy, P.M.; Khera, S.; Lerakis, S.; Anastasius, M.; Dangas, G.D.; Sharma, S.K.; et al. Cusp Overlap Technique: Should it become the Standard Implantation Technique for Self-Expanding Valves? Curr. Cardiol. Rep. 2022, 23, 154. [Google Scholar] [CrossRef] [PubMed]
- Doldi, P.M.; Stolz, L.; Escher, F.; Steffen, J.; Gmeiner, J.; Roden, D.; Linnemann, M.; Löw, K.; Deseive, S.; Stocker, T.J.; et al. Transcatheter Aortic Valve Replacement with the Self-Expandable Core Valve Evolut Prosthesis Using the Cusp-Overlap vs. Tricusp-View. J. Clin. Med. 2022, 11, 1561. [Google Scholar] [CrossRef] [PubMed]
- Immè, S.; Attizzani, G.F.; Sgroi, C.; Barbanti, M.; Patané, M.; Capodanno, D.; Tamburino, C. Pre-defining optimal C-arm position for TAVI with CT-scan using free software. EuroIntervention 2013, 9, 878–879. [Google Scholar] [CrossRef] [PubMed]
- Samim, M.; Stella, P.R.; Agostoni, P.; Kluin, J.; Ramjankhan, F.; Budde, R.P.J.; Sieswerda, G.; Algeri, E.; van Belle, C.; Elkalioubie, A.; et al. Automated 3D Analysis of Pre-Procedural MDCT to Predict Annulus Plane Angulation and C-Arm Positioning. JACC Cardiovasc. Imaging 2013, 6, 238–248. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) with the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Knecht, S.; Schaer, B.; Reichlin, T.; Spies, F.; Madaffari, A.; Vischer, A.; Fahmi, G.; Jeger, R.; Kaiser, C.; Osswald, S.; et al. Electrophysiology Testing to Stratify Patients with Left Bundle Branch Block after Transcatheter Aortic Valve Implantation. J. Am. Heart Assoc. 2020, 9, e014446. [Google Scholar] [CrossRef]
- van Steenberger, G.J.; van Straten, B.; Lam, K.Y.; van Veghel, D.; Dekker, L.; Tonino, P.A. Report on outcomes of valve-in-valve transcatheter aortic valve implantation and redo surgical aortic valve replacement in the Netherlands. Neth. Heart J. 2022, 30, 106–112. [Google Scholar] [CrossRef]
- Majmundar, M.; Doshi, R.; Kumar, A.; Johnston, D.; Brockett, J.; Kanaa’N, A.; Lahorra, J.A.; Svensson, L.G.; Krishnaswamy, A.; Reed, G.W.; et al. Valve-in-valve transcatheter aortic valve implantation versus repeat surgical aortic valve replacement in patients with a failed aortic bioprosthesis. EuroIntervention 2022, 17, 1227–12237. [Google Scholar] [CrossRef]
- van Nieuwkerk, A.C.; Santos, R.B.; Fernandez-Nofrerias, E.; Tchétché, D.; de Brito, F.S., Jr.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A.; Ribichini, F.; et al. Outcomes in Valve-in-Valve Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2022, 172, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Fovino, L.N.; Blitzer, D.; Doulamis, I.P.; Guariento, A.; Salvador, L.; Tagliari, A.P.; Ferrari, E. Transcatheter aortic valve replacement for structural degeneration of previously implanted transcatheter valves (TAVR-in-TAVR): A systematic review. Eur. J. Cardio-Thorac. Surg. 2022, 61, 967–976. [Google Scholar] [CrossRef]
- Williams, M.R.; Jilaihawi, H.; Makkar, R.; O’Neill, W.W.; Guyton, R.; Malaisrie, C.; Brown, D.L.; Blanke, P.; Leipsic, J.A.; Pibarot, P.; et al. The PARTNER 3 Bicuspid Registry for Transcatheter Aortic Valve Replacement in Low-Surgical-Risk Pati. J. Am. Coll. Cardiol. Interv. 2022, 15, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Shantl, A.E.; Verhulst, A.; Neven, E.; Behets, G.J.; D’Haese, P.C.; Maillard, M.; Mordasini, D.; Phan, O.; Burnier, M.; Spaggiari, D.; et al. Inhibition of vascular calcification by inositol phosphates derivatized with ethylene glycol oligomers. Nat. Commun. 2020, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Despres, A.A.; Perrot, N.; Poulin, A.; Tastet, L.; Shen, M.; Chen, H.Y.; Bourgeois, R.; Trottier, M.; Tessier, M.; Guimond, J.; et al. Lipoprotein(a), Oxidized Phospholipids, and Aortic Valve Microcalcification Assessed by 18F-Sodium Fluoride Positron Emission Tomography and Computed Tomography. CJC Open 2019, 1, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, Y.; Singh, S.S.; Zheng, K.H.; Verbeek, R.; Kavousi, M.; Pinto, S.J.; Vernooij, M.W.; Sijbrands, E.J.G.; Boekholdt, S.M.; de Rijke, Y.B.; et al. Lipoprotein(a) is robustly associated with aortic valve calcium. Heart 2021, 107, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
| Valve Type | ||||
|---|---|---|---|---|
| Balloon- Expandable | Self-Expandable | Mechanical-Expandable | ||
| Generation | First/Early | Edwards Sapien Edwards Sapien XT | Medtronic CoreValve | |
| Second/Newer | Edwards Sapien 3 Edwards Sapien 3 Ultra Meril Life Sciences Myval | Medtronic CoreValve Evolut R Medtronic Evolut PRO Medtronic Evolut PRO+ Abbott Portico Abbott Navitor Boston Scientific ACURATE neo Boston Scientific ACURATE neo2 NVT ALLEGRAJenavalve Trilogy | Boston Scientific Lotus | |
| Company and Valve | Clinical Study Name | STS | PTS | All Cause Mortality 30 Days | All Cause Mortality 1 Year | Disabling Stroke 30 Days | Pacemaker Rate 30 Days | Moderate & Severe PVL 30 Days | Gradient 30 Days (mmHg) | Vascular Complications 30 Days |
|---|---|---|---|---|---|---|---|---|---|---|
 MEDTRONIC EVOLUTR/EVOLUTPRO | EvolutR CE | 7.0 | 60 | 0.0% | 5.7% | 0.0% | 11.7% | 3.4% | 8.1 | 8.3% |
| SURTAVI | 4.1 | 275 | 0.0% | 5.5% | 0.4% | 16.1% | 1.1% | 8.9 | 3.6% | |
| Forward | 5.5 | 1038 | 3.94% | 8.9% | 1.7% | 17.5% | 1.9% | 8.5 | 5.5% | |
| EvolutPro US | 6.4 | 60 | 1.7% | - | 1.7% | 11.25% | 0.0% | 6.4 | 10.0% | |
| NEOPRO | 5.3 | 258 | 1.8% | - | 2.5% | 13.26% | 5.7% | 7.5 | 3.5% | |
| Solve | 7.7 | 219 | 2.6% | 17.6% | 0.5% | 22.98% | 1.9% | - | - | |
| ForwardPro | 4.7 | 629 | 3.2% | 9.7% | 2.5% | 18.6% | 1.8% | 7.0 | 2.4% | |
| EvolutLowRisk | 1.9 | 729 | 0.5% | 2.4% | 0.5% | 17.4% | 3.5% | 8.4 | 3.8% | |
| Scope II | 4.5 | 380 | - | 9.0% | - | 16.04% | 2.9% | - | - | |
 EDWARDS SAPIEN 3 | Sapien 3 CE | 7.5 | 95 | 2.5% | 8.4% | 0.0% | 12.5% | 2.6% | 10.7 | 5.3% |
| PARTNER 2 S3 | 8.4 | 491 | 3.5% | 10.7% | 2.1% | 13.24% | 2.9% | 11.111.3 | 5.5% | |
| Source 3 | 4.8 | 1065 | 3.9% | 11.2% | 0.5% | 12.5% | 3.1% | 11.0 | 4.1% | |
| Scope 1 | 3.4 | 357 | 0.8% | - | 5.0% | 10–32% | 2.8% | 11.5 | 5.5% | |
| SOLVE | 7.6 | 219 | 2.3% | 17.0% | 4.7% | 19.23% | 1.4% | - | - | |
| PARTNER 3 | 1.9 | 496 | 0.4% | 8.5% | 0.5% | 6.8% | 0.8% | 0.8 | 2.2% | |
 Boston Scientific Lotus Edge | REPRISE CE | 6.5 | 250 | 4.0% | 11.6% | 2.5% | 28.6% | 0.6% | 11.7 | 5.2% |
| REPRISE II | 6.7 | 248 | 2.5% | 11.9% | 2.0% | 29.1% | - | 12.0 | 7.0% | |
| RESPOND | 6.0 | 996 | 2.2% | 11.7% | 2.3% | 34.6% | 0.3% | 10.6 | 3.0% | |
| RESPOND Edge | 3.2 | 100 | 0.0% | - | 3.0% | 22.6% | 0.0% | 10.1 | 4.0% | |
| Edge Euro Reg | 3.2 | 256 | 2.4% | - | 2.0% | 30.8% | 2.0% | 11.2 | 2.1% | |
| REPRISE III | 3.9 | 100 | 0.0% | 6.0% | 2.0% | 20.1% | 0.0% | 14.0 | 6.0% | |
| Edge | ||||||||||
 Boston Scientific Acurate Neo & Neo 2 | Neo CE | 6.8 | 82 | 3.4% | 22.5% | 2.2% | 9.0% | 4.9% | 8.0 | - |
| SAVI TF Reg | 6.0 | 924 | 1.5% | 8.4% | 1.9% | 8.2% | 4.1% | 8.5 | 5.6% | |
| NEOPRO | 5.0 | 1235 | 3.0% | - | 2.096% | 8.8% | 5.2% | 8.3 | 5.0% | |
| Scope I | 3.7 | 372 | 2.6% | - | 1.9% | 11.5% | 9.4% | 7.0 | 7.0% | |
| Neo 2 CE | 4.8 | 118 | 3.3% | 12.2% | 1.7% | 15.05% | 3.0% | 7.9 | 3.3% | |
| PROGRESS PVL | 6.0 | 490 | 2.2% | 11.3% | 2.4% | 11.6% | 5.0% | 6.7 | 3.6% | |
| Scope II | 4.0 | 390 | - | 13.0% | - | 11.09% | 9.6% | 6.5 | - | |
| ITAL – NEO | 1.1% | 11.0% | ||||||||
| EARLY – NEO 2 | 1.3% | 2.1% | 6.0% | 1.3% | 9 | |||||
 | PORTICO CE | 5.8 | 222 | 3.6% | - | 3.2% | 13.1% | 5.5% | 8.5 | 5.9% |
| PORTICO 2 | 5.8 | 941 | 2.7% | 12.1% | 1.6% | 18.7% | 3.9% | 8.6 | 5.5% | |
| PORTICO DIE | 6.4 | 381 | 3.5% | 14.3% | 1.5% | 13.4% | 6.3% | 8.4 | 9.0% | |
| FLEXNAV CE | 3.3 | 150 | 0.6% | - | 1.2% | 15.4% | 4.1% | 7.1 | 5.0% | |
| Abbott | ||||||||||
| PORTICO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauten, P.; Costello-Boerrigter, L.C.; Goebel, B.; Gonzalez-Lopez, D.; Schreiber, M.; Kuntze, T.; Al Jassem, M.; Lapp, H. Transcatheter Aortic Valve Implantation: Addressing the Subsequent Risk of Permanent Pacemaker Implantation. J. Cardiovasc. Dev. Dis. 2023, 10, 230. https://doi.org/10.3390/jcdd10060230
Lauten P, Costello-Boerrigter LC, Goebel B, Gonzalez-Lopez D, Schreiber M, Kuntze T, Al Jassem M, Lapp H. Transcatheter Aortic Valve Implantation: Addressing the Subsequent Risk of Permanent Pacemaker Implantation. Journal of Cardiovascular Development and Disease. 2023; 10(6):230. https://doi.org/10.3390/jcdd10060230
Chicago/Turabian StyleLauten, Philipp, Lisa C. Costello-Boerrigter, Björn Goebel, David Gonzalez-Lopez, Matthias Schreiber, Thomas Kuntze, Mahmoud Al Jassem, and Harald Lapp. 2023. "Transcatheter Aortic Valve Implantation: Addressing the Subsequent Risk of Permanent Pacemaker Implantation" Journal of Cardiovascular Development and Disease 10, no. 6: 230. https://doi.org/10.3390/jcdd10060230
APA StyleLauten, P., Costello-Boerrigter, L. C., Goebel, B., Gonzalez-Lopez, D., Schreiber, M., Kuntze, T., Al Jassem, M., & Lapp, H. (2023). Transcatheter Aortic Valve Implantation: Addressing the Subsequent Risk of Permanent Pacemaker Implantation. Journal of Cardiovascular Development and Disease, 10(6), 230. https://doi.org/10.3390/jcdd10060230





