Association between Neuron-Specific Enolase, Memory Function, and Postoperative Delirium after Transfemoral Aortic Valve Replacement
Abstract
1. Introduction
2. Material and Methods
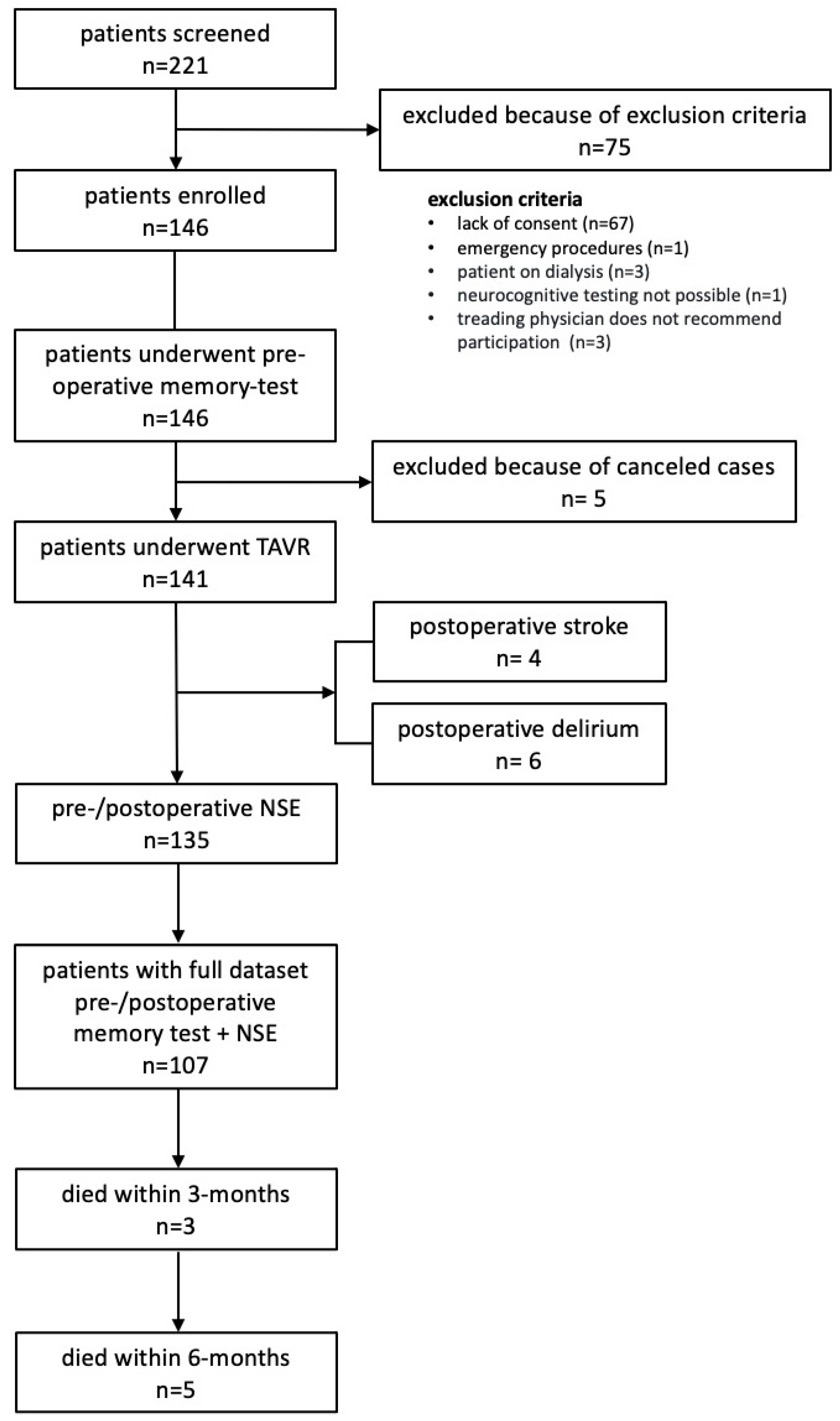
2.1. Patients
2.2. TAVR Procedure
2.3. Neurocognitive Function Assessment
2.4. Screening for Postoperative Delirium (POD)
2.5. Neuron-Specific Enolase (NSE) Measurement
2.6. Data Collection
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics and Outcome
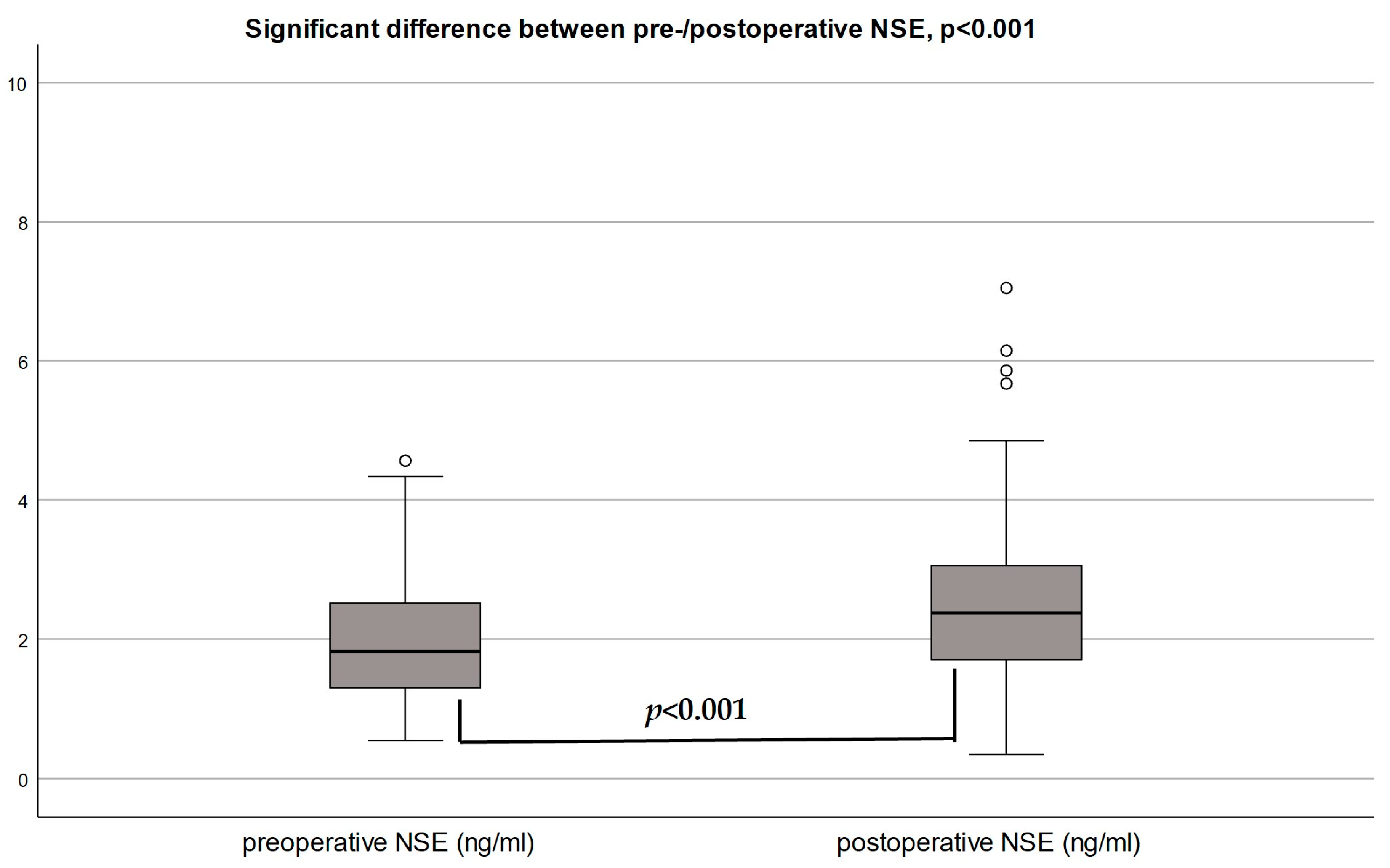
3.2. Neuron-Specific Enolase (NSE)
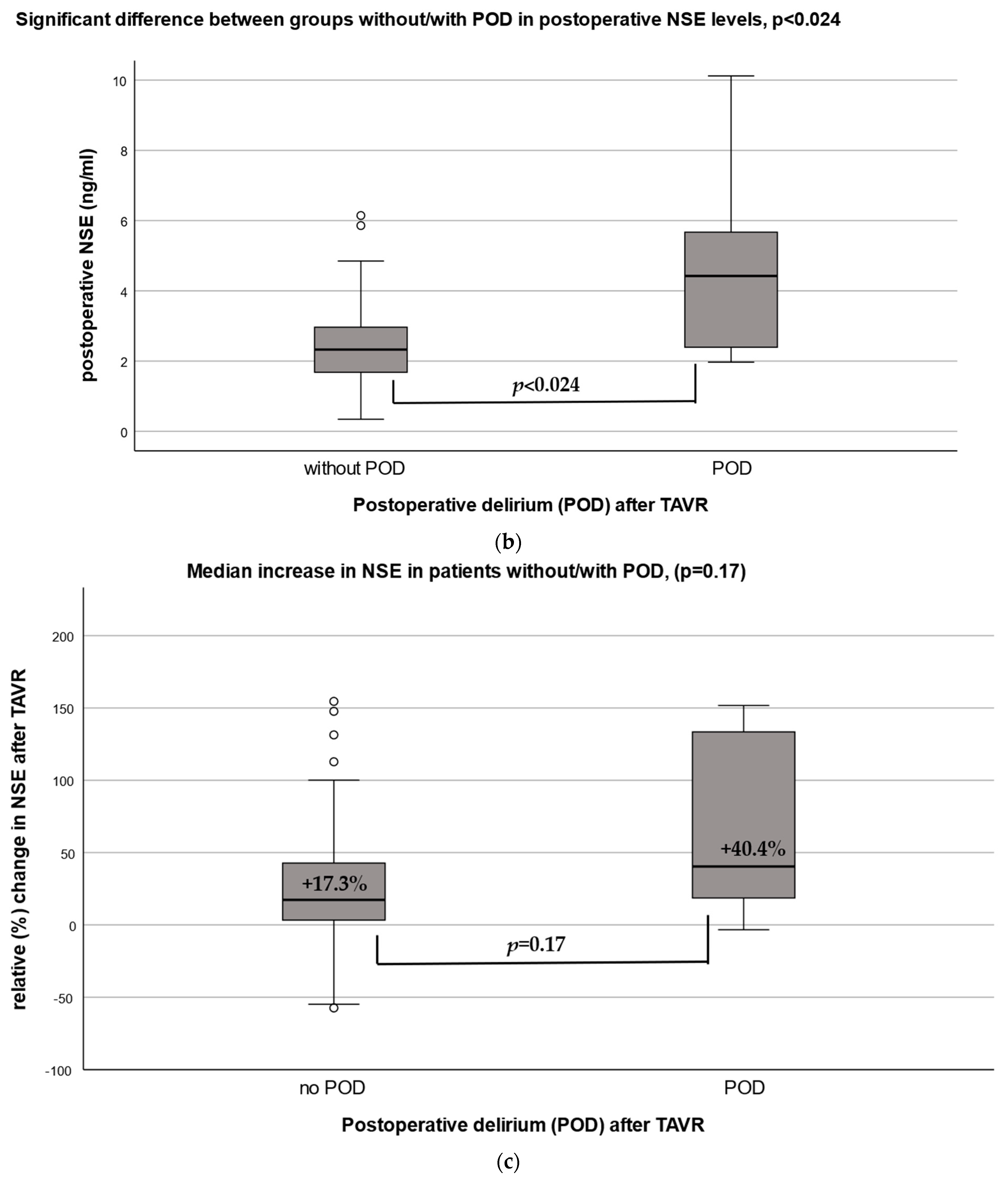
3.3. Post-NSE in Patients with and without POD
3.4. Preoperative and Postoperative Memory Function
3.5. Neuron-Specific Enolase (NSE) and Memory Function
3.6. Binary Memory Performance in CERAD and Neuron-Specific Enolase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahlert, P.; Al-Rashid, F.; Dottger, P.; Mori, K.; Plicht, B.; Wendt, D.; Bergmann, L.; Kottenberg, E.; Schlamann, M.; Mummel, P.; et al. Cerebral embolization during transcatheter aortic valve im-plantation: A transcranial Doppler study. Circulation 2012, 126, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Fanning, J.P.; Wesley, A.J.; Platts, D.G.; Walters, D.L.; Eeles, E.M.; Seco, M.; Tronstad, O.; Strugnell, W.; Barnett, A.G.; Clarke, A.J.; et al. The silent and apparent neurological injury in transcatheter aortic valve implantation study (SANITY): Concept, design and rationale. BMC Cardiovasc. Disord. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.; Muller, A.; Nahle, C.P.; Kocurek, J.; Werner, N.; Hammerstingl, C.; Schild, H.H.; Schwab, J.O.; Mellert, F.; Fimmers, R.; et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: A prospective pilot study with diffusion-weighted magnetic resonance imaging. J. Am. Coll. Cardiol. 2010, 55, 1427–1432. [Google Scholar] [CrossRef]
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Ma, X.; Chu, H.; Han, K.; Shao, Q.; Yu, Y.; Jia, S.; Wang, D.; Wang, Z.; Zhou, Y. Postoperative delirium after transcatheter aortic valve replacement: An updated systematic review and meta-analysis. J. Am. Geriatr. Soc. 2023, 71, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Gunther, M.L.; Morandi, A.; Krauskopf, E.; Pandharipande, P.; Girard, T.D.; Jackson, J.C.; Thompson, J.; Shintani, A.K.; Geevarghese, S.; Miller, R.R., 3rd; et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: The VISIONS cohort magnetic resonance imaging study. Crit. Care Med. 2012, 40, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Fanning, J.P.; See Hoe, L.E.; Passmore, M.R.; Barnett, A.G.; Obonyo, N.G.; Millar, J.E.; Wesley, A.J.; Suen, J.Y.; Fraser, J.F. Neuron-Specific Enolase and Matrix Metalloproteinase 9 Signal Perioperative Silent Brain Infarction during or after Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2019, 123, 434–439. [Google Scholar] [CrossRef]
- Isgro, M.A.; Bottoni, P.; Scatena, R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 125–143. [Google Scholar] [CrossRef]
- Cheng, F.; Yuan, Q.; Yang, J.; Wang, W.; Liu, H. The prognostic value of serum neuron-specific enolase in traumatic brain injury: Systematic review and meta-analysis. PLoS ONE 2014, 9, e106680. [Google Scholar] [CrossRef]
- Amacher, S.A.; Blatter, R.; Briel, M.; Appenzeller-Herzog, C.; Bohren, C.; Becker, C.; Beck, K.; Gross, S.; Tisljar, K.; Sutter, R.; et al. Predicting neurological outcome in adult patients with cardiac arrest: Systematic review and meta-analysis of prediction model performance. Crit. Care 2022, 26, 382. [Google Scholar] [CrossRef]
- Herrmann, M.; Ebert, A.D.; Galazky, I.; Wunderlich, M.T.; Kunz, W.S.; Huth, C. Neurobehavioral outcome prediction after cardiac surgery: Role of neurobiochemical markers of damage to neuronal and glial brain tissue. Stroke 2000, 31, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Kimura, F.; Kadohama, T.; Kitahara, H.; Ise, H.; Nakanishi, S.; Akasaka, N.; Kamiya, H. Serum Neuron-Specific Enolase Level as Predictor of Neurologic Outcome after Aortic Surgery. Thorac. Cardiovasc. Surg. 2020, 68, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Wu, F.; Liu, Y.; Yang, Y.; Chen, W.; Pan, Z.; Hu, W.; Zheng, F.; He, H. Relationship between postoperative biomarkers of neuronal injury and postoperative cognitive dysfunction: A meta-analysis. PLoS ONE 2023, 18, e0284728. [Google Scholar] [CrossRef] [PubMed]
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef]
- Maki, Y.; Yamaguchi, T.; Yamagami, T.; Murai, T.; Hachisuka, K.; Miyamae, F.; Ito, K.; Awata, S.; Ura, C.; Takahashi, R.; et al. The impact of subjective memory complaints on quality of life in community-dwelling older adults. Psychogeriatrics 2014, 14, 175–181. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Humpstone, H.J. Memory Span Tests. Psychol. Clin. 1919, 12, 196–200. [Google Scholar]
- Tobar, E.; Romero, C.; Galleguillos, T.; Fuentes, P.; Cornejo, R.; Lira, M.T.; de la Barrera, L.; Sanchez, J.E.; Bozan, F.; Bugedo, G.; et al. Confusion Assessment Method for diagnosing delirium in ICU patients (CAM-ICU): Cultural adaptation and validation of the Spanish version. Med. Intensiv. 2010, 34, 4–13. [Google Scholar] [CrossRef]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Ghanem, A.; Muller, A.; Sinning, J.M.; Kocurek, J.; Becker, B.V.; Vogel, M.; Vasa-Nicotera, M.; Hammerstingl, C.; Schwab’, J.O.; Nähle’, C.P.; et al. Prognostic value of cerebral injury following transfemoral aortic valve implantation. EuroIntervention 2013, 8, 1296–1306. [Google Scholar] [CrossRef]
- Deiner, S.; Silverstein, J.H. Postoperative delirium and cognitive dysfunction. Br. J. Anaesth. 2009, 103 (Suppl. S1), i41–i46. [Google Scholar] [CrossRef]
- Devlin, J.W.; Skrobik, Y.; Gelinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Yokoyama, K.; Yamauchi, N.; Koizumi, M.; Harasawa, N.; Yasuda, T.; Mimura, C.; Igita, H.; Suzuki, E.; Uchiide, Y.; et al. Sensitivity and Specificity of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and the Intensive Care Delirium Screening Checklist (ICDSC) for Detecting Post-Cardiac Surgery Delirium: A Single-Center Study in Japan. Heart Lung 2016, 45, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Bula, C.J.; Muller, O.; Krief, H.; Monney, P. Delirium in older patients undergoing aortic valve replacement: Incidence, predictors, and cognitive prognosis. BMC Geriatr. 2021, 21, 153. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Hu, J.; Ma, D. Postoperative delirium: Perioperative assessment, risk reduction, and management. Br. J. Anaesth. 2020, 125, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Gailiusas, M.; Andrejaitiene, J.; Sirvinskas, E.; Krasauskas, D.; Svagzdiene, M.; Kumpaitiene, B. Association between serum biomarkers and postoperative delirium after cardiac surgery. Acta Med. Litu. 2019, 26, 8–10. [Google Scholar] [CrossRef][Green Version]
- Mietani, K.; Hasegawa-Moriyama, M.; Inoue, R.; Ogata, T.; Shimojo, N.; Kurano, M.; Sumitani, M.; Uchida, K. Elevated neuron-specific enolase level is associated with postoperative delirium and detection of phosphorylated neurofilament heavy subunit: A prospective observational study. PLoS ONE 2021, 16, e0259217. [Google Scholar] [CrossRef]
- Burkhart, C.S.; Birkner-Binder, D.; Gagneux, A.; Berres, M.; Strebel, S.P.; Monsch, A.U.; Steiner, L.A. Evaluation of a summary score of cognitive performance for use in trials in perioperative and critical care. Dement. Geriatr. Cogn. Disord. 2011, 31, 451–459. [Google Scholar] [CrossRef]
- Khan, M.M.; Herrmann, N.; Gallagher, D.; Gandell, D.; Fremes, S.E.; Wijeysundera, H.C.; Radhakrishnan, S.; Sun, Y.R.; Lanctôt, K.L. Cognitive Outcomes after Transcatheter Aortic Valve Implantation: A Metaanalysis. J. Am. Geriatr. Soc. 2018, 66, 254–262. [Google Scholar] [CrossRef]
- Lai, K.S.; Herrmann, N.; Saleem, M.; Lanctot, K.L. Cognitive Outcomes following Transcatheter Aortic Valve Implantation: A Systematic Review. Cardiovasc. Psychiatry Neurol. 2015, 2015, 209569. [Google Scholar] [CrossRef]
- Talbot-Hamon, C.; Afilalo, J. Cognitive Function after Transcatheter Aortic Valve Replacement: Reassuring Findings for Now. J. Am. Geriatr. Soc. 2018, 66, 227–228. [Google Scholar] [CrossRef]
- Shen, J.; Sherman, M.; Souza, P.E. Test Administration Methods and Cognitive Test Scores in Older Adults with Hearing Loss. Gerontology 2020, 66, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, J.G.; Yao, W.X.; Tian, X.; Lv, S.P.; Zhang, T.Y.; Jin, S.H.; Bai, Y.J.; Ma, H. Systematic review and meta-analysis of the efficacy of serum neuron-specific enolase for early small cell lung cancer screening. Oncotarget 2017, 8, 64358–64372. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Zwetsloot, P.P.; Voskuil, M.; Stella, P.; Leiner, T.; Kraaijeveld, A. Clinically Significant Incidental Findings on CT Imaging during TAVI Work-up: A Systematic Review and Meta-Analysis. J. Invasive Cardiol. 2022, 34, E218–E225. [Google Scholar] [PubMed]
- Chung-Esaki, H.M.; Mui, G.; Mlynash, M.; Eyngorn, I.; Catabay, K.; Hirsch, K.G. The neuron specific enolase (NSE) ratio offers benefits over absolute value thresholds in post-cardiac arrest coma prognosis. J. Clin. Neurosci. 2018, 57, 99–104. [Google Scholar] [CrossRef]
- Zandbergen, E.G.; Hijdra, A.; Koelman, J.H.; Hart, A.A.; Vos, P.E.; Verbeek, M.M.; de Haan, R.J.; Group, P.S. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology 2006, 66, 62–68. [Google Scholar] [CrossRef]
| With POD (n = 6) | Without POD (n = 135) | Overall (n = 141) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 79 (72.0–84.0) | 82 (78.0–85.0) | 82 (77.5–85.0) | 0.33 |
| Female | 3 (50%) | 57 (42.2%) | 60 (42.6%) | 0.70 |
| Body mass index, kg/m2 | 29.0 (24.3–34.4) | 27.0 (24.1–31.0) | 27.0 (24.2–31.0) | 0.43 |
| Smoker | 0 (0%) | 16 (11.9%) | 16 (12.8%) | 0.49 |
| EuroScore II (%) | 6.5 (4.0–15.1) | 8.0 (5.2–4.0) | 8.0 (5.1–13.9) | 0.50 |
| Education in years | 8.0 (8.0–13.5) | 10.0 (8.0–14.0) | 10 (8.0–13.5) | 0.42 |
| Comorbities | ||||
| Arterial hypertension | 5 (83.3%) | 109 (80.5%) | 114 (80.9%) | >0.99 |
| Coronary heart disease | 6 (100%) | 85 (63.0%) | 91 (64.5%) | 0.09 |
| Atrial fibrillation | 2 (33.3%) | 46 (34.1%) | 48 (34.0%) | >0.99 |
| Peripheral arterial occlusive disease | 0 (0%) | 11 (8.1%) | 11 (7.8%) | >0.99 |
| Previous myocardial infarction (6 months) | 1 (16.7%) | 12 (8.9%) | 13 (9.2%) | 0.45 |
| Stroke in history | 0 (0%) | 9 (6.7%) | 9 (6.4%) | >0.99 |
| Acute decompensated heart failure | 0 (0%) | 5 (3.7%) | 5 (3.5%) | >0.99 |
| Chronic kidney disease | 4 (66.7%) | 50 (37.0%) | 54 (38.3%) | 0.21 |
| Hypertensive heart disease | 1 (16.7%) | 17 (12.6%) | 18 (12.8%) | 0.57 |
| Hyperlipoproteinemia | 5 (83.3%) | 58 (43.0%) | 63 (44.7%) | 0.09 |
| Diabetes type II (Insulin) | 3 (50%) | 30 (22.2%) | 33 (23.4%) | 0.14 |
| Asthma | 0 (0%) | 6 (4.4%) | 6 (4.3%) | >0.99 |
| COPD | 0 (0%) | 14 (10.4%) | 14 (9.9%) | >0.99 |
| Previous PTCA | 5 (83.3%) | 48 (35.6%) | 53 (37.6%) | 0.03 * |
| Previous coronary artery bypass grafting | 1 (16.7%) | 13 (9.6%) | 14 (9.9%) | 0.47 |
| Cardiac device | 2 (33.3%) | 14 (10.4%) | 16 (11.3%) | 0.14 |
| New York Heart Association Classification (NYHA) (n = 132) | ||||
| NYHA-Classification I | 0 (0%) | 2 (1.6%) | 2 (1.5%) | 0.38 |
| NYHA-Classification II | 0 (0%) | 28 (22.2%) | 28 (21%) | |
| NYHA-Classification III | 5 (83.3%) | 90 (71.4%) | 95 (71.3%) | |
| NYHA-Classification IV | 1 (16.7%) | 6 (4.8%) | 7 (5.3%) | |
| Laboratory parameters before TAVR | ||||
| Serum creatinine, µmol/L | 139.0 (108.3–199.3) | 87.0 (72.0–114.5) | 90.0 (73.0–116.0) | 0.003 * |
| eGFR, mL/min | 38.5 (23.0–46.8) | 63.0 (47.0–81.5) | 62.0 (46.0–80.0) | 0.003 * |
| NT-proBNP, pg/mL | 11,486 (2161–22,900) | 1343 (714–2959) | 1385 (751–3502) | 0.06 |
| Hemoglobin, mmol/L | 8.2 (7.1–8.8) | 8.1 (7.2–8.6) | 8.1 (7.2–8.6) | 0.77 |
| Serum NSE, ng/mL | 2.50 (1.95–4.33) | 1.78 (1.25–2.44) | 1.85 (1.30–2.53) | 0.04 * |
| Echocardiographic parameters before TAVR | ||||
| LVEF, % | 40 (35–40) | 45 (40–60) | 55 (40–60) | 0.15 |
| TAPSE, mm | 18 (17–21) | 21 (17–24) | 21 (17–24) | 0.36 |
| With POD (n = 6) | Without POD (n = 135) | Overall (n = 141) | p-Value | |
|---|---|---|---|---|
| Procedural Characteristics | ||||
| Days to TAVI | 3 (2–7) | 4 (2–6) | 4 (2–6) | 0.82 |
| Valve type (n = 136) | ||||
| Evolut R Evolut Pro Sapien 3 ** | 2 (33.3%) 0 (0%) 4 (66.6%) | 81 (62.3%) 25 (19.2%) 24 (18.5%) | 83 (58.9%) 25 (17.7%) 28 (19.9%) | 0.03 * |
| Balloon-expanding valve | 4/6 (66.6%) | 25/134 (18.7%) | 29 (20.7%) | 0.02 * |
| Self-expanding valve | 2/2 (33.3%) | 109/134 (81.3%) | 111 (79.3%) | 0.02 * |
| Predilatation | 4/6 (66.6%) | 92/134 (68.7%) | 96 (68.6%) | >0.99 |
| Postdilation | 0/6 (0%) | 29/134 (21.6%) | 29 (20.7%) | 0.35 |
| Valve-in-valve | 0/6 (0%) | 6/134 (4.5%) | 6 (4.3%) | >0.99 |
| General anesthesia | 6/6 (100%) | 104/134 (77.6%) | 110 (78.6%) | 0.34 |
| Sedation | 0/6 (0%) | 30/134 (22.4) | 30 (21.4%) | 0.34 |
| Procedural time, min. | 47.0 (40.5–70.0) | 51.0 (43.0–62.3) | 51.0 (43.0–62.8) | 0.68 |
| Volume of contrast media, mL | 120 (97–134) | 113 (87–148) | 113 (87–148) | 0.77 |
| Amount of rapid ventricular pacings (RVPs) | 3 (2–5) | 3 (2–4) | 3 (2–4) | 0.56 |
| Cumulative RVP time, sec. | 52 (39–102) | 49.5 (32–67) | 50 (32–67) | 0.51 |
| Laboratory markers after TAVR | ||||
| Change in serum NSE, ng/mL ** | 1.4 (0.26–3.44) | 0.3 (0.05–0.81) | 0.3 (0.05–0.86) | 0.065 |
| Serum creatinine, µmol/L ** | 131.0 (114.0–323.5) | 79.0 (62.0–96.8) | 80.0 (62.0–98.0) | <0.001 * |
| eGFR, mL/min *** | 46.5 (33.0–55.5) | 73.0 (59.0–85.5) | 72.0 (56.5–82.0) | 0.009 * |
| NT-proBNP, pg/mL *** | 5194 (988–5194) | 833 (422–2023) | 893 (434–2215) | 0.062 |
| Hemoglobin, mmol/L *** | 6.7 (5.1–7.4) | 6.8 (6.0–7.6) | 6.8 (6.0–7.5) | 0.74 |
| Outcome | ||||
| Postoperative pacemaker implantation | 0 (0%) | 21 (15.6%) | 21 (14.9%) | 0.59 |
| Postoperative stroke | 1 (16.7%) | 3 (2.2%) | 4 (2.8%) | 0.16 |
| Bleeding requiring transfusion (n = 139) | 1 (16.7%) | 9 (6.8%) | 10 (7.2%) | 0.37 |
| Acute kidney injury (n = 95) | 2 (40%) | 16 (17.8%) | 18 (12,8%) | 0.24 |
| Hemodynamic relevant pericardial effusion | 1 (16.7%) | 0 (0%) | 1 (0.7%) | 0.04 * |
| Died during procedure | 0 (0%) | 0 (0%) | 0 (0%) | >0.99 |
| ICU admission (n = 134) | 1 (16.7%) | 6 (4.7%) | 7 (5.2%) | 0.28 |
| Died in hospital | 0 (0%) | 1 0(.7) | 1 (0.7%) | >0.99 |
| Length of stay in hospital, days | 15.0 (13/26) | 10 (8/13) | 10 (8/13) | 0.003 * |
| Discharge home | 3 (50%) | 122 (90.4%) | 125 (88,7%) | 0.02 * |
| Discharge rehabilitation | 2 (33.3%) | 3 (2.2%) | 5 (3,5%) | 0.01 * |
| Discharge to nursing home | 0 (0%) | 1 (0.7%) | 1 (0,7%) | >0.99 |
| Discharge to another hospital | 1 (16.7%) | 8 (5.9%) | 9 (6,4%) | 0.33 |
| Follow-Up | ||||
| Subjective feeling of no change in cognitive function | 1/1 (100%) | 28/114 (24.6%) | 87/116 (82.3%) | 0.44 |
| Died within 3 months | 0/5 (0%) | 3/128 (2.3%) | 3/133 (2.3%) | >0.99 |
| Rehospitalization within 3 months | 2/3 (66.7%) | 21/100 (21%) | 23/103 (22.3%) | 0.12 |
| Died within 6 months | 1/6 (16.7%) | 4/88 (4.5%) | 5/94 (5.4%) | 0.01 |
| With POD | Without POD | Overall | p-Value | |
|---|---|---|---|---|
| Preoperative memory function | ||||
| WL learning | n = 6 | n = 135 | n = 141 | |
| Number of recalled words | 13 ± 4.0 | 14 ± 3.8 | 14 ± 3.8 | 0.48 |
| z-value | −2.3 ± 1.6 | −1.4 ± 1.1 | −1.45 ± 1.1 | 0.08 |
| WL recall | n = 6 | n = 134 | n = 140 | |
| Number of recalled words | 2 ± 2.2 | 2 ± 2.2 | 2 ± 2.2 | 0.88 |
| z-value | −2.8 ± 1.3 | −2.3 ± 1.1 | −2.4 ± 1.1 | 0.48 |
| DST | n = 6 | n = 135 | n = 141 | |
| Digit span | 5 ± 0.8 | 5 ± 1.0 | 5 ± 1.0 | 0.11 |
| Pre-/postoperative difference scores (t1–t0) | ||||
| WL learning | n = 3 | n = 109 | n = 112 | |
| Difference score number of recalled words | 0 ± 5.0 | 2 ± 3.2 | 2 ± 3.2 | 0.66 |
| WL recall | n = 3 | n = 108 | n = 111 | |
| Difference score number of recalled words | 0 ± 0.6 | 0 ± 1.9 | 0 ± 1.9 | 0.25 |
| DST | n = 3 | n = 110 | n = 113 | |
| Difference score digit span | 0 ± 0.6 | 0 ± 0.9 | 0 ± 0.9 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nübel, J.; Buhre, C.; Hoffmeister, M.; Oess, S.; Labrenz, O.; Jost, K.; Hauptmann, M.; Schön, J.; Fritz, G.; Butter, C.; et al. Association between Neuron-Specific Enolase, Memory Function, and Postoperative Delirium after Transfemoral Aortic Valve Replacement. J. Cardiovasc. Dev. Dis. 2023, 10, 441. https://doi.org/10.3390/jcdd10110441
Nübel J, Buhre C, Hoffmeister M, Oess S, Labrenz O, Jost K, Hauptmann M, Schön J, Fritz G, Butter C, et al. Association between Neuron-Specific Enolase, Memory Function, and Postoperative Delirium after Transfemoral Aortic Valve Replacement. Journal of Cardiovascular Development and Disease. 2023; 10(11):441. https://doi.org/10.3390/jcdd10110441
Chicago/Turabian StyleNübel, Jonathan, Charlotte Buhre, Meike Hoffmeister, Stefanie Oess, Oliver Labrenz, Kerstin Jost, Michael Hauptmann, Julika Schön, Georg Fritz, Christian Butter, and et al. 2023. "Association between Neuron-Specific Enolase, Memory Function, and Postoperative Delirium after Transfemoral Aortic Valve Replacement" Journal of Cardiovascular Development and Disease 10, no. 11: 441. https://doi.org/10.3390/jcdd10110441
APA StyleNübel, J., Buhre, C., Hoffmeister, M., Oess, S., Labrenz, O., Jost, K., Hauptmann, M., Schön, J., Fritz, G., Butter, C., & Haase-Fielitz, A. (2023). Association between Neuron-Specific Enolase, Memory Function, and Postoperative Delirium after Transfemoral Aortic Valve Replacement. Journal of Cardiovascular Development and Disease, 10(11), 441. https://doi.org/10.3390/jcdd10110441





