Change in Right-to-Left Shunt Fraction in Patients with Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Endarterectomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurements
2.3. Outcomes
2.4. Statistics
3. Results
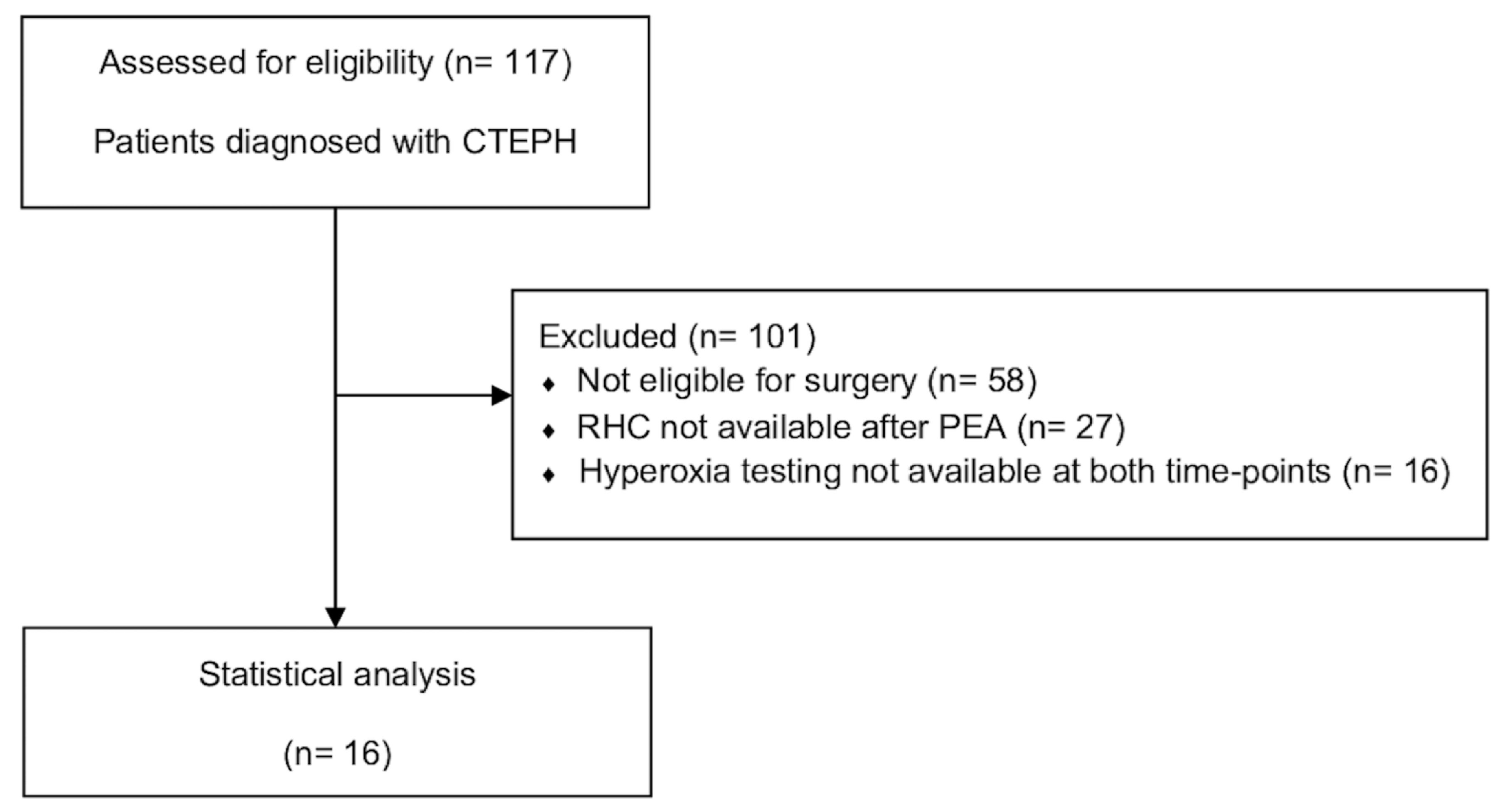
3.1. Study Population
3.2. Comparison of Patients with and without Improvement in Shunt Fraction
4. Discussion
5. Contribution to the Field Statement
6. Limitation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| BP | Blood pressure |
| BPA | Balloon pulmonary angioplasty |
| BSA | Body surface area |
| CI | Cardiac index |
| CO | Cardiac output |
| CTEPH | Chronic thromboembolic pulmonary hypertension |
| ERS | European Respiratory Society |
| ESC | European Society of Cardiology |
| FiO2 | Fraction of inspired oxygen |
| Hb | Hemoglobin |
| HR | Heart rate |
| mPAP | Mean pulmonary artery pressure |
| NT-proBNP | N-terminal prohormone of brain natriuretic peptide |
| PAH | Pulmonary arterial hypertension |
| PAWP | Pulmonary artery wedge pressure |
| PEA | Pulmonary endarterectomy |
| PH | Pulmonary hypertension |
| PVR | Pulmonary vascular resistance |
| Qs/Qt | Fraction of right-to-left shunt |
| RHC | Right heart catheterization |
| WHO | World Health Organization |
| WU | Wood unit |
| 6MWD | 6 min walking distance |
Appendix A
| Baseline | 1 Year after PEA | |
|---|---|---|
| Resting Hemodynamics during RHC | Hyperoxia (FiO2 1.0) | Hyperoxia (FiO2 1.0) |
| Oxygen saturation, % | 100 (99; 100) | 100 (100; 100) |
| Heart rate, min−1 | 63 (54; 71) | 65 (59; 75) |
| Systolic blood pressure, mmHg | 126 (118; 132) | 136 (120; 151) |
| Diastolic blood pressure, mmHg | 71 (63; 79) | 81 (67; 85) |
| Mean pulmonary artery pressure, mmHg | 33 (27; 39) | 20 (17; 24) |
| Pulmonary artery wedge pressure, mmHg | 11 (8.5; 14) | 10 (8; 12) |
| Right atrial pressure, mmHg | 7 (4; 7) | 5 (4; 8) |
| Cardiac index, L/min/m2 | 2.3 (1.9; 2.7) | 2.6 (2.3; 3.2) |
| Pulmonary vascular resistance, WU | 4.7 (3.5; 6.7) | 2.0 (1.1; 3.0) |
| Blood gases, arterial | ||
| Hemoglobin, g/dL | 14.6 (13.7; 15.7) | 15.0 (13.8; 15.6) |
| Oxygen saturation, % | 98 (98; 99) | 98 (98; 99) |
| pH | 7.44 (7.43; 7.50) | 7.45 (7.41; 7.47) |
| Partial pressure of oxygen, kPa | 62.8 (59.2; 67.9) | 69.2 (56.0; 71.8) |
| Partial pressure of carbon dioxide, kPa | 4.1 (3.6; 4.6) | 4.3 (3.9; 4.9) |
| Blood gases, mixed venous | ||
| Oxygen saturation, % | 78 (74; 83) | 80 (77; 82) |
| Partial pressure of oxygen, kPa | 5.8 (5.0; 6.6) | 6.1 (5.5; 6.7) |
| Partial pressure of carbon dioxide, kPa | 4.9 (4.4; 5.5) | 4.9 (4.5; 5.7) |
| Oxygen content and delivery | ||
| Oxygen delivery, mL/min | 904 (678; 995) | 962 (873; 1057) |
| Pulmonary capillary content of oxygen, mL/dL | 19.8 (18.7; 21.3) | 20.3 (18.7; 21.1) |
| Arterial content of oxygen, mL/dL | 19.5 (18.2; 20.9) | 19.8 (18.3; 20.7) |
| Mixed venous content of oxygen, mL/dL | 15.6 (14.4; 16.1) | 15.8 (15.0; 16.4) |
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic Definitions and Updated Clinical Classification of Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Lichtblau, M.; Saxer, S.; Latshang, T.D.; Aeschbacher, S.S.; Huber, F.; Scheiwiller, P.M.; Herzig, J.J.; Schneider, S.R.; Hasler, E.D.; Furian, M.; et al. Altitude Travel in Patients with Pulmonary Hypertension: Randomized Pilot-Trial Evaluating Nocturnal Oxygen Therapy. Front. Med. 2020, 7, 502. [Google Scholar] [CrossRef]
- Cenedese, E.; Speich, R.; Dorschner, L.; Ulrich, S.; Maggiorini, M.; Jenni, R.; Fischler, M. Measurement of Quality of Life in Pulmonary Hypertension and Its Significance. Eur. Respir. J. 2006, 28, 808–815. [Google Scholar] [CrossRef]
- Cima, K.; Twiss, J.; Speich, R.; McKenna, S.P.; Grünig, E.; Kähler, C.M.; Ehlken, N.; Treder, U.; Crawford, S.R.; Huber, L.C.; et al. The German Adaptation of the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR). Health Qual. Life Outcomes 2012, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Saxer, S.; Hasler, E.D.; Schwarz, E.I.; Schneider, S.R.; Furian, M.; Bader, P.R.; Lichtblau, M.; Bloch, K.E. Effect of Domiciliary Oxygen Therapy on Exercise Capacity and Quality of Life in Patients with Pulmonary Arterial or Chronic Thromboembolic Pulmonary Hypertension: A Randomised, Placebo-Controlled Trial. Eur. Respir. J. 2019, 54, 1900276. [Google Scholar] [CrossRef] [PubMed]
- Groth, A.; Saxer, S.; Bader, P.R.; Lichtblau, M.; Furian, M.; Schneider, S.R.; Schwarz, E.I.; Bloch, K.E.; Ulrich, S. Acute Hemodynamic Changes by Breathing Hypoxic and Hyperoxic Gas Mixtures in Pulmonary Arterial and Chronic Thromboembolic Pulmonary Hypertension. Int. J. Cardiol. 2018, 270, 262–267. [Google Scholar] [CrossRef]
- Madani, M.; Ogo, T.; Simonneau, G. The Changing Landscape of Chronic Thromboembolic Pulmonary Hypertension Management. Eur. Respir. Rev. 2017, 26, 170105. [Google Scholar] [CrossRef]
- Kapitan, K.S.; Buchbinder, M.; Wagner, P.D.; Moser, K.M. Mechanisms of Hypoxemia in Chronic Thromboembolic Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 1989, 139, 1149–1154. [Google Scholar] [CrossRef]
- Isabelle, O.; Silvia, U. Chronic Thromboembolic Pulmonary Hypertension. Swiss Med. Wkly. 2018, 148, w14702. [Google Scholar] [CrossRef]
- Carta, A.F.; Lichtblau, M.; Berlier, C.; Saxer, S.; Schneider, S.R.; Schwarz, E.I.; Furian, M.; Bloch, K.E.; Ulrich, S. The Impact of Breathing Hypoxic Gas and Oxygen on Pulmonary Hemodynamics in Patients with Pulmonary Hypertension. Front. Med. 2022, 9, 791423. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Hasler, E.D.; Saxer, S.; Furian, M.; Müller-Mottet, S.; Keusch, S.; Bloch, K.E. Effect of Breathing Oxygen-Enriched Air on Exercise Performance in Patients with Precapillary Pulmonary Hypertension: Randomized, Sham-Controlled Cross-over Trial. Eur. Heart J. 2017, 38, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Avian, A.; Pienn, M.; Naeije, R.; Olschewski, H. Reading Pulmonary Vascular Pressure Tracings: How to Handle the Problems of Zero Leveling and Respiratory Swings. Am. J. Respir. Crit. Care Med. 2014, 190, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Avian, A.; Olschewski, A.; Olschewski, H. Zero Reference Level for Right Heart Catheterisation. Eur. Respir. J. 2013, 42, 1586–1594. [Google Scholar] [CrossRef]
- Chiang, S.T. Anomogram for Venous Shunt (Qs-Qt) Calculation. Thorax 1968, 23, 563–565. [Google Scholar] [CrossRef]
- Boucly, A.; Weatherald, J.; Savale, L.; de Groote, P.; Cottin, V.; Prévot, G.; Chaouat, A.; Picard, F.; Horeau-Langlard, D.; Bourdin, A.; et al. External Validation of a Refined Four-Stratum Risk Assessment Score from the French Pulmonary Hypertension Registry. Eur. Respir. J. 2022, 59, 2102419. [Google Scholar] [CrossRef]
- Madani, M.M.; Auger, W.R.; Pretorius, V.; Sakakibara, N.; Kerr, K.M.; Kim, N.H.; Fedullo, P.F.; Jamieson, S.W. Pulmonary Endarterectomy: Recent Changes in a Single Institution’s Experience of More than 2700 Patients. Ann. Thorac. Surg. 2012, 94, 97–103. [Google Scholar] [CrossRef]
- Mayer, E.; Jenkins, D.; Lindner, J.; D’Armini, A.; Kloek, J.; Meyns, B.; Ilkjaer, L.B.; Klepetko, W.; Delcroix, M.; Lang, I.; et al. Surgical Management and Outcome of Patients with Chronic Thromboembolic Pulmonary Hypertension: Results from an International Prospective Registry. J. Thorac. Cardiovasc. Surg. 2011, 141, 702–710. [Google Scholar] [CrossRef]
- Kapitan, K.; Clausen, L.; Moser, K.M. Gas Exchange in Chronic Thromboembolism after Pulmonary Thromboendarterectomy. Clin. Investig. 1990, 98, 14–19. [Google Scholar] [CrossRef]
- Minatsuki, S.; Hatano, M.; Maki, H.; Takimoto, E.; Morita, H.; Komuro, I. Analysis of Oxygenation in Chronic Thromboembolic Pulmonary Hypertension Using Dead Space Ratio and Intrapulmonary Shunt Ratio. Int. Heart J. 2019, 60, 1137–1141. [Google Scholar] [CrossRef]
- Gossage, J.R.; Kanj, G. State of the Art Pulmonary Arteriovenous Malformations A State of the Art Review. Am. J. Respir. Crit. Care Med. 1998, 158, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Frost, A.E.; Langleben, D.; Oudiz, R.; Hill, N.; Horn, E.; McLaughlin, V.; Robbins, I.M.; Shapiro, S.; Tapson, V.F.; Zwicke, D.; et al. The 6-Min Walk Test (6MW) as an Efficacy Endpoint in Pulmonary Arterial Hypertension Clinical Trials: Demonstration of a Ceiling Effect. Vascul Pharmacol. 2005, 43, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Frantz, R.P.; Leopold, J.A.; Hassoun, P.M.; Hemnes, A.R.; Horn, E.M.; Mathai, S.C.; Rischard, F.P.; Larive, A.B.; Tang, W.h.W.; Park, M.M.; et al. Acute Vasoreactivity Testing during Right Heart Catheterization in Chronic Thromboembolic Pulmonary Hypertension: Results from the Pulmonary Vascular Disease Phenomics Study. Pulm. Circ. 2023, 13, e12181. [Google Scholar] [CrossRef] [PubMed]
- Scoccia, G.; Sfredda, S.; Miotti, C.; Luongo, F.; Toto, F.; Malerba, C.; Caputo, A.; Manguso, G.; Adamo, F.; Serino, G.; et al. Intrapulmonary Shunt Assessment in Pulmonary Arterial Hypertension. Eur. Heart J. Suppl. 2021, 23, suab133-022. [Google Scholar] [CrossRef]
- Takei, M.; Kawakami, T.; Kataoka, M.; Kuwahira, I.; Fukuda, K. Residual High Intrapulmonary Shunt Fraction Limits Exercise Capacity in Patients Treated with Balloon Pulmonary Angioplasty. Heart Vessels 2019, 34, 868–874. [Google Scholar] [CrossRef]
- Sandqvist, A.; Kylhammar, D.; Bartfay, S.E.; Hesselstrand, R.; Hjalmarsson, C.; Kavianipour, M.; Nisell, M.; Rådegran, G.; Wikström, G.; Kjellström, B.; et al. Risk Stratification in Chronic Thromboembolic Pulmonary Hypertension Predicts Survival. Scand. Cardiovasc. J. 2021, 55, 43–49. [Google Scholar] [CrossRef]
- Delcroix, M.; Staehler, G.; Gall, H.; Grünig, E.; Held, M.; Halank, M.; Klose, H.; Vonk-Noordegraaf, A.; Rosenkranz, S.; Pepke-Zaba, J.; et al. Risk Assessment in Medically Treated Chronic Thromboembolic Pulmonary Hypertension Patients. Eur. Respir. J. 2018, 52, 1800248. [Google Scholar] [CrossRef]
- Jamieson, S.W.; Kapelanski, D.P.; Sakakibara, N.; Manecke, G.R.; Thistlethwaite, P.A.; Kerr, K.M.; Channick, R.N.; Fedullo, P.F.; Auger, W.R.; Mcgregor, C.G.; et al. Pulmonary Endarterectomy: Experience and Lessons Learned in 1500 Cases. Ann. Thorac. Surg. 2003, 76, 1457–1464. [Google Scholar] [CrossRef]
- Bonderman, D.; Skoro-Sajer, N.; Jakowitsch, J.; Adlbrecht, C.; Dunkler, D.; Taghavi, S.; Klepetko, W.; Kneussl, M.; Lang, I.M. Predictors of Outcome in Chronic Thromboembolic Pulmonary Hypertension. Circulation 2007, 115, 2153–2158. [Google Scholar] [CrossRef]
- Plácido, R.; Guimarães, T.; Jenkins, D.; Cortez-Dias, N.; Pereira, S.C.; Campos, P.; Mineiro, A.; Lousada, N.; Martins, S.R.; Moreira, S.; et al. Chronic Thromboembolic Pulmonary Hypertension: Initial Experience of Patients Undergoing Pulmonary Thromboendarterectomy. Rev. Port. Cardiol. 2021, 40, 741–752. [Google Scholar] [CrossRef]
| Number | 16 |
|---|---|
| Female, n (%) | 6 (38) |
| Deceased, n (%) | 0 (0) |
| Age, years | 66 (55; 74) |
| Height, cm | 174 (165; 180) |
| Weight, kg | 81 (60; 91) |
| Body mass index, kg/m2 | 25.9 (22.1; 29.4) |
| Body surface area, m2 | 1.9 (1.7; 2.1) |
| 6 min walking distance, m | 512 (443; 570) |
| Oxygen saturation after 6 min walking test, % | 88 (85; 93) |
| NT-proBNP, ng/L | 409 (125; 968) |
| World Health Organization functional class | |
| WHO-FC II | 8 (50) |
| WHO-FC III | 7 (44) |
| WHO-FC IV | 1 (6) |
| Pulmonary hypertension treatment before PEA | |
| Endothelin receptor antagonist | 6 (38) |
| Phosphodiesterase-5 inhibitors | 0 (0) |
| Soluble guanylate cyclase stimulator | 7 (44) |
| Prostacyclin receptor agonist | 0 (0) |
| Combination therapy | 4 (25) |
| No therapy | 8 (50) |
| Risk score | 1.67 (1.08; 2.25) |
| Four-strata risk category | |
| Low | 6 (38) |
| Intermediate low | 7 (44) |
| Intermediate high | 3 (19) |
| High | 0 (0) |
| Pulmonary function tests, % predicted, n = 14 | |
| FEV1 | 89.0 (79.5; 100.5) |
| FVC | 91.5 (86.3; 105.5) |
| TLC | 94.5 (85.8; 103.3) |
| DLCO | 69.0 (63.8; 76.3) |
| Kco | 80.5 (72.0; 89.3) |
| Fraction of Right-to-Left Shunt | Baseline | 1 Year after PEA | p-Value |
|---|---|---|---|
| Shunt fraction, % | 13.7 (10.0; 17.5) | 13.0 (11.2; 15.6) | 0.679 |
| Shunt fraction > 5% | 16 (100) | 16 (100) | |
| Shunt fraction > 10% | 12 (75) | 14 (88) | |
| Patients with improved shunt by >10% of baseline value | 7 (44) | ||
| Patients that did not improve in shunt | 9 (56) |
| Baseline | 1 Year after PEA | ||
|---|---|---|---|
| Resting Hemodynamics during RHC | Normoxia (FiO2 0.21) | Normoxia (FiO2 0.21) | p-Value |
| Oxygen saturation, % | 93 (90; 96) | 98 (95; 99) | 0.002 * |
| Heart rate, min−1 | 70 (58; 78) | 64 (60; 79) | 0.641 |
| Systolic blood pressure, mmHg | 126 (119; 135) | 141 (118; 146) | 0.438 |
| Diastolic blood pressure, mmHg | 73 (66; 76) | 77 (63; 86) | 0.277 |
| Mean pulmonary artery pressure, mmHg | 38 (32; 41) | 24 (18; 28) | <0.001 * |
| Pulmonary artery wedge pressure, mmHg | 11 (10; 14) | 11 (8; 12) | 0.507 |
| Right atrial pressure, mmHg | 7 (6; 10) | 6 (5; 8) | 0.580 |
| Cardiac index, L/min/m2 | 2.7 (2.1; 3.2) | 2.7 (2.3; 3.0) | 0.682 |
| Pulmonary vascular resistance, WU | 5.7 (4.0; 6.8) | 2.5 (1.4; 3.8) | <0.001 * |
| Blood gases, arterial | |||
| Hemoglobin, g/dL | 15.1 (13.5; 15.7) | 14.8 (13.9; 15.5) | 0.972 |
| Oxygen saturation, % | 92 (88; 93) | 94 (93; 95) | 0.001 * |
| pH | 7.44 (7.42; 7.47) | 7.43 (7.40; 7.45) | 0.423 |
| Partial pressure of oxygen, kPa | 8.5 (6.9; 9.3) | 10.2 (9.4; 11.0) | 0.002 * |
| Partial pressure of carbon dioxide, kPa | 4.4 (3.9; 4.7) | 4.6 (4.3; 5.2) | 0.034 * |
| Blood gases, mixed venous | |||
| Oxygen saturation, % | 64 (57; 68) | 69 (65; 72) | 0.003 * |
| Partial pressure of oxygen, kPa | 4.5 (4.1; 4.7) | 5.0 (4.6; 5.2) | <0.001 * |
| Partial pressure of carbon dioxide, kPa | 5.1 (4.6; 5.4) | 5.4 (4.7; 5.5) | 0.215 |
| Oxygen content and delivery | |||
| Oxygen delivery, mL/min | 873 (680; 1029) | 898 (808; 998) | 0.352 |
| Arterial content of oxygen, mL/dL | 17.5 (16.5; 19.1) | 18.7 (17.7; 19.5) | 0.796 |
| Mixed venous content of oxygen, mL/dL | 12.6 (11.4; 13.6) | 13.8 (12.5; 14.8) | 0.034 * |
| Baseline | 1 Year after PEA | p-Value | |
|---|---|---|---|
| WHO functional class | 0.002 * | ||
| WHO-FC I | 0 (0) | 6 (38) | |
| WHO-FC II | 8 (50) | 10 (63) | |
| WHO-FC III | 7 (44) | 0 (0) | |
| WHO-FC IV | 1 (6) | 0 (0) | |
| 6 min walking test (breathing ambient air) | |||
| 6MWD, m | 512 (443; 570) | 518 (462; 596) | 0.221 |
| SpO2 at end exercise, % | 88 (85; 93) | 93 (87; 96) | 0.319 |
| NT-proBNP, ng/L | 409 (125; 968) | 206 (94; 437) | 0.233 |
| Four-strata risk category | |||
| Low | 6 (38) | 12 (75) | |
| Intermediate low | 7 (44) | 4 (25) | |
| Intermediate high | 3 (19) | 0 (0) | |
| High | 0 (0) | 0 (0) | |
| Risk score | 1.67 (1.08; 2.25) | 1.00 (1.00; 1.59) | 0.015 * |
| Median (IQR) | Test Statistics | ||||
|---|---|---|---|---|---|
| n | Baseline | n | 1 Year after PEA | p-Value | |
| Improvement in shunt fraction | |||||
| Oxygen saturation, % | 7 | 92 (90; 97) | 7 | 97 (95; 99) | 0.028 * |
| Mean pulmonary artery pressure, mmHg | 7 | 37 (29; 48) | 7 | 24 (19; 28) | 0.018 * |
| Pulmonary vascular resistance, WU | 7 | 6.5 (3.4; 6.8) | 7 | 2.8 (1.5; 3.8) | 0.043 * |
| Cardiac index, L/min/m2 | 7 | 2.8 (2.0; 3.2) | 7 | 2.4 (2.2; 3.3) | 0.610 |
| WHO functional class | 7 | 3 (2; 3) | 7 | 2 (1; 2) | 0.038 * |
| NT-proBNP, ng/L | 7 | 436 (100; 976) | 7 | 125 (76; 349) | 0.115 |
| 6 min walking distance, m | 7 | 552 (420; 618) | 6 | 557 (511; 602) | 0.173 |
| SpO2 at end 6MWD, % | 7 | 88 (87; 93) | 6 | 93 (88; 97) | 0.916 |
| Risk score | 7 | 1.67 (1.0; 2.33) | 7 | 1.00 (1.00; 1.33) | 0.042 * |
| No improvement in shunt fraction | |||||
| Oxygen saturation, % | 9 | 94 (90; 96) | 9 | 98 (95; 99) | 0.021 * |
| Mean pulmonary artery pressure, mmHg | 9 | 38 (33; 41) | 23 (17; 29) | 0.008 * | |
| Pulmonary vascular resistance, WU | 9 | 4.2 (4.1; 7.9) | 9 | 2.4 (1.3; 3.2) | 0.008 * |
| Cardiac index, L/min/m2 | 9 | 2.7 (2.1; 2.9) | 9 | 2.7 (2.6; 2.9) | 0.174 |
| WHO functional class | 9 | 2 (2; 3) | 9 | 2 (1; 2) | 0.023 * |
| NT-proBNP, ng/L | 9 | 381 (141; 1058) | 9 | 220 (170; 611) | 0.678 |
| 6 min walking distance, m | 9 | 508 (446; 566) | 9 | 490 (387; 630) | 0.674 |
| SpO2 at end 6MWT, % | 9 | 86 (81; 93) | 9 | 93 (85; 96) | 0.286 |
| Risk score | 9 | 1.67 (1.17; 2.34) | 9 | 1.00 (1.00; 1.67) | 0.118 |
| Variables | Overall Sample | Improvement in Shunt Fraction | No Improvement in Shunt Fraction | p-Value |
|---|---|---|---|---|
| (n = 16) | (n = 7) | (n = 9) | ||
| ∆ Mean pulmonary artery pressure, mmHg | −14 (−21; −7) | −14 (−21; −4) | −14 (−21; −9) | 0.837 |
| ∆ Pulmonary vascular resistance, WU | −2.7 (−4.9; −0.7) | −2.9 (−5.0; −0.6) | −2.4 (−6.5; −0.9) | 0.681 |
| ∆ 6MWD, m | 28 (−21; 106) | 36 (−13; 145) | 0 (−61; 107) | 0.689 |
| ∆ NT-proBNP, ng/L | −19 (−704; 60) | −19 (−692; 0) | −1 (−733; 273) | 0.681 |
| WHO functional class | 1.000 | |||
| Improvement (n, %) | 11 (69) | 5 (71) | 6 (67) | |
| No improvement (n, %) | 5 (31) | 2 (29) | 3 (33) | |
| NT-proBNP, ng/L | 0.633 | |||
| Improvement (n, %) | 10 (63) | 5 (71) | 5 (56) | |
| No improvement (n, %) | 6 (38) | 2 (29) | 4 (44) | |
| 6 min walking distance, m | 0.608 | |||
| Improvement (n, %) | 8 (53) | 4 (67) | 4 (44) | |
| No improvement (n, %) | 7 (47) | 2 (33) | 5 (56) | |
| SpO2 end 6MWD, % | 0.608 | |||
| Improvement (n, %) | 7 (47) | 2 (33) | 5 (56) | |
| No improvement (n, %) | 8 (53) | 4 (67) | 4 (44) | |
| Risk score | 1.000 | |||
| Improvement (n, %) | 11 (69) | 5 (71) | 6 (67) | |
| No improvement (n, %) | 5 (31) | 2 (29) | 3 (33) | |
| Four-strata risk category | 0.633 | |||
| Improvement (n, %) | 10 (63) | 5 (71) | 5 (56) | |
| No improvement (n, %) | 6 (38) | 2 (29) | 4 (44) | |
| Residual PH | 1.000 | |||
| Yes (n, %) | 8 (50) | 4 (57) | 4 (44) | |
| No (n, %) | 8 (50) | 3 (43) | 5 (56) | |
| Pulmonary hypertension treatment before PEA | 1.000 | |||
| Yes (n, %) | 8 (50) | 3 (43) | 5 (56) | |
| No (n, %) | 8 (50) | 4 (57) | 4 (44) | |
| Pulmonary hypertension treatment after PEA | 1.000 | |||
| Yes (n, %) | 5 (31) | 2 (29) | 3 (33) | |
| No (n, %) | 11 (69) | 5 (71) | 6 (67) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reimann, L.; Mayer, L.; Schneider, S.R.; Schwarz, E.I.; Müller, J.; Titz, A.; Furian, M.; Carta, A.F.; Etienne, H.; Battilana, B.; et al. Change in Right-to-Left Shunt Fraction in Patients with Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Endarterectomy. J. Cardiovasc. Dev. Dis. 2023, 10, 442. https://doi.org/10.3390/jcdd10110442
Reimann L, Mayer L, Schneider SR, Schwarz EI, Müller J, Titz A, Furian M, Carta AF, Etienne H, Battilana B, et al. Change in Right-to-Left Shunt Fraction in Patients with Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Endarterectomy. Journal of Cardiovascular Development and Disease. 2023; 10(11):442. https://doi.org/10.3390/jcdd10110442
Chicago/Turabian StyleReimann, Lena, Laura Mayer, Simon Raphael Schneider, Esther I. Schwarz, Julian Müller, Anna Titz, Michael Furian, Arcangelo F. Carta, Harry Etienne, Bianca Battilana, and et al. 2023. "Change in Right-to-Left Shunt Fraction in Patients with Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Endarterectomy" Journal of Cardiovascular Development and Disease 10, no. 11: 442. https://doi.org/10.3390/jcdd10110442
APA StyleReimann, L., Mayer, L., Schneider, S. R., Schwarz, E. I., Müller, J., Titz, A., Furian, M., Carta, A. F., Etienne, H., Battilana, B., Saxer, S., Pfammatter, T., Frauenfelder, T., Opitz, I., Ulrich, S., & Lichtblau, M. (2023). Change in Right-to-Left Shunt Fraction in Patients with Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Endarterectomy. Journal of Cardiovascular Development and Disease, 10(11), 442. https://doi.org/10.3390/jcdd10110442






