Hedinger Syndrome—Lessons Learnt: A Single-Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Cohort
2.2. Perioperative Strategies
2.3. Surgical Methods
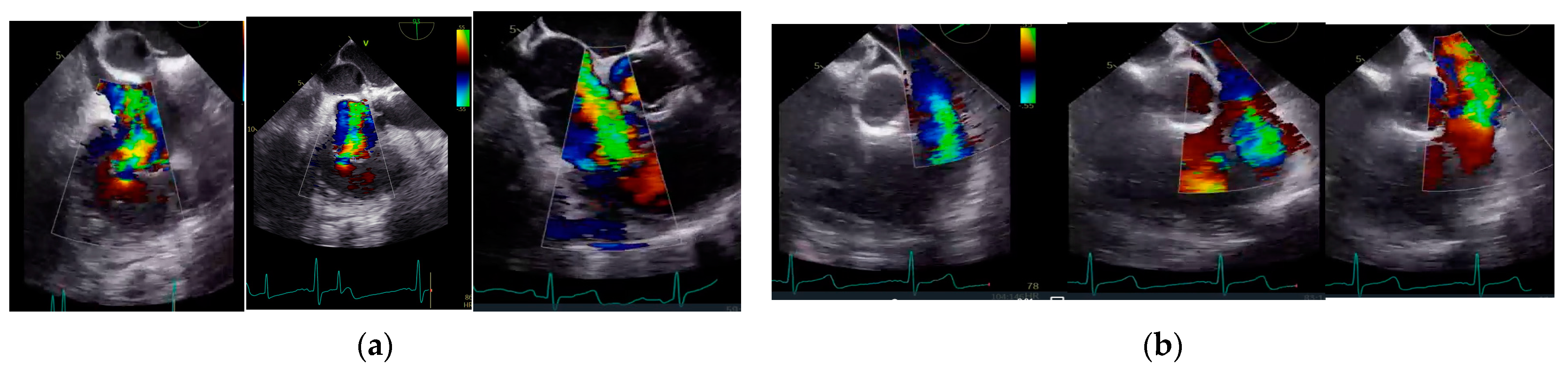
2.4. Echocardiographic Analysis
3. Results
3.1. Baseline, Procedural, and Clinical Characteristics
3.2. Surgery
3.3. Tricuspid Surgical Techniques through the Years
3.4. Additional Surgical Strategies
3.5. Clinical Outcomes and Follow Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| AKI | acute kidney injury |
| AVL | aortic valve lesion |
| CD | carcinoid disease |
| CBP | cardiopulmonary bypass |
| COPD | chronic obstructive pulmonary disease |
| CS | carcinoid syndrome |
| DM | diabetes mellitus |
| ENETS | European Neuroendocrine Tumor Society |
| GEP-NETs | gastroenteropancreatic NETs |
| HLP | hyperlipidaemia |
| IRVF | impaired right ventricular function |
| LV-EF | left ventricular ejection fraction |
| MVL | mitral valve lesion |
| NETs | neuroendocrine tumours |
| NYHA | New York Heart Association |
| PAH | pulmonary arterial hypertension |
| PCS | previous cardiac surgery |
| pNETs | pancreatic NETs |
| PFO | patent foramen ovale |
| PPI | permanent pacemaker implantation |
| PTR | pulmonary trunk reconstruction |
| PTL | primary tumour location |
| PV | pulmonary valve |
| PVL | pulmonary valve lesion |
| RD | renal disease |
| RVOTR | right ventricle outflow tract |
| TOE | transoesophageal echocardiogram |
| TVL | tricuspid valve lesion |
| TVI | tricuspid valve implantation |
| V-A ECMO | veno-arterial extracorporeal mechanical oxygenation |
| VHD | valvular heart disease |
| XCL | aortic cross-clamp time |
References
- Ghukasyan, H. Hedinger Syndrome: A Rare Cardiac Manifestation of Carcinoid Syndrome. Cureus 2022, 14, e26528. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Villanacci, V.; Ubiali, A. Biological and molecular aspects of gastroenteropancreatic neuroendocrine tumors. Digestion 2000, 62 (Suppl. S1), 19–26. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2011, 18 (Suppl. S1), S1–S16. [Google Scholar] [CrossRef] [PubMed]
- Halperin, D.M.; Shen, C.; Dasari, A.; Xu, Y.; Chu, Y.; Zhou, S.; Shih, Y.-C.T.; Yao, J.C. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: A population-based study. Lancet Oncol. 2017, 18, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Strosberg, J. An update on gastroenteropancreatic neuroendocrine tumors. Oncology 2014, 28, 749–756, 758. [Google Scholar]
- Grozinsky-Glasberg, S.; Grossman, A.B.; Gross, D.J. Carcinoid Heart Disease: From Pathophysiology to Treatment—’Something in the Way It Moves’. Neuroendocrinology 2015, 101, 263–273. [Google Scholar] [CrossRef]
- Grozinsky-Glasberg, S.; Davar, J.; Hofland, J.; Dobson, R.; Prasad, V.; Pascher, A.; Denecke, T.; Tesselaar, M.E.T.; Panzuto, F.; Albåge, A.; et al. European Neuroendocrine Tumor Society (ENETS) 2022 Guidance Paper for Carcinoid Syndrome and Carcinoid Heart Disease. J. Neuroendocrinol. 2022, 34, e13146. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Tajik, A.J.; Khandheria, B.K.; Seward, J.B.; Callahan, J.A.; Pitot, H.C.; Kvols, L.K. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation 1993, 87, 1188–1196. [Google Scholar] [CrossRef]
- Davar, J.; Connolly, H.M.; Caplin, M.E.; Pavel, M.; Zacks, J.; Bhattacharyya, S.; Cuthbertson, D.J.; Dobson, R.; Grozinsky-Glasberg, S.; Steeds, R.P.; et al. Diagnosing and Managing Carcinoid Heart Disease in Patients with Neuroendocrine Tumors: An Expert Statement. J. Am. Coll. Cardiol. 2017, 69, 1288–1304. [Google Scholar] [CrossRef]
- Feldman, J.M.; O’Dorisio, T.M. Role of neuropeptides and serotonin in the diagnosis of carcinoid tumors. Am. J. Med. 1986, 81, 41–48. [Google Scholar] [CrossRef]
- Janson, E.T.; Holmberg, L.; Stridsberg, M.; Eriksson, B.; Theodorsson, E.; Wilander, E.; Oberg, K. Carcinoid tumors: Analysis of prognostic factors and survival in 301 patients from a referral center. Ann. Oncol. 1997, 8, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Arghami, A.; Connolly, H.M.; Abel, M.D.; Schaff, H.V. Quadruple valve replacement in patients with carcinoid heart disease. J. Thorac. Cardiovasc. Surg. 2010, 140, 1432–1434. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.S.; Kong, G.; Ludhani, P.; Michael, M.; Morgan, J.; Hofman, M.S.; Hicks, R.J.; Larobina, M. Early Outcomes of Surgery for Carcinoid Heart Disease. Heart Lung Circ. 2020, 29, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.M.; Sousa, L.G.; Riechelmann, R.P. Complications from carcinoid syndrome: Review of the current evidence. Ecancermedicalscience 2016, 10, 662. [Google Scholar] [CrossRef]
- Connolly, H.M.; Nishimura, R.A.; Smith, H.C.; Pellikka, P.A.; Mullany, C.J.; Kvols, L.K. Outcome of cardiac surgery for carcinoid heart disease. J. Am. Coll. Cardiol. 1995, 25, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Kidd, M.; Latich, I.; Zikusoka, M.N.; Shapiro, M.D. Current Status of Gastrointestinal Carcinoids. Gastroenterology 2005, 128, 1717–1751. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.J.; Khattar, R.S. Carcinoid heart disease: Presentation, diagnosis, and management. Heart 2004, 90, 1224–12288. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Toumpanakis, C.; Chilkunda, D.; Caplin, M.E.; Davar, J. Risk Factors for the Development and Progression of Carcinoid Heart Disease. Am. J. Cardiol. 2011, 107, 1221–1226. [Google Scholar] [CrossRef]
- Zuetenhorst, J.M.; Bonfrer, J.M.G.M.; Korse, C.M.; Bakker, R.; van Tinteren, H.; Taal, B.G. Carcinoid heart disease: The role of urinary 5-hydroxyindoleacetic acid excretion and plasma levels of atrial natriuretic peptide, transforming growth factor-beta and fibroblast growth factor. Cancer 2003, 97, 1609–1615. [Google Scholar] [CrossRef]
- Pandya, U.H.; Pellikka, P.A.; Enriquez-Sarano, M.; Edwards, W.D.; Schaff, H.V.; Connolly, H.M. Metastatic carcinoid tumor to the heart: Echocardiographic-pathologic study of 11 patients. J. Am. Coll. Cardiol. 2002, 40, 1328–1332. [Google Scholar] [CrossRef]
- Silaschi, M.; Barr, J.; Chaubey, S.; Nicou, N.; Srirajaskanthan, R.; Byrne, J.; Ramage, J.; MacCarthy, P.; Wendler, O. Optimized Outcomes Using a Standardized Approach for the Treatment of Patients with Carcinoid Heart Disease. Neuroendocrinology 2017, 104, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Connolly, H.M.; Schaff, H.V.; Abel, M.D.; Rubin, J.; Askew, J.W.; Li, Z.; Inda, J.J.; Luis, S.A.; Nishimura, R.A.; Pellikka, P.A. Early and Late Outcomes of Surgical Treatment in Carcinoid Heart Disease. J. Am. Coll. Cardiol. 2015, 66, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Honan, K.A.; Hassan, S.; Deswal, A.; Herrmann, J.; Song, J.; Monlezun, D.; Halperin, D.; Mahvash, A.; Dasari, A.; Koutroumpakis, E.; et al. Bioprosthetic valve monitoring in patients with carcinoid heart disease. Front. Cardiovasc. Med. 2022, 9, 1072890. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, G.; Caplin, M.; Davies, P.; Ferone, D.; Garcia-Carbonero, R.; Grozinsky-Glasberg, S.; Hörsch, D.; Janson, E.T.; Kianmanesh, R.; Kos-Kudla, B.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pre- and Perioperative Therapy in Patients with Neuroendocrine Tumors. Neuroendocrinology 2017, 105, 245–254. [Google Scholar] [CrossRef]
- Manoly, I.; McAnelly, S.-L.; Sriskandarajah, S.; McLaughlin, K.E. Prognosis of patients with carcinoid heart disease after valvular surgery. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 302–305. [Google Scholar] [CrossRef]
- Møller, J.E.; Pellikka, P.A.; Bernheim, A.M.; Schaff, H.V.; Rubin, J.; Connolly, H.M. Prognosis of carcinoid heart disease: Analysis of 200 cases over two deCVDes. Circulation 2005, 112, 3320–3327. [Google Scholar] [CrossRef]
- Mokhles, P.; van Herwerden, L.A.; de Jong, P.L.; de Herder, W.W.; Siregar, S.; Constantinescu, A.A.; van Domburg, R.T.; Roos-Hesselink, J.W. Carcinoid heart disease: Outcomes after surgical valve replacement. Eur. J. Cardiothorac. Surg. 2012, 41, 1278–1283. [Google Scholar] [CrossRef]
- Castillo, J.G.; Filsoufi, F.; Rahmanian, P.B.; Anyanwu, A.; Zacks, J.S.; Warner, R.R.; Adams, D.H. Early and late results of valvular surgery for carcinoid heart disease. J. Am. Coll. Cardiol. 2008, 51, 1507–1509. [Google Scholar] [CrossRef]
- Robiolio, P.A.; Rigolin, V.H.; Harrison, J.K.; Lowe, J.E.; Moore, J.O.; Bashore, T.M.; Feldman, J.M. Predictors of outcome of tricuspid valve replacement in carcinoid heart disease. Am. J. Cardiol. 1995, 75, 485–488. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Raja, S.G.; Toumpanakis, C.; Caplin, M.E.; Dreyfus, G.D.; Davar, J. Outcomes, risks and complications of cardiac surgery for carcinoid heart disease. Eur. J. Cardiothorac. Surg. 2011, 40, 168–172. [Google Scholar] [CrossRef]
- El Gabry, M.; Shehada, S.-E.; Mourad, F.; Ruhparwar, A.; Lahner, H.; Dirkmann, D.; Thielmann, M.; Jakob, H.; Wendt, D. Hedinger syndrome: First experience and two-year follow-up in patients with carcinoid heart disease. J. Thorac. Dis. 2019, 11, 3234–3240. [Google Scholar] [CrossRef] [PubMed]



| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||||
| Age, years | 41 | 60 | 49 | 65 | 54 | 54 | 79 | 74 | 67 | 45 | 55 |
| Female gender | Yes | No | Yes | No | No | No | Yes | Yes | Yes | No | No |
| PTL | SI | SI | SI | SI | SI | SI | SI | SI | SI | SI | SI |
| Metastasis | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| COPD | No | No | No | Yes | Yes | No | Yes | Yes | No | No | No |
| Hypertension | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| AF | No | Yes | No | No | No | No | No | No | No | No | No |
| DM Type II | Yes | No | Yes | No | No | No | Yes | Yes | No | Yes | No |
| HLP | No | Yes | No | No | No | Yes | Yes | Yes | No | No | No |
| RD (>200 µmol/L) | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| EuroSCOREII (%) | 3.7 | 4.3 | 8.7 | 36.1 | 6.1 | 2.4 | 29.8 | 18.1 | 6.4 | 8.6 | 5.4 |
| NYHA | III | IV | III | IV | III | IV | III | IV | IV | IV | IV |
| PCS | No | No | No | Yes | No | No | No | No | No | No | No |
| PAH | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Preoperative echocardiographic findings | |||||||||||
| LV-EF (%) | 55 | 57 | 45 | 40 | 45 | 50 | 55 | 50 | 50 | 50 | 55 |
| TVL | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| PVL | Yes | Yes | Yes | No | No | Yes | No | No | Yes | Yes | No |
| MVL | No | No | No | Yes | No | No | No | No | No | No | No |
| AVL | No | No | No | Yes | Yes | No | No | No | No | No | Yes |
| PFO | No | Yes | No | No | No | No | No | No | No | Yes | Yes |
| IRVF | Moderate | Moderate | Moderate | Moderate | Moderate | Severe | Moderate | Severe | No | Severe | No |
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Valvular Lesions Before Surgery | |||||||||||
| TV | Combined | Combined | Combined | Severe Regurgitation | Combined | Combined | Severe Regurgitation | Severe Regurgitation | Severe Regurgitation | Combined | Combined |
| PV | Combined | Combined | Severe Regurgitation | No | No | Combined | No | No | Severe Regurgitation | No | No |
| MV | No | No | No | Severe Regurgitation | No | No | No | No | No | No | No |
| AV | No | No | No | Severe regurgitation | Severe regurgitation | No | No | No | No | No | Severe Regurgitation |
| Surgical Strategy | |||||||||||
| TV | Replacement | Replacement | Replacement | Repair | Repair | Replacement | Repair | Implantation | Implantation | Implantation | Implantation |
| PV | Replacement | Replacement | Replacement | No | No | Replacement | No | No | Implantation | No | No |
| MV | No | No | No | Repair | No | No | No | No | No | No | No |
| AV | No | No | No | suAVR | suAVR | No | No | No | No | No | suAVR |
| RVOTR | No | No | No | No | No | No | No | No | Yes | Yes | No |
| PTR | No | No | Yes | No | No | Yes | No | No | No | Yes | No |
| PFO closure | No | Yes | No | Yes | No | No | No | No | Yes | Yes | |
| Assist Device needed | No | No | No | No | No | No | No | Yes; v.a.ECMO | No | Yes; TandemHeart | No |
| XCL, min | 95 | 78 | 79 | 74 | 90 | 124 | 0 | 0 | 95 | 107 | 84 |
| CPB time, min | 131 | 120 | 146 | 101 | 123 | 256 | 74 | 87 | 118 | 151 | 90 |
| Variable | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AV Block III | Yes | No | No | No | No | No | No | No | No | No | No |
| PPI | Yes | No | No | No | No | No | No | No | No | No | No |
| AKNI (Dialysis) | No | Yes | Yes | Yes | No | Yes | No | Yes | No | Yes | No |
| Delir | No | No | No | Yes | No | No | No | No | No | No | No |
| Redo (Major Bleeding) | No | Yes | No | No | No | No | No | No | No | Yes | No |
| Pneumonia | No | No | No | No | No | No | No | No | No | Yes | No |
| Sepsis | No | Yes | No | No | No | No | No | No | No | Yes | No |
| 30-day Survival | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Re-Do (Heart Valve Lesion) | No | No | No | No | Yes, Recurrent TV stenosis | No | Yes, Recurrent TV stenosis | No | No | No | No |
| Status at Last Follow up | Dead | Dead | Dead | Alive, NYHA I–II | Dead | Dead | Dead | Dead | Alive, NYHA I–II | Dead | Alive |
| Survival * | 15 Months | 1 Day | 18 Months | 72 Months, Alive | 60 Months | 4 Months | 6 Months | 4 Days | 50 Months, Alive | 1.5 Month | 1.5 Month, Alive |
| Cause of death | Carcinoid Disease | Cardiogenic Shock | Accident | Carcinoid Disease | Carcinoid Disease | Accident | Carcinoid Disease | Carcinoid Crisis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Gabry, M.; Arends, S.; Shehada, S.-E.; Lahner, H.; Kamler, M.; Wendt, D.; Spetsotaki, K. Hedinger Syndrome—Lessons Learnt: A Single-Center Experience. J. Cardiovasc. Dev. Dis. 2023, 10, 413. https://doi.org/10.3390/jcdd10100413
El Gabry M, Arends S, Shehada S-E, Lahner H, Kamler M, Wendt D, Spetsotaki K. Hedinger Syndrome—Lessons Learnt: A Single-Center Experience. Journal of Cardiovascular Development and Disease. 2023; 10(10):413. https://doi.org/10.3390/jcdd10100413
Chicago/Turabian StyleEl Gabry, Mohamed, Sven Arends, Sharaf-Eldin Shehada, Harald Lahner, Markus Kamler, Daniel Wendt, and Konstantina Spetsotaki. 2023. "Hedinger Syndrome—Lessons Learnt: A Single-Center Experience" Journal of Cardiovascular Development and Disease 10, no. 10: 413. https://doi.org/10.3390/jcdd10100413
APA StyleEl Gabry, M., Arends, S., Shehada, S.-E., Lahner, H., Kamler, M., Wendt, D., & Spetsotaki, K. (2023). Hedinger Syndrome—Lessons Learnt: A Single-Center Experience. Journal of Cardiovascular Development and Disease, 10(10), 413. https://doi.org/10.3390/jcdd10100413








