Positron Emission Tomography in Heart Failure: From Pathophysiology to Clinical Application
Abstract
1. Introduction
2. Pathophysiology
2.1. Metabolic Derangements
2.2. Microvascular Dysfunction
2.3. Inflammation and Fibrosis
2.4. Sympathetic Denervation
3. Clinical Applications
3.1. Prognosis and Risk Stratification
3.2. Response to Heart Failure Therapy
3.3. Infiltrative Cardiomyopathies
3.4. Cardiac Sarcoidosis
| Diet | Description |
|---|---|
| A | High-fat, low-carbohydrate diet beginning 24 h before the study. Nothing by mouth, except water and oral pills, 6 h before the examination. |
| B | 18 h of fasting before the study. Nothing by mouth, except water and oral pills, for 18 h before the study. |
| C | High-fat, low-carbohydrate ketogenic diet beginning 72 h before the study. Nothing by mouth, except water and oral pills, for 3 overnight fasts before the exam. First 2 nights from 08:00 PM until at least 08:00 AM the next morning. The night before the test from 08:00 PM until the time of the test. For diabetics, consider avoiding prolonged fasting, preferably complete the examination during afternoon hours after ketogenic breakfast and morning insulin, followed by 6-h fasting. |
3.5. Cardio-Oncology
4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Quail, M.A.; Sinusas, A.J. PET-CMR in Heart Failure—Synergistic or Redundant Imaging? Heart Fail. Rev. 2017, 22, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Dilsizian, V.; Bacharach, S.L.; Beanlands, R.S.; Bergmann, S.R.; Delbeke, D.; Dorbala, S.; Gropler, R.J.; Knuuti, J.; Schelbert, H.R.; Travin, M.I. ASNC Imaging Guidelines/SNMMI Procedure Standard for Positron Emission Tomography (PET) Nuclear Cardiology Procedures. J. Nucl. Cardiol. 2016, 23, 1187–1226. [Google Scholar] [CrossRef]
- Saraste, A.; Knuuti, J. PET Imaging in Heart Failure: The Role of New Tracers. Heart Fail. Rev. 2017, 22, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.R.; Fox, K.A.; Rand, A.L.; McElvany, K.D.; Welch, M.J.; Markham, J.; Sobel, B.E. Quantification of Regional Myocardial Blood Flow in Vivo with H215O. Circulation 1984, 70, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.L.; Naya, M.; Foster, C.R.; Hainer, J.; Gaber, M.; Di Carli, G.; Blankstein, R.; Dorbala, S.; Sitek, A.; Pencina, M.J.; et al. Improved Cardiac Risk Assessment with Noninvasive Measures of Coronary Flow Reserve. Circulation 2011, 124, 2215–2224. [Google Scholar] [CrossRef]
- Czernin, J.; Müller, P.; Chan, S.; Brunken, R.C.; Porenta, G.; Krivokapich, J.; Chen, K.; Chan, A.; Phelps, M.E.; Schelbert, H.R. Influence of Age and Hemodynamics on Myocardial Blood Flow and Flow Reserve. Circulation 1993, 88, 62–69. [Google Scholar] [CrossRef]
- Di Carli, M.; Czernin, J.; Hoh, C.K.; Gerbaudo, V.H.; Brunken, R.C.; Huang, S.-C.; Phelps, M.E.; Schelbert, H.R. Relation Among Stenosis Severity, Myocardial Blood Flow, and Flow Reserve in Patients With Coronary Artery Disease. Circulation 1995, 91, 1944–1951. [Google Scholar] [CrossRef]
- Schindler, T.H.; Nitzsche, E.U.; Olschewski, M.; Magosaki, N.; Mix, M.; Prior, J.O.; Facta, A.D.; Solzbach, U.; Just, H.; Schelbert, H.R. Chronic Inflammation and Impaired Coronary Vasoreactivity in Patients With Coronary Risk Factors. Circulation 2004, 110, 1069–1075. [Google Scholar] [CrossRef]
- Prior, J.O.; Quiñones, M.J.; Hernandez-Pampaloni, M.; Facta, A.D.; Schindler, T.H.; Sayre, J.W.; Hsueh, W.A.; Schelbert, H.R. Coronary Circulatory Dysfunction in Insulin Resistance, Impaired Glucose Tolerance, and Type 2 Diabetes Mellitus. Circulation 2005, 111, 2291–2298. [Google Scholar] [CrossRef]
- Moody, J.B.; Poitrasson-Rivière, A.; Hagio, T.; Buckley, C.; Weinberg, R.L.; Corbett, J.R.; Murthy, V.L.; Ficaro, E.P. Added Value of Myocardial Blood Flow Using 18F-Flurpiridaz PET to Diagnose Coronary Artery Disease: The Flurpiridaz 301 Trial. J. Nucl. Cardiol. 2021, 28, 2313–2329. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.T.; Yeatman, L.A.; Buxton, D.B.; Chen, K.; Johnson, J.A.; Huang, S.C.; Kofoed, K.F.; Weismueller, S.; Czernin, J.; Phelps, M.E.; et al. Simultaneous Measurement of Myocardial Oxygen Consumption and Blood Flow Using [1-Carbon-11]Acetate. J. Nucl. Med. 1998, 39, 272–280. [Google Scholar] [PubMed]
- Grassi, I.; Nanni, C.; Allegri, V.; Morigi, J.J.; Montini, G.C.; Castellucci, P.; Fanti, S. The Clinical Use of PET with (11)C-Acetate. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 33–47. [Google Scholar] [PubMed]
- Hansen, K.B.; Sörensen, J.; Hansson, N.H.; Nielsen, R.; Larsen, A.H.; Frøkiær, J.; Tolbod, L.P.; Gormsen, L.C.; Harms, H.J.; Wiggers, H. Myocardial Efficiency in Patients with Different Aetiologies and Stages of Heart Failure. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 328–337. [Google Scholar] [CrossRef]
- Lindner, O.; Vogt, J.; Kammeier, A.; Wielepp, P.; Holzinger, J.; Baller, D.; Lamp, B.; Hansky, B.; Körfer, R.; Horstkotte, D.; et al. Effect of Cardiac Resynchronization Therapy on Global and Regional Oxygen Consumption and Myocardial Blood Flow in Patients with Non-Ischaemic and Ischaemic Cardiomyopathy. Eur. Heart J. 2005, 26, 70–76. [Google Scholar] [CrossRef]
- Song, W.; Zhang, X.; He, S.; Gai, Y.; Qin, C.; Hu, F.; Wang, Y.; Wang, Z.; Bai, P.; Wang, J.; et al. 68Ga-FAPI PET Visualize Heart Failure: From Mechanism to Clinic. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 475–485. [Google Scholar] [CrossRef]
- Sun, F.; Wang, C.; Du, X. [18F] AlF-NOTA-FAPI-04 PET imaging of fibroblast activation protein in heart failure with preserved ejection fraction. J. Nucl. Med. 2022, 63, 3330. [Google Scholar]
- Wang, G.; Yang, Q.; Wu, S.; Xu, X.; Li, X.; Liang, S.; Pan, G.; Zuo, C.; Zhao, X.; Cheng, C.; et al. Molecular Imaging of Fibroblast Activity in Pressure Overload Heart Failure Using [68 Ga]Ga-FAPI-04 PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 465–474. [Google Scholar] [CrossRef]
- Li, M.; Younis, M.H.; Zhang, Y.; Cai, W.; Lan, X. Clinical Summary of Fibroblast Activation Protein Inhibitor-Based Radiopharmaceuticals: Cancer and Beyond. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2844–2868. [Google Scholar] [CrossRef]
- Aikawa, T.; Naya, M.; Obara, M.; Oyama-Manabe, N.; Manabe, O.; Magota, K.; Ito, Y.M.; Katoh, C.; Tamaki, N. Regional Interaction between Myocardial Sympathetic Denervation, Contractile Dysfunction, and Fibrosis in Heart Failure with Preserved Ejection Fraction: 11C-Hydroxyephedrine PET Study. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1897–1905. [Google Scholar] [CrossRef]
- Zelt, J.G.E.; Wang, J.Z.; Mielniczuk, L.M.; Beanlands, R.S.B.; Fallavollita, J.A.; Canty, J.M.; deKemp, R.A. Positron Emission Tomography Imaging of Regional Versus Global Myocardial Sympathetic Activity to Improve Risk Stratification in Patients With Ischemic Cardiomyopathy. Circ Cardiovasc. Imaging 2021, 14, e012549. [Google Scholar] [CrossRef] [PubMed]
- Skali, H.; Schulman, A.R.; Dorbala, S. 18F-FDG PET/CT for the Assessment of Myocardial Sarcoidosis. Curr. Cardiol. Rep. 2013, 15, 352. [Google Scholar] [CrossRef] [PubMed]
- Chareonthaitawee, P.; Beanlands, R.S.; Chen, W.; Dorbala, S.; Miller, E.J.; Murthy, V.L.; Birnie, D.H.; Chen, E.S.; Cooper, L.T.; Tung, R.H.; et al. Joint SNMMI–ASNC Expert Consensus Document on the Role of 18 F-FDG PET/CT in Cardiac Sarcoid Detection and Therapy Monitoring. J. Nucl. Med. 2017, 58, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Martineau, P.; Grégoire, J.; Harel, F.; Pelletier-Galarneau, M. Assessing Cardiovascular Infection and Inflammation with FDG-PET. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 46–58. [Google Scholar]
- Bateman, T.; Heller, G.; Mcghie, A.; Friedman, J.; Case, J.; Bryngelson, J.; Hertenstein, G.; Moutray, K.; Reid, K.; Cullom, S. Diagnostic Accuracy of Rest/Stress ECG-Gated Rb-82 Myocardial Perfusion PET: Comparison with ECG-Gated Tc-99m Sestamibi SPECT. J. Nucl. Cardiol. 2006, 13, 24–33. [Google Scholar] [CrossRef]
- Higgins, A.R.; Jaber, W. SPECT and PET MPI: The Future Has Arrived but It Is Unevenly Distributed. J. Nucl. Cardiol. 2020, 27, 417–418. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Ćorović, A.; Wall, C.; Gopalan, D.; Rudd, J.H. Positron Emission Tomography Imaging in Cardiovascular Disease. Heart 2020, 106, 1712–1718. [Google Scholar] [CrossRef]
- AbouEzzeddine, O.F.; Kemp, B.J.; Borlaug, B.A.; Mullan, B.P.; Behfar, A.; Pislaru, S.V.; Fudim, M.; Redfield, M.M.; Chareonthaitawee, P. Myocardial Energetics in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2019, 12, e006240. [Google Scholar] [CrossRef]
- Siebermair, J.; Köhler, M.I.; Kupusovic, J.; Nekolla, S.G.; Kessler, L.; Ferdinandus, J.; Guberina, N.; Stuschke, M.; Grafe, H.; Siveke, J.T.; et al. Cardiac Fibroblast Activation Detected by Ga-68 FAPI PET Imaging as a Potential Novel Biomarker of Cardiac Injury/Remodeling. J. Nucl. Cardiol. 2021, 28, 812–821. [Google Scholar] [CrossRef]
- Venkateshvaran, A.; Faxen, U.L.; Hage, C.; Michaëlsson, E.; Svedlund, S.; Saraste, A.; Beussink-Nelson, L.; Fermer, M.L.; Gan, L.; Tromp, J.; et al. Association of Epicardial Adipose Tissue with Proteomics, Coronary Flow Reserve, Cardiac Structure and Function, and Quality of Life in Heart Failure with Preserved Ejection Fraction: Insights from the PROMIS-HFpEF Study. Eur. J. Heart Fail. 2022, 24, 2251–2260. [Google Scholar] [CrossRef]
- Sabbah, M.S.; Fayyaz, A.U.; de Denus, S.; Felker, G.M.; Borlaug, B.A.; Dasari, S.; Carter, R.E.; Redfield, M.M. Obese-Inflammatory Phenotypes in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2020, 13, e006414. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.M. Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2016, 375, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Tersalvi, G.; Gasperetti, A.; Schiavone, M.; Dauw, J.; Gobbi, C.; Denora, M.; Krul, J.D.; Cioffi, G.M.; Mitacchione, G.; Forleo, G.B. Acute Heart Failure in Elderly Patients: A Review of Invasive and Non-Invasive Management. J. Geriatr Cardiol 2021, 18, 560–576. [Google Scholar] [CrossRef]
- Neubauer, S. The Failing Heart--an Engine out of Fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Burrage, M.K.; Lewis, A.J.; Miller, J.J.J. Functional and Metabolic Imaging in Heart Failure with Preserved Ejection Fraction: Promises, Challenges, and Clinical Utility. Cardiovasc. Drugs 2022, 37, 379–399. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Taylor, M.; Wallhaus, T.R.; Degrado, T.R.; Russell, D.C.; Stanko, P.; Nickles, R.J.; Stone, C.K. An Evaluation of Myocardial Fatty Acid and Glucose Uptake Using PET with [18F]Fluoro-6-Thia-Heptadecanoic Acid and [18F]FDG in Patients with Congestive Heart Failure. J. Nucl. Med. 2001, 42, 55–62. [Google Scholar]
- Schwartz, B.; Gjini, P.; Gopal, D.M.; Fetterman, J.L. Inefficient Batteries in Heart Failure. JACC Basic Transl. Sci. 2022, 7, 1161–1179. [Google Scholar] [CrossRef]
- van Bilsen, M.; van Nieuwenhoven, F.A.; van der Vusse, G.J. Metabolic Remodelling of the Failing Heart: Beneficial or Detrimental? Cardiovasc. Res. 2008, 81, 420–428. [Google Scholar] [CrossRef]
- Vom Dahl, J.; Herman, W.H.; Hicks, R.J.; Ortiz-Alonso, F.J.; Lee, K.S.; Allman, K.C.; Wolfe, E.R.; Kalff, V.; Schwaiger, M. Myocardial Glucose Uptake in Patients with Insulin-Dependent Diabetes Mellitus Assessed Quantitatively by Dynamic Positron Emission Tomography. Circulation 1993, 88, 395–404. [Google Scholar] [CrossRef]
- Eisenberg, J.D.; Sobel, B.E.; Geltman, E.M. Differentiation of Ischemic from Nonischemic Cardiomyopathy with Positron Emission Tomography. Am. J. Cardiol. 1987, 59, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Mody, F.V.; Brunken, R.C.; Stevenson, L.W.; Nienaber, C.A.; Phelps, M.E.; Schelbert, H.R. Differentiating Cardiomyopathy of Coronary Artery Disease from Nonischemic Dilated Cardiomyopathy Utilizing Positron Emission Tomography. J. Am. Coll. Cardiol. 1991, 17, 373–383. [Google Scholar] [CrossRef]
- O’Farrell, A.C.; Evans, R.; Silvola, J.M.U.; Miller, I.S.; Conroy, E.; Hector, S.; Cary, M.; Murray, D.W.; Jarzabek, M.A.; Maratha, A.; et al. A Novel Positron Emission Tomography (PET) Approach to Monitor Cardiac Metabolic Pathway Remodeling in Response to Sunitinib Malate. PLoS ONE 2017, 12, e0169964. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The Obligatory Role of Endothelial Cells in the Relaxation of Arterial Smooth Muscle by Acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Zuchi, C.; Ambrosio, G.; Lüscher, T.F.; Landmesser, U. Nutraceuticals in Cardiovascular Prevention: Lessons from Studies on Endothelial Function: Nutraceuticals in Cardiovascular Prevention. Cardiovasc. Ther. 2010, 28, 187–201. [Google Scholar] [CrossRef]
- Breitenstein, S.; Roessig, L.; Sandner, P.; Lewis, K.S. Novel SGC Stimulators and SGC Activators for the Treatment of Heart Failure. In Heart Failure; Handbook of Experimental Pharmacology; Bauersachs, J., Butler, J., Sandner, P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 243, pp. 225–247. ISBN 978-3-319-59658-7. [Google Scholar]
- Kubo, S.H.; Rector, T.S.; Bank, A.J.; Williams, R.E.; Heifetz, S.M. Endothelium-Dependent Vasodilation Is Attenuated in Patients with Heart Failure. Circulation 1991, 84, 1589–1596. [Google Scholar] [CrossRef]
- Paolocci, N.; Biondi, R.; Bettini, M.; Lee, C.-I.; Berlowitz, C.O.; Rossi, R.; Xia, Y.; Ambrosio, G.; L»Abbate, A.; Kass, D.A.; et al. Oxygen Radical-Mediated Reduction in Basal and Agonist-Evoked NO Release in Isolated Rat Heart. J. Mol. Cell. Cardiol. 2001, 33, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Srivaratharajah, K.; Coutinho, T.; deKemp, R.; Liu, P.; Haddad, H.; Stadnick, E.; Davies, R.A.; Chih, S.; Dwivedi, G.; Guo, A.; et al. Reduced Myocardial Flow in Heart Failure Patients With Preserved Ejection Fraction. Circ. Heart Fail. 2016, 9, e002562. [Google Scholar] [CrossRef]
- Dandekar, V.K.; Bauml, M.A.; Ertel, A.W.; Dickens, C.; Gonzalez, R.C.; Farzaneh-Far, A. Assessment of global myocardial perfusion reserve using cardiovascular magnetic resonance of coronary sinus flow at 3 Tesla. J. Cardiovasc. Magn. Reson. 2014, 16, 24. [Google Scholar] [CrossRef]
- Wöhrle, J.; Nusser, T.; Merkle, N.; Kestler, H.A.; Grebe, O.C.; Marx, N.; Höher, M.; Kochs, M.; Hombach, V. Myocardial Perfusion Reserve in Cardiovascular Magnetic Resonance: Correlation to Coronary Microvascular Dysfunction. J. Cardiovasc. Magn Reson 2006, 8, 781–787. [Google Scholar] [CrossRef]
- Murthy, V.L.; Naya, M.; Taqueti, V.R.; Foster, C.R.; Gaber, M.; Hainer, J.; Dorbala, S.; Blankstein, R.; Rimoldi, O.; Camici, P.G.; et al. Effects of Sex on Coronary Microvascular Dysfunction and Cardiac Outcomes. Circulation 2014, 129, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Konerman, M.C.; Greenberg, J.C.; Kolias, T.J.; Corbett, J.R.; Shah, R.V.; Murthy, V.L.; Hummel, S.L. Reduced Myocardial Flow Reserve Is Associated With Diastolic Dysfunction and Decreased Left Atrial Strain in Patients With Normal Ejection Fraction and Epicardial Perfusion. J. Card. Fail. 2018, 24, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Tonet, E.; Pompei, G.; Faragasso, E.; Cossu, A.; Pavasini, R.; Passarini, G.; Tebaldi, M.; Campo, G. Coronary Microvascular Dysfunction: PET, CMR and CT Assessment. J. Clin. Med. 2021, 10, 1848. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N. International Standardization of Diagnostic Criteria for Microvascular Angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Maaniitty, T.; Knuuti, J.; Saraste, A. 15O-Water PET MPI: Current Status and Future Perspectives. Semin. Nucl. Med. 2020, 50, 238–247. [Google Scholar] [CrossRef]
- Bilak, J.M.; Alam, U.; Miller, C.A.; McCann, G.P.; Arnold, J.R.; Kanagala, P. Microvascular Dysfunction in Heart Failure with Preserved Ejection Fraction: Pathophysiology, Assessment, Prevalence and Prognosis. Card Fail. Rev. 2022, 8, e24. [Google Scholar] [CrossRef]
- Pfisterer, M.; Buser, P.; Rickli, H.; Gutmann, M.; Erne, P.; Rickenbacher, P.; Vuillomenet, A.; Jeker, U.; Dubach, P.; Beer, H.; et al. BNP-Guided vs Symptom-Guided Heart Failure Therapy: The Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) Randomized Trial. JAMA 2009, 301, 383. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Starling, R.C.; Hernandez, A.F.; Armstrong, P.W.; Dickstein, K.; Hasselblad, V.; Heizer, G.M.; Komajda, M.; Massie, B.M.; McMurray, J.J.V.; et al. Effect of Nesiritide in Patients with Acute Decompensated Heart Failure. N. Engl. J. Med. 2011, 365, 32–43. [Google Scholar] [CrossRef]
- Tersalvi, G.; Vicenzi, M.; Calabretta, D.; Biasco, L.; Pedrazzini, G.; Winterton, D. Elevated Troponin in Patients With Coronavirus Disease 2019: Possible Mechanisms. J. Card. Fail. 2020, 26, 470–475. [Google Scholar] [CrossRef]
- Rauchhaus, M.; Doehner, W.; Francis, D.P.; Davos, C.; Kemp, M.; Liebenthal, C.; Niebauer, J.; Hooper, J.; Volk, H.-D.; Coats, A.J.S.; et al. Plasma Cytokine Parameters and Mortality in Patients With Chronic Heart Failure. Circulation 2000, 102, 3060–3067. [Google Scholar] [CrossRef]
- Mehta, D.; Lubitz, S.A.; Frankel, Z.; Wisnivesky, J.P.; Einstein, A.J.; Goldman, M.; Machac, J.; Teirstein, A. Cardiac Involvement in Patients with Sarcoidosis. Chest 2008, 133, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Munoz, C.; Schneider, A.; Botnar, R.M.; Prieto, C. Recent Advances in PET-MRI for Cardiac Sarcoidosis. Front. Nucl. Med. 2022, 2, 1032444. [Google Scholar] [CrossRef]
- Cheung, E.; Ahmad, S.; Aitken, M.; Chan, R.; Iwanochko, R.M.; Balter, M.; Metser, U.; Veit-Haibach, P.; Billia, F.; Moayedi, Y.; et al. Combined Simultaneous FDG-PET/MRI with T1 and T2 Mapping as an Imaging Biomarker for the Diagnosis and Prognosis of Suspected Cardiac Sarcoidosis. Eur. J. Hybrid. Imaging 2021, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Nensa, F.; Kloth, J.; Tezgah, E.; Poeppel, T.D.; Heusch, P.; Goebel, J.; Nassenstein, K.; Schlosser, T. Feasibility of FDG-PET in Myocarditis: Comparison to CMR Using Integrated PET/MRI. J. Nucl. Cardiol. 2018, 25, 785–794. [Google Scholar] [CrossRef]
- Thackeray, J.T.; Bengel, F.M. Molecular Imaging of Myocardial Inflammation With Positron Emission Tomography Post-Ischemia. JACC Cardiovasc. Imaging 2018, 11, 1340–1355. [Google Scholar] [CrossRef]
- Glasenapp, A.; Derlin, K.; Wang, Y.; Bankstahl, M.; Meier, M.; Wollert, K.C.; Bengel, F.M.; Thackeray, J.T. Multimodality Imaging of Inflammation and Ventricular Remodeling in Pressure-Overload Heart Failure. J. Nucl. Med. 2020, 61, 590–596. [Google Scholar] [CrossRef]
- Varasteh, Z.; Mohanta, S.; Robu, S.; Braeuer, M.; Li, Y.; Omidvari, N.; Topping, G.; Sun, T.; Nekolla, S.G.; Richter, A.; et al. Molecular Imaging of Fibroblast Activity After Myocardial Infarction Using a 68 Ga-Labeled Fibroblast Activation Protein Inhibitor, FAPI-04. J. Nucl. Med. 2019, 60, 1743–1749. [Google Scholar] [CrossRef]
- Florea, V.G.; Cohn, J.N. The Autonomic Nervous System and Heart Failure. Circ. Res. 2014, 114, 1815–1826. [Google Scholar] [CrossRef]
- Zelt, J.G.E.; deKemp, R.A.; Rotstein, B.H.; Nair, G.M.; Narula, J.; Ahmadi, A.; Beanlands, R.S.; Mielniczuk, L.M. Nuclear Imaging of the Cardiac Sympathetic Nervous System: A Disease-Specific Interpretation in Heart Failure. JACC Cardiovasc. Imaging 2020, 13, 1036–1054. [Google Scholar] [CrossRef]
- Fallavollita, J.A.; Heavey, B.M.; Luisi, A.J.; Michalek, S.M.; Baldwa, S.; Mashtare, T.L.; Hutson, A.D.; Dekemp, R.A.; Haka, M.S.; Sajjad, M.; et al. Regional Myocardial Sympathetic Denervation Predicts the Risk of Sudden Cardiac Arrest in Ischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 141–149. [Google Scholar] [CrossRef]
- Boutagy, N.E.; Sinusas, A.J. Recent Advances and Clinical Applications of PET Cardiac Autonomic Nervous System Imaging. Curr. Cardiol. Rep. 2017, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Dickfeld, T.; Lei, P.; Dilsizian, V.; Jeudy, J.; Dong, J.; Voudouris, A.; Peters, R.; Saba, M.; Shekhar, R.; Shorofsky, S. Integration of Three-Dimensional Scar Maps for Ventricular Tachycardia Ablation with Positron Emission Tomography-Computed Tomography. JACC Cardiovasc. Imaging 2008, 1, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Argulian, E.; Narula, J. Advanced Cardiovascular Imaging in Clinical Heart Failure. JACC Heart Fail. 2021, 9, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Hassan, O.K.A.; Higgins, A.R. The Role of Multimodality Imaging in Patients with Heart Failure with Reduced and Preserved Ejection Fraction. Curr. Opin. Cardiol. 2022, 37, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Slart, R.H.J.A.; Glaudemans, A.W.J.M.; Gheysens, O.; Lubberink, M.; Kero, T.; Dweck, M.R.; Habib, G.; Gaemperli, O.; Saraste, A.; Gimelli, A.; et al. Procedural Recommendations of Cardiac PET/CT Imaging: Standardization in Inflammatory-, Infective-, Infiltrative-, and Innervation (4Is)-Related Cardiovascular Diseases: A Joint Collaboration of the EACVI and the EANM. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1016–1039. [Google Scholar] [CrossRef]
- Bax, J.J.; Maddahi, J.; Poldermans, D.; Elhendy, A.; Schinkel, A.; Boersma, E.; Valkema, R.; Krenning, E.P.; Roelandt, J.R.T.C.; van der Wall, E.E. Preoperative Comparison of Different Noninvasive Strategies for Predicting Improvement in Left Ventricular Function after Coronary Artery Bypass Grafting. Am. J. Cardiol. 2003, 92, 1–4. [Google Scholar] [CrossRef]
- Tillisch, J.; Brunken, R.; Marshall, R.; Schwaiger, M.; Mandelkern, M.; Phelps, M.; Schelbert, H. Reversibility of Cardiac Wall-Motion Abnormalities Predicted by Positron Tomography. N. Engl. J. Med. 1986, 314, 884–888. [Google Scholar] [CrossRef]
- Benz, D.C.; Kaufmann, P.A.; von Felten, E.; Benetos, G.; Rampidis, G.; Messerli, M.; Giannopoulos, A.A.; Fuchs, T.A.; Gräni, C.; Gebhard, C.; et al. Prognostic Value of Quantitative Metrics From Positron Emission Tomography in Ischemic Heart Failure. JACC Cardiovasc. Imaging 2021, 14, 454–464. [Google Scholar] [CrossRef]
- Gulati, V.; Ching, G.; Heller, G.V. The Role of Radionuclide Imaging in Heart Failure. J. Nucl. Cardiol. 2013, 20, 1173–1183. [Google Scholar] [CrossRef]
- Tio, R.A.; Dabeshlim, A.; Siebelink, H.-M.J.; de Sutter, J.; Hillege, H.L.; Zeebregts, C.J.; Dierckx, R.A.J.O.; van Veldhuisen, D.J.; Zijlstra, F.; Slart, R.H.J.A. Comparison between the Prognostic Value of Left Ventricular Function and Myocardial Perfusion Reserve in Patients with Ischemic Heart Disease. J. Nucl. Med. 2009, 50, 214–219. [Google Scholar] [CrossRef]
- Beanlands, R.S.B.; Nichol, G.; Huszti, E.; Humen, D.; Racine, N.; Freeman, M.; Gulenchyn, K.Y.; Garrard, L.; deKemp, R.; Guo, A.; et al. F-18-Fluorodeoxyglucose Positron Emission Tomography Imaging-Assisted Management of Patients With Severe Left Ventricular Dysfunction and Suspected Coronary Disease. J. Am. Coll. Cardiol. 2007, 50, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 ACC/AHA/HFSA Guideline for the Management of Heart Failure. J. Card. Fail. 2022, 28, e1–e167. [Google Scholar] [CrossRef]
- Neglia, D.; Michelassi, C.; Trivieri, M.G.; Sambuceti, G.; Giorgetti, A.; Pratali, L.; Gallopin, M.; Salvadori, P.; Sorace, O.; Carpeggiani, C.; et al. Prognostic Role of Myocardial Blood Flow Impairment in Idiopathic Left Ventricular Dysfunction. Circulation 2002, 105, 186–193. [Google Scholar] [CrossRef]
- Neglia, D.; Parodi, O.; Gallopin, M.; Sambuceti, G.; Giorgetti, A.; Pratali, L.; Salvadori, P.; Michelassi, C.; Lunardi, M.; Pelosi, G. Myocardial Blood Flow Response to Pacing Tachycardia and to Dipyridamole Infusion in Patients with Dilated Cardiomyopathy without Overt Heart Failure. A Quantitative Assessment by Positron Emission Tomography. Circulation 1995, 92, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, F.; Olivotto, I.; Gistri, R.; Lorenzoni, R.; Chiriatti, G.; Camici, P.G. Coronary Microvascular Dysfunction and Prognosis in Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2003, 349, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Kusuoka, H.; Maruyama, K.; Nishimura, T.; Hori, M.; Hatazawa, J. Myocardial Positron Emission Computed Tomographic Images Obtained with Fluorine-18 Fluoro-2-Deoxyglucose Predict the Response of Idiopathic Dilated Cardiomyopathy Patients to Beta-Blockers. J. Am. Coll. Cardiol. 2004, 43, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Auricchio, A.; Martens, P.; Witte, K.; Cowie, M.R.; Delgado, V.; Dickstein, K.; Linde, C.; Vernooy, K.; Leyva, F.; et al. Optimized Implementation of Cardiac Resynchronization Therapy: A Call for Action for Referral and Optimization of Care: A Joint Position Statement from the Heart Failure Association (HFA), European Heart Rhythm Association (EHRA), and European Association of Cardiovascular Imaging (EACVI) of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 2349–2369. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.M.; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef]
- Tang, A.S.L.; Wells, G.A.; Talajic, M.; Arnold, M.O.; Sheldon, R.; Connolly, S.; Hohnloser, S.H.; Nichol, G.; Birnie, D.H.; Sapp, J.L.; et al. Cardiac-Resynchronization Therapy for Mild-to-Moderate Heart Failure. N. Engl. J. Med. 2010, 363, 2385–2395. [Google Scholar] [CrossRef]
- Knaapen, P.; van Campen, L.C.M.; de Cock, C.C.; Götte, M.J.W.; Visser, C.A.; Lammertsma, A.A.; Visser, F.C. Effects of Cardiac Resynchronization Therapy on Myocardial Perfusion Reserve. Circulation 2004, 110, 646–651. [Google Scholar] [CrossRef]
- Nowak, B.; Stellbrink, C.; Sinha, A.M.; Kaiser, H.-J.; Reinartz, P.; Koos, R.; Markus, K.; Hanrath, P.; Buell, U.; Schaefer, W.M. Effects of Cardiac Resynchronization Therapy on Myocardial Blood Flow Measured by Oxygen-15 Water Positron Emission Tomography in Idiopathic-Dilated Cardiomyopathy and Left Bundle Branch Block. Am. J. Cardiol 2004, 93, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Sundell, J.; Engblom, E.; Koistinen, J.; Ylitalo, A.; Naum, A.; Stolen, K.Q.; Kalliokoski, R.; Nekolla, S.G.; Airaksinen, K.E.J.; Bax, J.J.; et al. The Effects of Cardiac Resynchronization Therapy on Left Ventricular Function, Myocardial Energetics, and Metabolic Reserve in Patients with Dilated Cardiomyopathy and Heart Failure. J. Am. Coll. Cardiol. 2004, 43, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, F.; Sörensen, J.; von Bibra, H.; Olsson, A.; Rydén, L.; Långström, B.; Linde, C. Effects of Biventricular Pacing on Myocardial Blood Flow and Oxygen Consumption Using Carbon-11 Acetate Positron Emission Tomography in Patients with Heart Failure. Am. J. Cardiol. 2003, 92, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Neri, G.; Zanco, P.; Zanon, F.; Buchberger, R. Effect of Biventricular Pacing on Metabolism and Perfusion in Patients Affected by Dilated Cardiomyopathy and Left Bundle Branch Block: Evaluation by Positron Emission Tomography. Europace 2003, 5, 111–115. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Bøttcher, M.; Jensen, H.K.; Nielsen, T.T.; Pedersen, A.K.; Mortensen, P.T. Regional Myocardial Perfusion during Chronic Biventricular Pacing and after Acute Change of the Pacing Mode in Patients with Congestive Heart Failure and Bundle Branch Block Treated with an Atrioventricular Sequential Biventricular Pacemaker. Eur. J. Heart Fail. 2003, 5, 179–186. [Google Scholar] [CrossRef]
- Ohkusu, Y.; Takahashi, N.; Ishikawa, T.; Sumita, S.; Kobayashi, T.; Matsushita, K.; Yamakawa, Y.; Uchino, K.; Kimura, K.; Inoue, T.; et al. Effect of Biventricular Pacing on Myocardial Glucose Metabolism in Patients with Heart Failure Using Fluoro-18-Deoxyglucose Positron Emission Tomography. Pacing Clin. Electrophysiol. 2003, 26, 144–147. [Google Scholar] [CrossRef]
- Ukkonen, H.; Sundell, J.; Knuuti, J. Effects of CRT on Myocardial Innervation, Perfusion and Metabolism. Europace 2008, 10, iii114–iii117. [Google Scholar] [CrossRef]
- Nelson, G.S.; Berger, R.D.; Fetics, B.J.; Talbot, M.; Spinelli, J.C.; Hare, J.M.; Kass, D.A. Left Ventricular or Biventricular Pacing Improves Cardiac Function at Diminished Energy Cost in Patients with Dilated Cardiomyopathy and Left Bundle-Branch Block. Circulation 2000, 102, 3053–3059. [Google Scholar] [CrossRef]
- Knuuti, J.; Sundell, J.; Naum, A.; Engblom, E.; Koistinen, J.; Ylitalo, A.; Stolen, K.Q.; Kalliokoski, R.; Nekolla, S.G.; Bax, K.E.J.J.; et al. Assessment of Right Ventricular Oxidative Metabolism by PET in Patients with Idiopathic Dilated Cardiomyopathy Undergoing Cardiac Resynchronization Therapy. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1592–1598. [Google Scholar] [CrossRef]
- Boriani, G.; Regoli, F.; Saporito, D.; Martignani, C.; Toselli, T.; Biffi, M.; Francolini, G.; Diemberger, I.; Bacchi, L.; Rapezzi, C.; et al. Neurohormones and Inflammatory Mediators in Patients with Heart Failure Undergoing Cardiac Resynchronization Therapy: Time Courses and Prediction of Response. Peptides 2006, 27, 1776–1786. [Google Scholar] [CrossRef]
- Martignani, C.; Diemberger, I.; Nanni, C.; Biffi, M.; Ziacchi, M.; Boschi, S.; Corzani, A.; Fanti, S.; Sambuceti, G.; Boriani, G. Cardiac Resynchronization Therapy and Cardiac Sympathetic Function. Eur. J. Clin. Invest. 2015, 45, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, S.; Nanni, C.; Marini, C.; Bonfiglioli, R.; Martignani, C.; Dib, B.; Fuccio, C.; Boriani, G.; Picori, L.; Boschi, S.; et al. Heterogeneous Response of Cardiac Sympathetic Function to Cardiac Resynchronization Therapy in Heart Failure Documented by 11[C]-Hydroxy-Ephedrine and PET/CT. Nucl. Med. Biol. 2015, 42, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Vidula, M.K.; Bravo, P.E. Multimodality Imaging for the Diagnosis of Infiltrative Cardiomyopathies. Heart 2022, 108, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Trivieri, M.G.; Dweck, M.R.; Abgral, R.; Robson, P.M.; Karakatsanis, N.A.; Lala, A.; Contreras, J.; Sahni, G.; Gopalan, R.; Gorevic, P.; et al. 18F-Sodium Fluoride PET/MR for the Assessment of Cardiac Amyloidosis. J. Am. Coll. Cardiol 2016, 68, 2712–2714. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, D.; Vergaro, G.; Giorgetti, A.; Marzullo, P.; Scipioni, M.; Santarelli, M.F.; Pucci, A.; Buda, G.; Volpi, E.; Emdin, M. [18F]-Florbetaben PET/CT for Differential Diagnosis Among Cardiac Immunoglobulin Light Chain, Transthyretin Amyloidosis, and Mimicking Conditions. JACC Cardiovasc. Imaging 2021, 14, 246–255. [Google Scholar] [CrossRef]
- Takasone, K.; Katoh, N.; Takahashi, Y.; Abe, R.; Ezawa, N.; Yoshinaga, T.; Yanagisawa, S.; Yazaki, M.; Oguchi, K.; Koyama, J.; et al. Non-Invasive Detection and Differentiation of Cardiac Amyloidosis Using 99m Tc-Pyrophosphate Scintigraphy and 11 C-Pittsburgh Compound B PET Imaging. Amyloid 2020, 27, 266–274. [Google Scholar] [CrossRef]
- Lee, S.-P.; Suh, H.-Y.; Park, S.; Oh, S.; Kwak, S.-G.; Kim, H.-M.; Koh, Y.; Park, J.-B.; Kim, H.-K.; Cho, H.-J.; et al. Pittsburgh B Compound Positron Emission Tomography in Patients With AL Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2020, 75, 380–390. [Google Scholar] [CrossRef]
- Gambarin, F.I.; Disabella, E.; Narula, J.; Diegoli, M.; Grasso, M.; Serio, A.; Favalli, B.M.E.V.; Agozzino, M.; Tavazzi, L.; Fraser, A.G.; et al. When Should Cardiologists Suspect Anderson-Fabry Disease? Am. J. Cardiol. 2010, 106, 1492–1499. [Google Scholar] [CrossRef]
- Patel, M.R.; Cecchi, F.; Cizmarik, M.; Kantola, I.; Linhart, A.; Nicholls, K.; Strotmann, J.; Tallaj, J.; Tran, T.C.; West, M.L.; et al. Cardiovascular Events in Patients With Fabry Disease. J. Am. Coll. Cardiol. 2011, 57, 1093–1099. [Google Scholar] [CrossRef]
- Imbriaco, M.; Pisani, A.; Spinelli, L.; Cuocolo, A.; Messalli, G.; Capuano, E.; Marmo, M.; Liuzzi, R.; Visciano, B.; Cianciaruso, B.; et al. Effects of Enzyme-Replacement Therapy in Patients with Anderson-Fabry Disease: A Prospective Long-Term Cardiac Magnetic Resonance Imaging Study. Heart 2009, 95, 1103–1107. [Google Scholar] [CrossRef]
- Thurberg, B.L.; Fallon, J.T.; Mitchell, R.; Aretz, T.; Gordon, R.E.; O’Callaghan, M.W. Cardiac Microvascular Pathology in Fabry Disease: Evaluation of Endomyocardial Biopsies Before and After Enzyme Replacement Therapy. Circulation 2009, 119, 2561–2567. [Google Scholar] [CrossRef]
- Weidemann, F.; Niemann, M.; Breunig, F.; Herrmann, S.; Beer, M.; Störk, S.; Voelker, W.; Ertl, G.; Wanner, C.; Strotmann, J. Long-Term Effects of Enzyme Replacement Therapy on Fabry Cardiomyopathy: Evidence for a Better Outcome With Early Treatment. Circulation 2009, 119, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Linhart, A.; Germain, D.P.; Olivotto, I.; Akhtar, M.M.; Anastasakis, A.; Hughes, D.; Namdar, M.; Pieroni, M.; Hagège, A.; Cecchi, F.; et al. An Expert Consensus Document on the Management of Cardiovascular Manifestations of Fabry Disease. Eur. J. Heart Fail. 2020, 22, 1076–1096. [Google Scholar] [CrossRef] [PubMed]
- Nappi, C.; Altiero, M.; Imbriaco, M.; Nicolai, E.; Giudice, C.A.; Aiello, M.; Diomiaiuti, C.T.; Pisani, A.; Spinelli, L.; Cuocolo, A. First Experience of Simultaneous PET/MRI for the Early Detection of Cardiac Involvement in Patients with Anderson-Fabry Disease. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1025–1031. [Google Scholar] [CrossRef]
- Slart, R.H.J.A.; Glaudemans, A.W.J.M.; Lancellotti, P.; Hyafil, F.; Blankstein, R.; Schwartz, R.G.; Jaber, W.A.; Russell, R.; Gimelli, A.; Rouzet, F.; et al. A Joint Procedural Position Statement on Imaging in Cardiac Sarcoidosis: From the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. J. Nucl. Cardiol. 2018, 25, 298–319. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, J.; Uusitalo, V.; Pöyhönen, P.; Mäyränpää, M.I.; Kupari, M. Cardiac Sarcoidosis: Phenotypes, Diagnosis, Treatment, and Prognosis. Eur. Heart J. 2023, 44, 1495–1510. [Google Scholar] [CrossRef]
- Saric, P.; Young, K.; Rodriguez-Porcel, M.; Chareonthaitawee, P. PET Imaging in Cardiac Sarcoidosis: A Narrative Review with Focus on Novel PET Tracers. Pharmaceuticals 2021, 14, 1286. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Cosedis Nielsen, J.; et al. HRS Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated with Cardiac Sarcoidosis. Heart Rhythm 2014, 11, 1305–1323. [Google Scholar] [CrossRef]
- Terasaki, F.; Azuma, A.; Anzai, T.; Ishizaka, N.; Ishida, Y.; Isobe, M.; Inomata, T.; Ishibashi-Ueda, H.; Eishi, Y.; Kitakaze, M.; et al. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis―Digest Version―. Circ. J. 2019, 83, 2329–2388. [Google Scholar] [CrossRef]
- Subramanian, M.; Swapna, N.; Ali, A.Z.; Saggu, D.K.; Yalagudri, S.; Kishore, J.; Swamy, L.T.N.; Narasimhan, C. Pre-Treatment Myocardial 18FDG Uptake Predicts Response to Immunosuppression in Patients With Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2021, 14, 2008–2016. [Google Scholar] [CrossRef]
- Osborne, M.T.; Hulten, E.A.; Singh, A.; Waller, A.H.; Bittencourt, M.S.; Stewart, G.C.; Hainer, J.; Murthy, V.L.; Skali, H.; Dorbala, S.; et al. Reduction in 18F-Fluorodeoxyglucose Uptake on Serial Cardiac Positron Emission Tomography Is Associated with Improved Left Ventricular Ejection Fraction in Patients with Cardiac Sarcoidosis. J. Nucl. Cardiol. 2014, 21, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Giblin, G.T.; Murphy, L.; Stewart, G.C.; Desai, A.S.; Di Carli, M.F.; Blankstein, R.; Givertz, M.M.; Tedrow, U.B.; Sauer, W.H.; Hunninghake, G.M.; et al. Cardiac Sarcoidosis: When and How to Treat Inflammation. Card Fail. Rev. 2021, 7, e17. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.I.; Abebe, A.T.; Han, Y.; Alnabelsi, T.; Agrawal, T.; Kassi, M.; Aljizeeri, A.; Taylor, A.; Tleyjeh, I.M.; Al-Mallah, M.H. The Prognostic Role of Cardiac Positron Emission Tomography Imaging in Patients with Sarcoidosis: A Systematic Review. J. Nucl. Cardiol. 2021, 28, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.N.; Pieper, J.A.; Poitrasson-Rivière, A.; Kopin, D.; Cascino, T.; Aaronson, K.; Murthy, V.L.; Koelling, T. The Prognostic Value of Positron Emission Tomography in the Evaluation of Suspected Cardiac Sarcoidosis. J. Nucl. Cardiol. 2022, 29, 2460–2470. [Google Scholar] [CrossRef]
- Gowani, Z.; Habibi, M.; Okada, D.R.; Smith, J.; Derakhshan, A.; Zimmerman, S.L.; Misra, S.; Gilotra, N.A.; Berger, R.D.; Calkins, H.; et al. Utility of Cardiac Magnetic Resonance Imaging Versus Cardiac Positron Emission Tomography for Risk Stratification for Ventricular Arrhythmias in Patients With Cardiac Sarcoidosis. Am. J. Cardiol. 2020, 134, 123–129. [Google Scholar] [CrossRef]
- Gilotra, N.; Okada, D.; Sharma, A.; Chrispin, J. Management of Cardiac Sarcoidosis in 2020. Arrhythm. Electrophysiol. Rev. 2020, 9, 182–188. [Google Scholar] [CrossRef]
- Cacoub, P.; Chapelon-Abric, C.; Resche-Rigon, M.; Saadoun, D.; Desbois, A.C.; Biard, L. Cardiac Sarcoidosis: A Long Term Follow up Study. PLoS ONE 2020, 15, e0238391. [Google Scholar] [CrossRef]
- Grunewald, J.; Grutters, J.C.; Arkema, E.V.; Saketkoo, L.A.; Moller, D.R.; Müller-Quernheim, J. Sarcoidosis. Nat. Rev. Dis. Prim. 2019, 5, 45. [Google Scholar] [CrossRef]
- Asatryan, B.; Asimaki, A.; Landstrom, A.P.; Khanji, M.Y.; Odening, K.E.; Cooper, L.T.; Marchlinski, F.E.; Gelzer, A.R.; Semsarian, C.; Reichlin, T.; et al. Inflammation and Immune Response in Arrhythmogenic Cardiomyopathy: State-of-the-Art Review. Circulation 2021, 144, 1646–1655. [Google Scholar] [CrossRef]
- Wicks, E.C.; Menezes, L.J.; Barnes, A.; Mohiddin, S.A.; Sekhri, N.; Porter, J.C.; Booth, H.L.; Garrett, E.; Patel, R.S.; Pavlou, M.; et al. Diagnostic Accuracy and Prognostic Value of Simultaneous Hybrid 18F-Fluorodeoxyglucose Positron Emission Tomography/Magnetic Resonance Imaging in Cardiac Sarcoidosis. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 757–767. [Google Scholar] [CrossRef]
- Greulich, S.; Gatidis, S.; Gräni, C.; Blankstein, R.; Glatthaar, A.; Mezger, K.; Müller, K.A.L.; Castor, T.; Mahrholdt, H.; Häntschel, M.; et al. Hybrid Cardiac Magnetic Resonance/Fluorodeoxyglucose Positron Emission Tomography to Differentiate Active From Chronic Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2022, 15, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Özütemiz, C.; Koksel, Y.; Froelich, J.W.; Rubin, N.; Bhargava, M.; Roukoz, H.; Cogswell, R.; Markowitz, J.; Perlman, D.M.; Steinberger, D. Comparison of the Effect of Three Different Dietary Modifications on Myocardial Suppression in 18 F-FDG PET/CT Evaluation of Patients for Suspected Cardiac Sarcoidosis. J. Nucl. Med. 2021, 62, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Babich, J.W. PET Tracers for Imaging Cardiac Function in Cardio-Oncology. Curr. Cardiol. Rep. 2022, 24, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Jingu, K.; Kaneta, T.; Nemoto, K.; Ichinose, A.; Oikawa, M.; Takai, Y.; Ogawa, Y.; Nakata, E.; Sakayauchi, T.; Takai, K.; et al. The Utility of 18F-Fluorodeoxyglucose Positron Emission Tomography for Early Diagnosis of Radiation-Induced Myocardial Damage. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 845–851. [Google Scholar] [CrossRef]
- Sarocchi, M.; Bauckneht, M.; Arboscello, E.; Capitanio, S.; Marini, C.; Morbelli, S.; Miglino, M.; Congiu, A.G.; Ghigliotti, G.; Balbi, M.; et al. An Increase in Myocardial 18-Fluorodeoxyglucose Uptake Is Associated with Left Ventricular Ejection Fraction Decline in Hodgkin Lymphoma Patients Treated with Anthracycline. J. Transl. Med. 2018, 16, 295. [Google Scholar] [CrossRef]
- Jo, I.Y.; Lee, J.W.; Kim, W.C.; Min, C.K.; Kim, E.S.; Yeo, S.-G.; Lee, S.M. Relationship Between Changes in Myocardial F-18 Fluorodeoxyglucose Uptake and Radiation Dose After Adjuvant Three-Dimensional Conformal Radiotherapy in Patients with Breast Cancer. J. Clin. Med. 2020, 9, 666. [Google Scholar] [CrossRef]
- Croteau, E.; Gascon, S.; Bentourkia, M.; Langlois, R.; Rousseau, J.A.; Lecomte, R.; Bénard, F. [11C]Acetate Rest-Stress Protocol to Assess Myocardial Perfusion and Oxygen Consumption Reserve in a Model of Congestive Heart Failure in Rats. Nucl. Med. Biol. 2012, 39, 287–294. [Google Scholar] [CrossRef]
- Christensen, N.L.; Jakobsen, S.; Schacht, A.C.; Munk, O.L.; Alstrup, A.K.O.; Tolbod, L.P.; Harms, H.J.; Nielsen, S.; Gormsen, L.C. Whole-Body Biodistribution, Dosimetry, and Metabolite Correction of [11C]Palmitate: A PET Tracer for Imaging of Fatty Acid Metabolism. Mol. Imaging 2017, 16, 1536012117734485. [Google Scholar] [CrossRef]
- Harms, H.J.; Hansson, N.H.S.; Kero, T.; Baron, T.; Tolbod, L.P.; Kim, W.Y.; Frøkiær, J.; Flachskampf, F.A.; Wiggers, H.; Sörensen, J. Automatic Calculation of Myocardial External Efficiency Using a Single 11C-Acetate PET Scan. J. Nucl. Cardiol. 2018, 25, 1937–1944. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Boughdad, S.; Latifyan, S.; Fenwick, C.; Bouchaab, H.; Suffiotti, M.; Moslehi, J.J.; Salem, J.-E.; Schaefer, N.; Nicod-Lalonde, M.; Costes, J.; et al. 68Ga-DOTATOC PET/CT to Detect Immune Checkpoint Inhibitor-Related Myocarditis. J. Immunother. Cancer 2021, 9, e003594. [Google Scholar] [CrossRef]
- Finke, D.; Heckmann, M.B.; Herpel, E.; Katus, H.A.; Haberkorn, U.; Leuschner, F.; Lehmann, L.H. Early Detection of Checkpoint Inhibitor-Associated Myocarditis Using 68Ga-FAPI PET/CT. Front. Cardiovasc. Med. 2021, 8, 614997. [Google Scholar] [CrossRef] [PubMed]
- Murabito, A.; Hirsch, E.; Ghigo, A. Mechanisms of Anthracycline-Induced Cardiotoxicity: Is Mitochondrial Dysfunction the Answer? Front. Cardiovasc. Med. 2020, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, S.P.; Haslop, A.; Coello, C.; Gunn, R.N.; Tate, E.W.; Southworth, R.; Plisson, C.; Long, N.J.; Wells, L.A. Imaging of Chemotherapy-Induced Acute Cardiotoxicity with 18F-Labeled Lipophilic Cations. J. Nucl. Med. 2019, 60, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Valenta, I.; Szabo, Z.; Mathews, W.B.; Abraham, T.P.; Abraham, M.R.; Schindler, T.H. PET/CT Imaging of Cardiac Angiotensin II Type 1 Receptors in Nonobstructive Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2019, 12, 1895–1896. [Google Scholar] [CrossRef]
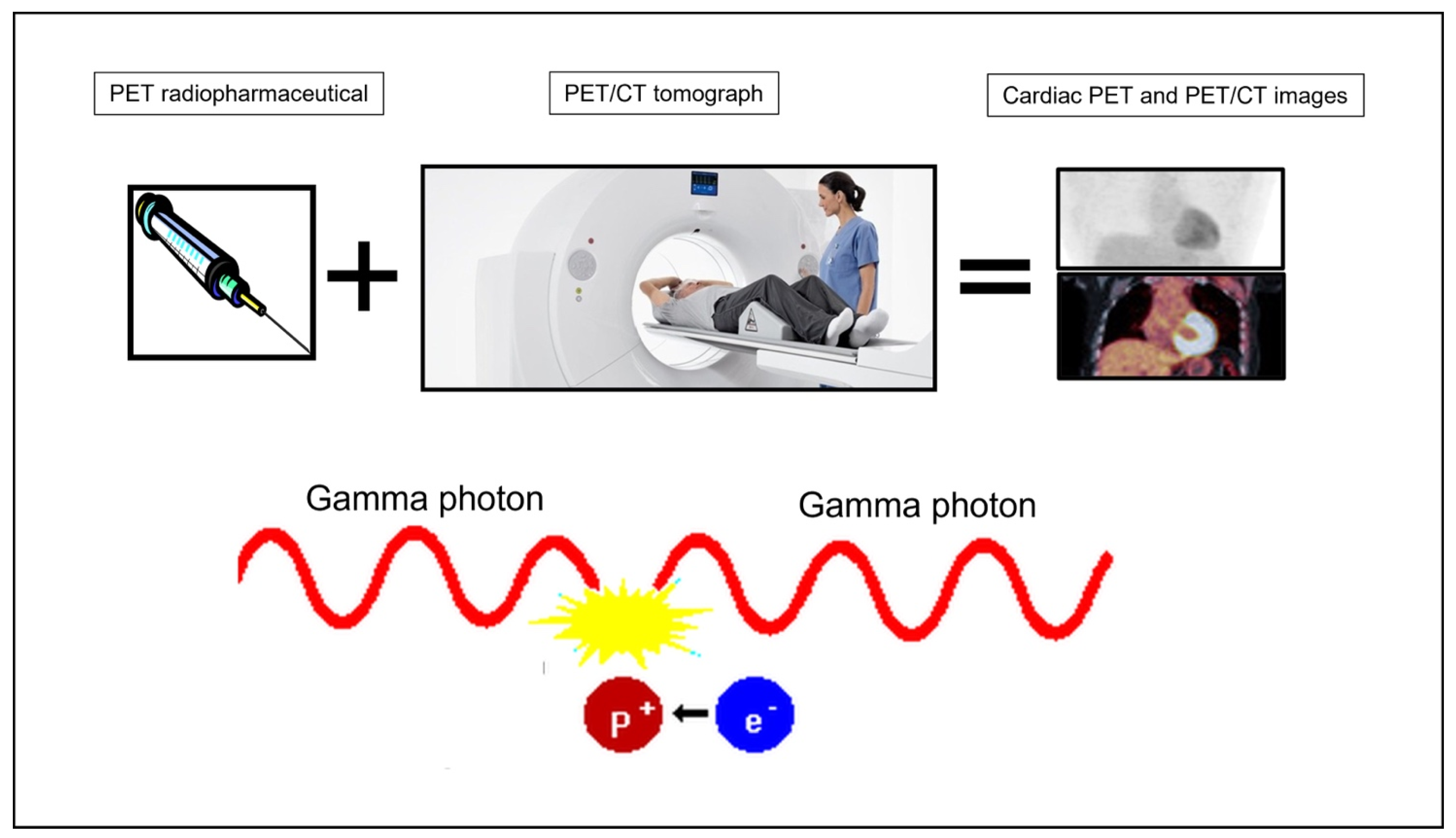

| Tracer | Name | Function |
|---|---|---|
| H2[15O] | H215O-water | Myocardial blood flow quantification (gold standard) [5] |
| 82Rb | Rubidium-82 | Myocardial blood flow quantification [6] |
| [13N]H3 | 13N-Ammonia | Myocardial blood flow quantification [7,8,9,10] |
| [18F]Flurpiridaz | 18F-Flurpiridaz | Myocardial blood flow quantification [11] |
| [11C]Acetate | Carbon-11 labeled acetate | Myocardial blood flow quantification, myocardial oxygen consumption, and cardiac efficiency [12,13,14,15] |
| [68Ga]FAPI (i.e., FAPI-04, FAPI-46, etc.) | Gallium-68 labeled fibroblast activation protein inhibitor | Detection of fibrosis through targeting activated fibroblast response [16,17,18,19] |
| [18F]AlF-NOTA-FAPI-04 | Aluminum-[18F]Fluoride labeled fibroblast activation protein inhibitor-04 chelated with NOTA | |
| [11C]HED | Carbon-11 labeled hydroxyephedrine | Global and regional sympathetic innervation quantification [20,21] |
| [18F]FDG | Fluorine-18 labeled fluorodeoxyglucose | Evaluation of increased metabolism (e.g., inflammation) [2,22,23,24] and myocardial viability |
| Indication | Purpose |
|---|---|
| Coronary artery disease | Viability assessment before revascularization |
| Cardiac sarcoidosis | Diagnosis; treatment monitoring |
| Cardiac amyloidosis | Diagnosis; research |
| Acute myocarditis | Additional diagnostic test |
| Prosthetic valve endocarditis | Diagnosis |
| Infection of CIED | Diagnosis; infection extent |
| Infection of LVAD | Diagnosis; infection extent |
| Myocardial innervation | Mainly research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tersalvi, G.; Beltrani, V.; Grübler, M.R.; Molteni, A.; Cristoforetti, Y.; Pedrazzini, G.; Treglia, G.; Biasco, L. Positron Emission Tomography in Heart Failure: From Pathophysiology to Clinical Application. J. Cardiovasc. Dev. Dis. 2023, 10, 220. https://doi.org/10.3390/jcdd10050220
Tersalvi G, Beltrani V, Grübler MR, Molteni A, Cristoforetti Y, Pedrazzini G, Treglia G, Biasco L. Positron Emission Tomography in Heart Failure: From Pathophysiology to Clinical Application. Journal of Cardiovascular Development and Disease. 2023; 10(5):220. https://doi.org/10.3390/jcdd10050220
Chicago/Turabian StyleTersalvi, Gregorio, Vittorio Beltrani, Martin R. Grübler, Alessandra Molteni, Yvonne Cristoforetti, Giovanni Pedrazzini, Giorgio Treglia, and Luigi Biasco. 2023. "Positron Emission Tomography in Heart Failure: From Pathophysiology to Clinical Application" Journal of Cardiovascular Development and Disease 10, no. 5: 220. https://doi.org/10.3390/jcdd10050220
APA StyleTersalvi, G., Beltrani, V., Grübler, M. R., Molteni, A., Cristoforetti, Y., Pedrazzini, G., Treglia, G., & Biasco, L. (2023). Positron Emission Tomography in Heart Failure: From Pathophysiology to Clinical Application. Journal of Cardiovascular Development and Disease, 10(5), 220. https://doi.org/10.3390/jcdd10050220







