Increased Proteoglycanases in Pulmonary Valves after Birth Correlate with Extracellular Matrix Maturation and Valve Sculpting
Abstract
1. Introduction
2. Materials and Methods
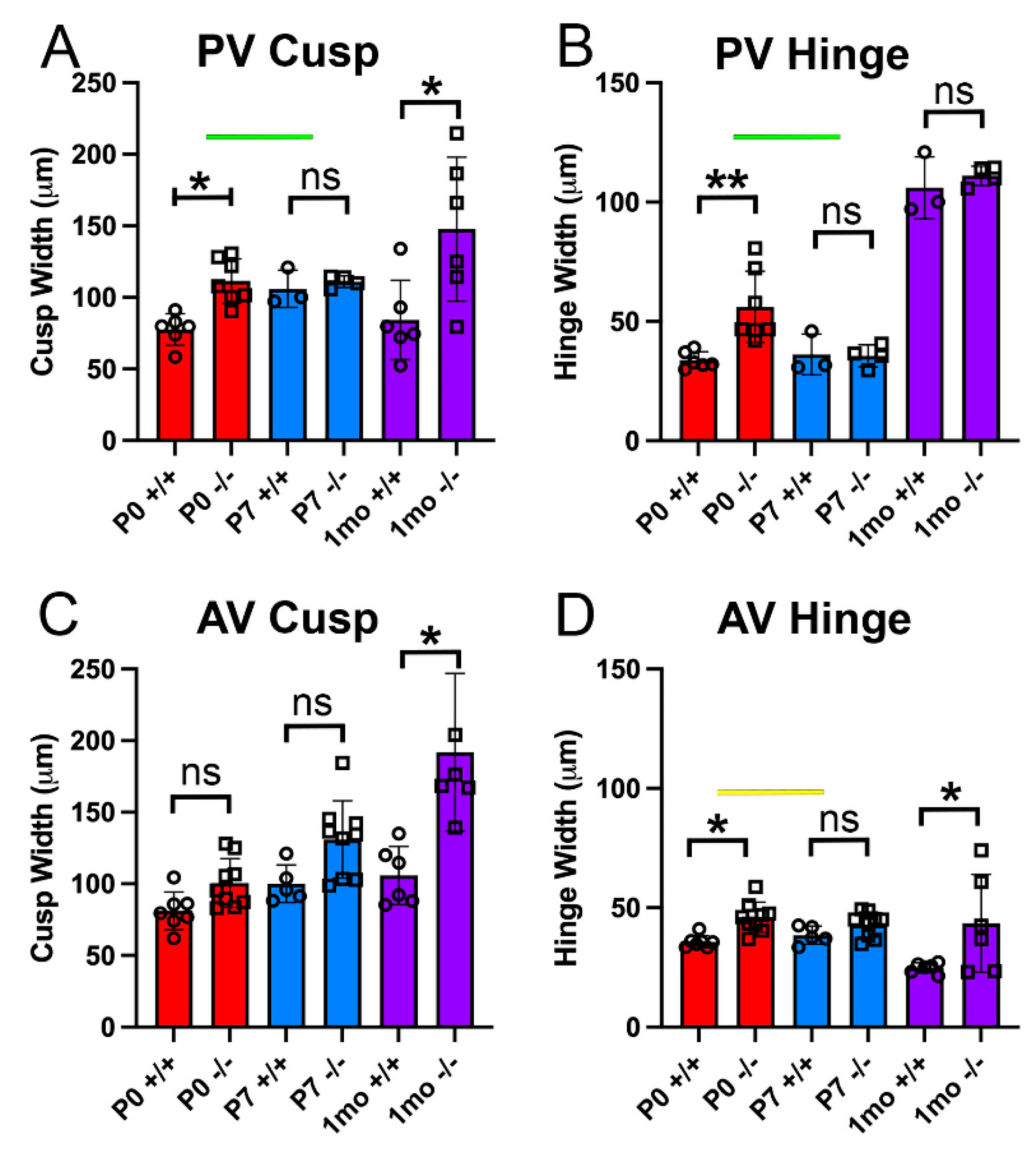
2.1. Valve Cusp and Hinge Quantification
2.2. Immunohistochemistry
2.3. Statistics
3. Results
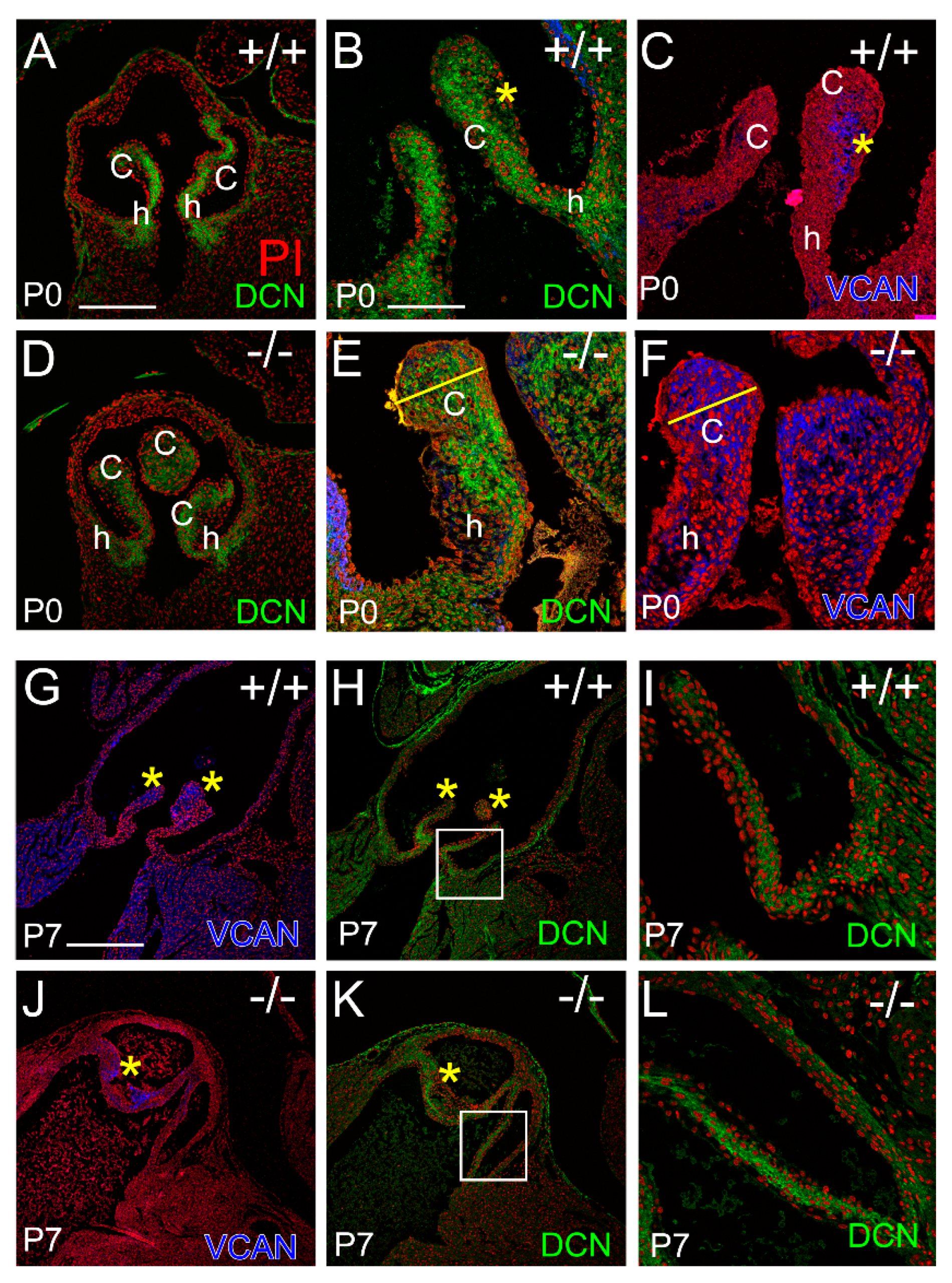
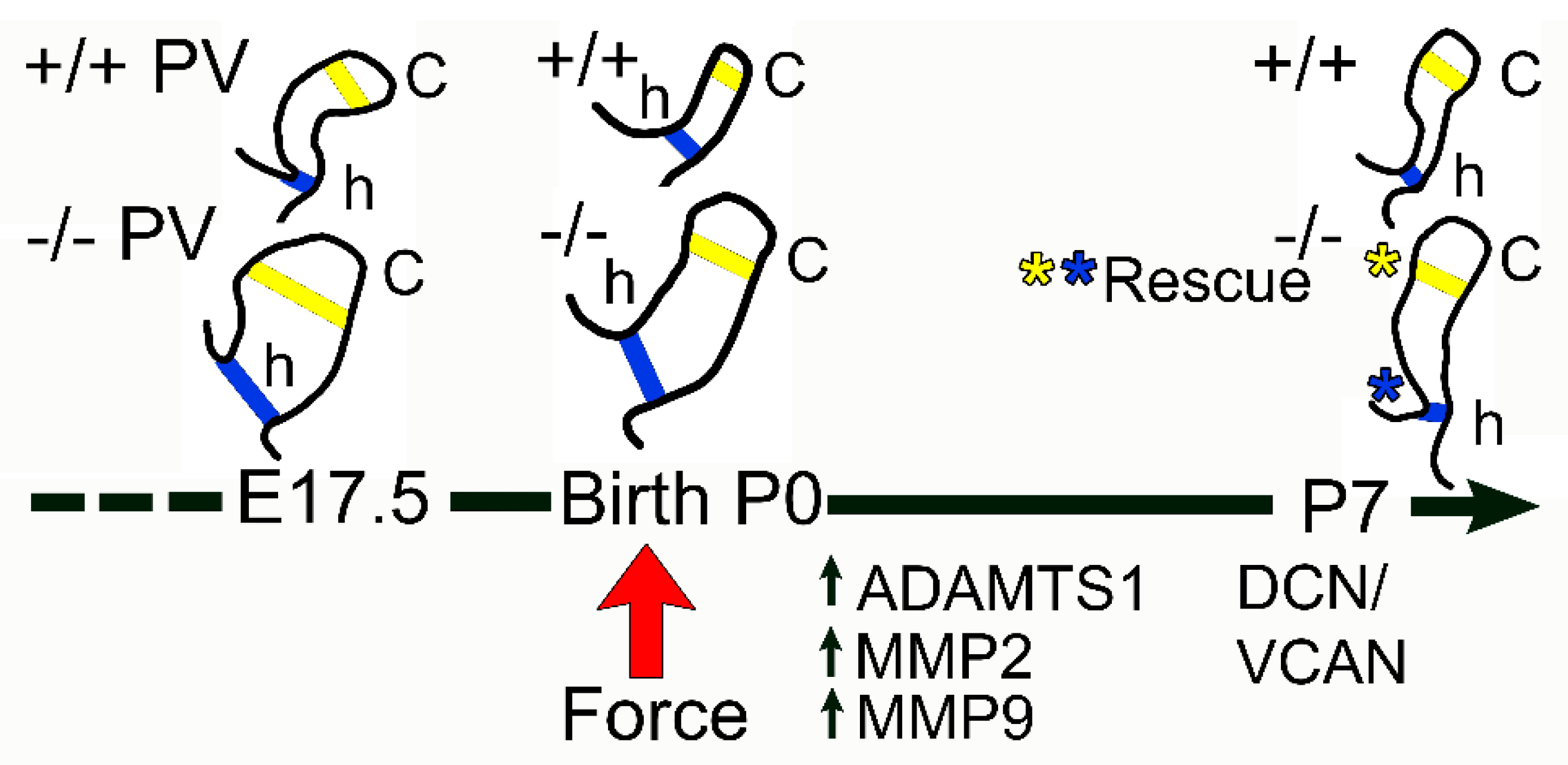
3.1. The Pulmonary Valves of Adamts5−/− Mice Exhibited a Transient Phenotypic Rescue at Postnatal Day 7
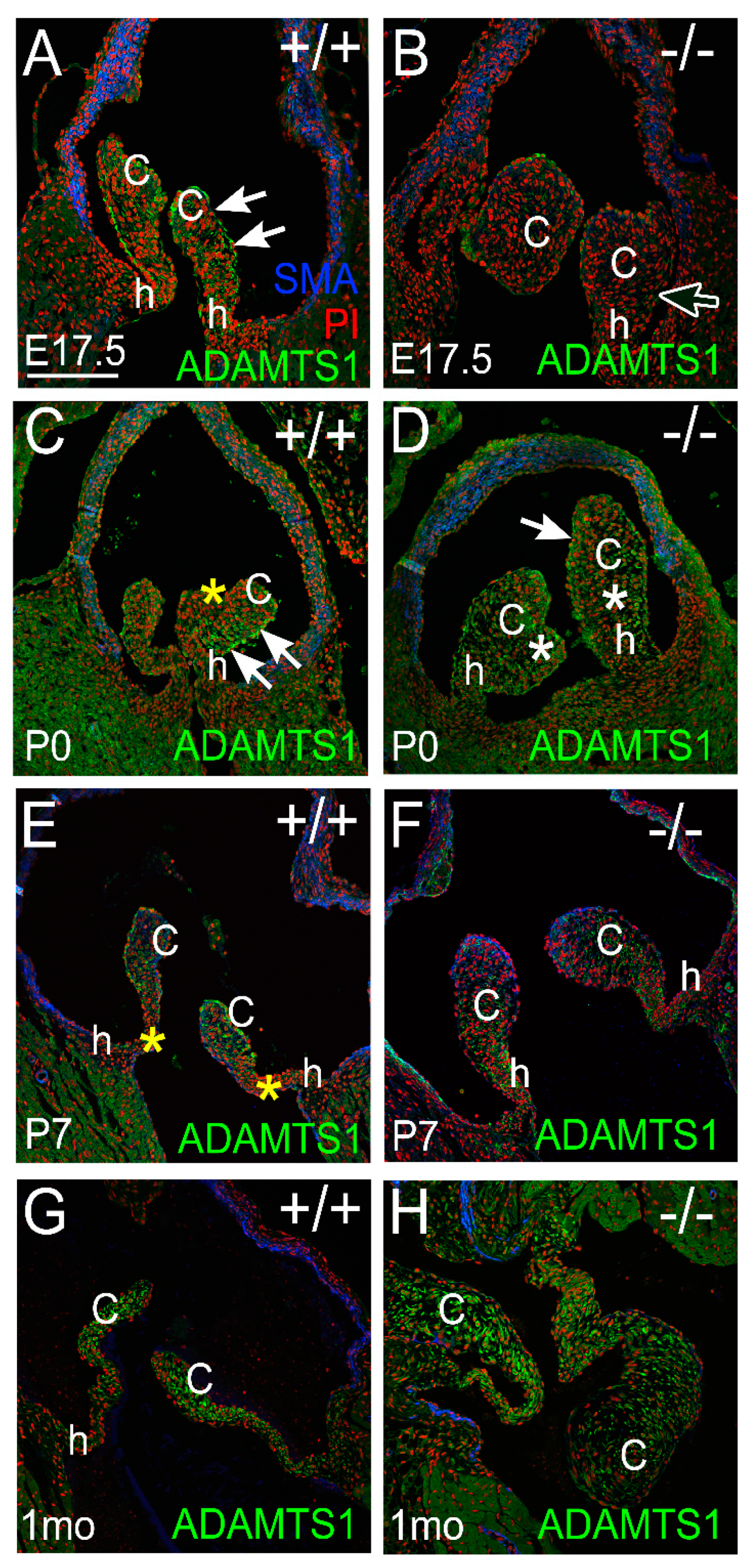
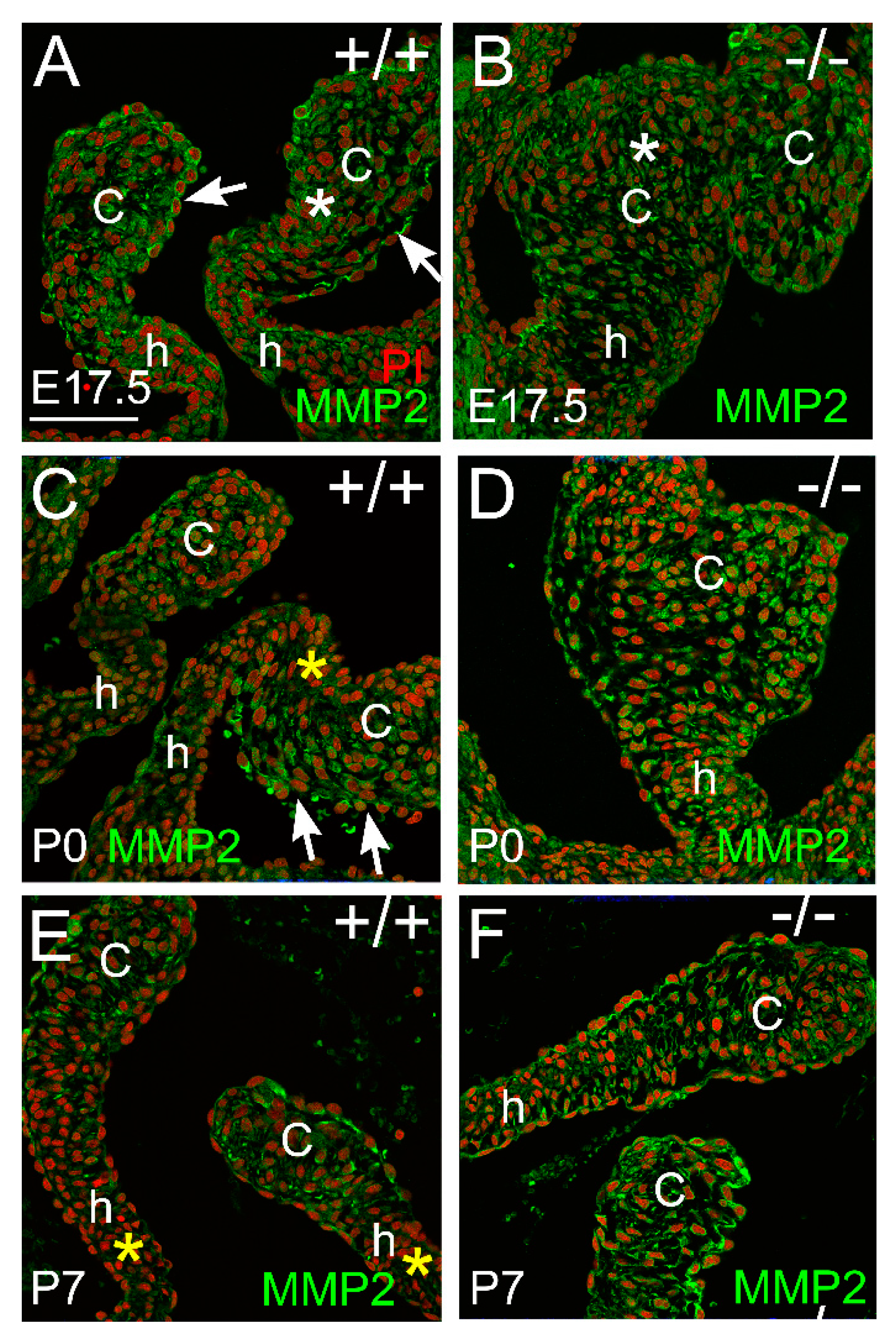
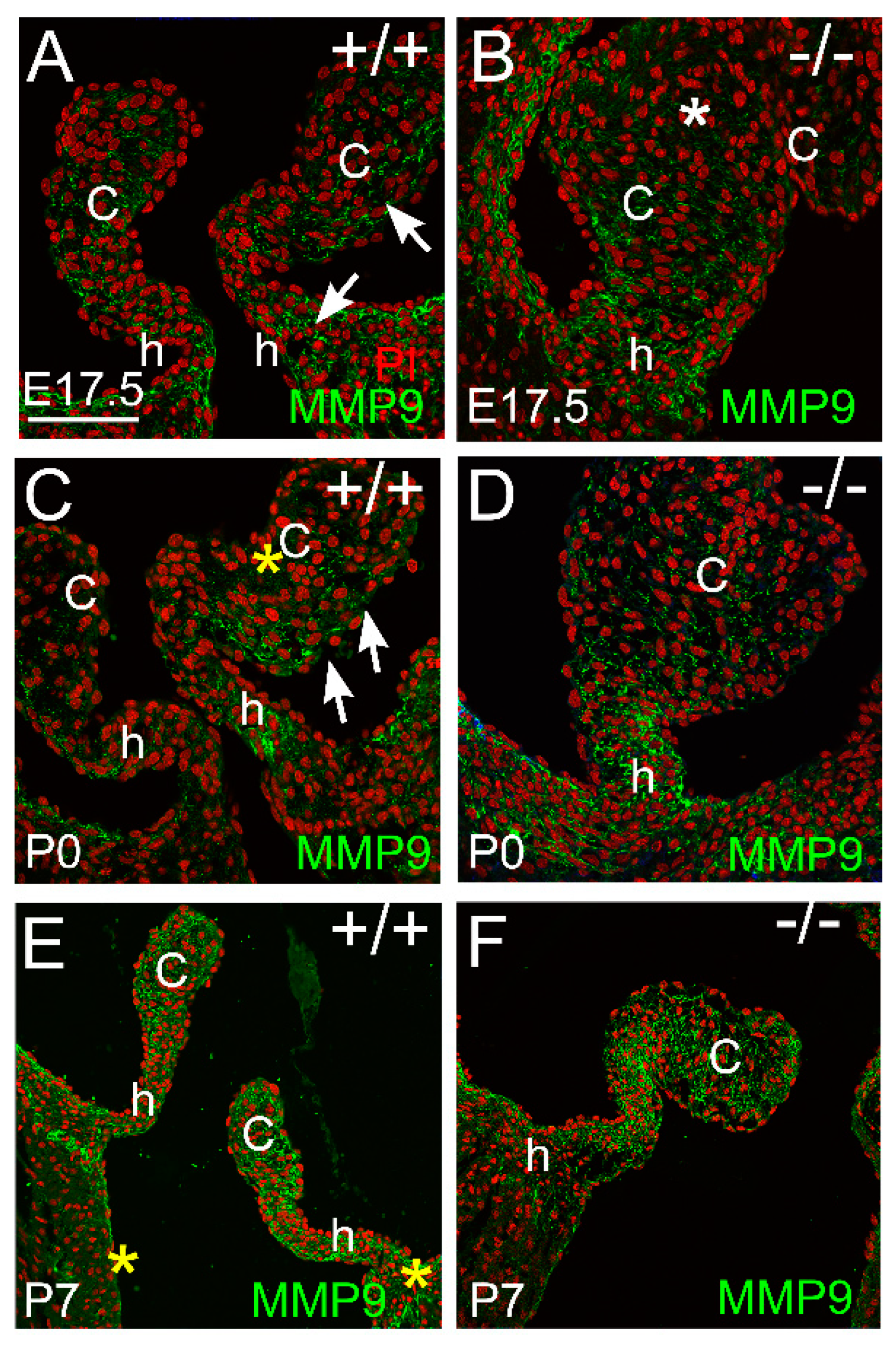
3.2. Proteoglycan Proteases ADAMTS1, MMP2, and MMP9 Were Present in Postnatal Cusp and Hinge Regions of the Pulmonary Valve
3.2.1. ADAMTS1 Is the Closest Family Member to ADAMTS5, Exhibits Catalytic Activity to VCAN, and May Compensate for the Loss of ADAMTS5 from P0 to P7
3.2.2. MMP2 Was Evident in the Pulmonary Valve and May Play a Role in Late-Staged Valve Maturation
3.2.3. MMP9 Was Localized in the Maturing Valve Cusps in Regions where the Mesenchymal Cells Were Highly Compacted
3.3. Localization of the Small Leucine-Rich Proteoglycan Decorin Was Overlapping with Versican at P0 in the Adamts5−/− Cusps, but at P7 DCN and VCAN Were Restricted to Separate Layers Consistent with ‘Phenotypic Rescue’
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, M.A.; Schaller, M.D.; Ginsberg, M.H. Integrins: Emerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 1995, 11, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Kodigepalli, K.M.; Thatcher, K.; West, T.; Howsmon, D.P.; Schoen, F.J.; Sacks, M.S.; Breuer, C.K.; Lincoln, J. Biology and Biomechanics of the Heart Valve Extracellular Matrix. J. Cardiovasc. Dev. Dis. 2020, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.; Yutzey, K.E. Mechanisms of heart valve development and disease. Development 2020, 147, dev183020. [Google Scholar] [CrossRef] [PubMed]
- Dye, B.; Lincoln, J. The Endocardium and Heart Valves. Cold Spring Harb. Perspect. Biol. 2020, 12, a036723. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Aggeler, J. Dynamic reciprocity: How do extracellular matrix and hormones direct gene expression? Prog. Clin. Biol. Res. 1987, 249, 251–262. [Google Scholar]
- Mead, T.J.; Bhutada, S.; Martin, D.R.; Apte, S.S. Proteolysis: A key post-translational modification regulating proteoglycans. Am. J. Physiol. Cell Physiol. 2022, 323, C651–C665. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, D.R.; Nelson, C.M.; Dixon, L.J.; Silver, D.L.; Wylie, J.D.; Lindner, V.; Sasaki, T.; Cooley, M.A.; Argraves, W.S.; Apte, S.S. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev. Cell 2009, 17, 687–698. [Google Scholar] [CrossRef]
- Miller, R.E.; Ishihara, S.; Tran, P.B.; Golub, S.B.; Last, K.; Miller, R.J.; Fosang, A.J.; Malfait, A.M. An aggrecan fragment drives osteoarthritis pain through Toll-like receptor 2. J. Clin. Investig. 2018, 3, 1–9. [Google Scholar] [CrossRef]
- Lees, S.; Golub, S.B.; Last, K.; Zeng, W.; Jackson, D.C.; Sutton, P.; Fosang, A.J. Bioactivity in an Aggrecan 32-mer Fragment Is Mediated via Toll-like Receptor 2. Arthritis Rheumatol. 2015, 67, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Sandy, J.D.; Westling, J.; Kenagy, R.D.; Iruela-Arispe, M.L.; Verscharen, C.; Rodriguez-Mazaneque, J.C.; Zimmermann, D.R.; Lemire, J.M.; Fischer, J.W.; Wight, T.N.; et al. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J. Biol. Chem. 2001, 276, 13372–13378. [Google Scholar] [CrossRef] [PubMed]
- Fosang, A.J.; Last, K.; Gardiner, P.; Jackson, D.C.; Brown, L. Development of a cleavage-site-specific monoclonal antibody for detecting metalloproteinase-derived aggrecan fragments: Detection of fragments in human synovial fluids. Biochem. J. 1995, 310 Pt 1, 337–343. [Google Scholar] [CrossRef]
- Stanton, H.; Melrose, J.; Little, C.B.; Fosang, A.J. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim. Biophys. Acta 2011, 1812, 1616–1629. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.E.; McCulloch, D.R.; McGarity, J.D.; Bahan, A.; Wessels, A.; Weber, D.; Diminich, A.M.; Nelson, C.M.; Apte, S.S.; Kern, C.B. Altered versican cleavage in ADAMTS5 deficient mice; a novel etiology of myxomatous valve disease. Dev. Biol. 2011, 357, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.E.; Nelson, E.L.; Hozik, B.; Porto, S.C.; Rogers-DeCotes, A.; Fosang, A.; Kern, C.B. Adamts5 (−/−) Mice Exhibit Altered Aggrecan Proteolytic Profiles That Correlate With Ascending Aortic Anomalies. Arter. Thromb. Vasc. Biol. 2019, 39, 2067–2081. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.E.; Osinska, H.; Weinstein, M.B.; Hinton, R.B.; Kern, C.B. Insufficient versican cleavage and Smad2 phosphorylation results in bicuspid aortic and pulmonary valves. J. Mol. Cell. Cardiol. 2013, 60, 50–59. [Google Scholar] [CrossRef]
- McCulloch, D.R.; Wylie, J.D.; Longpre, J.M.; Leduc, R.; Apte, S.S. 10mM glucosamine prevents activation of proADAMTS5 (aggrecanase-2) in transfected cells by interference with post-translational modification of furin. Osteoarthr. Cartil. 2010, 18, 455–463. [Google Scholar] [CrossRef]
- Stankunas, K.; Hang, C.T.; Tsun, Z.Y.; Chen, H.; Lee, N.V.; Wu, J.I.; Shang, C.; Bayle, J.H.; Shou, W.; Iruela-Arispe, M.L.; et al. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev. Cell 2008, 14, 298–311. [Google Scholar] [CrossRef]
- Rogers, A.W.; Cisewski, S.E.; Kern, C.B. The Zonal Architecture of the Mandibular Condyle Requires ADAMTS5. J. Dent. Res. 2018, 97, 1383–1390. [Google Scholar] [CrossRef]
- Islam, S.; Chuensirikulchai, K.; Khummuang, S.; Keratibumrungpong, T.; Kongtawelert, P.; Kasinrerk, W.; Hatano, S.; Nagamachi, A.; Honda, H.; Watanabe, H. Accumulation of versican facilitates wound healing: Implication of its initial ADAMTS-cleavage site. Matrix Biol. 2020, 87, 77–93. [Google Scholar] [CrossRef]
- Nandadasa, S.; Burin des Roziers, C.; Koch, C.; Tran-Lundmark, K.; Dours-Zimmermann, M.T.; Zimmermann, D.R.; Valleix, S.; Apte, S.S. A new mouse mutant with cleavage-resistant versican and isoform-specific versican mutants demonstrate that proteolysis at the Glu(441)-Ala(442) peptide bond in the V1 isoform is essential for interdigital web regression. Matrix Biol. Plus 2021, 10, 100064. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Nelson, C.M.; Somerville, R.P.; Mielke, K.; Dixon, L.J.; Powell, K.; Apte, S.S. Cooperation of two ADAMTS metalloproteases in closure of the mouse palate identifies a requirement for versican proteolysis in regulating palatal mesenchyme proliferation. Development 2010, 137, 4029–4038. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.B.; Wessels, A.; McGarity, J.; Dixon, L.J.; Alston, E.; Argraves, W.S.; Geeting, D.; Nelson, C.M.; Menick, D.R.; Apte, S.S. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol. 2010, 29, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Massadeh, S.; Alhashem, A.; van de Laar, I.; Alhabshan, F.; Ordonez, N.; Alawbathani, S.; Khan, S.; Kabbani, M.S.; Chaikhouni, F.; Sheereen, A.; et al. ADAMTS19-associated heart valve defects: Novel genetic variants consolidating a recognizable cardiac phenotype. Clin. Genet. 2020, 98, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Song, R.; Ao, L.; Reece, T.B.; Cleveland, J.C., Jr.; Dong, N.; Fullerton, D.A.; Meng, X. ADAMTS5 Deficiency in Calcified Aortic Valves Is Associated With Elevated Pro-Osteogenic Activity in Valvular Interstitial Cells. Arter. Thromb. Vasc. Biol. 2017, 37, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Wunnemann, F.; Ta-Shma, A.; Preuss, C.; Leclerc, S.; van Vliet, P.P.; Oneglia, A.; Thibeault, M.; Nordquist, E.; Lincoln, J.; Scharfenberg, F.; et al. Loss of ADAMTS19 causes progressive non-syndromic heart valve disease. Nat. Genet. 2020, 52, 40–47. [Google Scholar] [CrossRef]
- Cikach, F.S.; Koch, C.D.; Mead, T.J.; Galatioto, J.; Willard, B.B.; Emerton, K.B.; Eagleton, M.J.; Blackstone, E.H.; Ramirez, F.; Roselli, E.E.; et al. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. J. Clin. Investig. 2018, 3. [Google Scholar] [CrossRef]
- Rajamannan, N.M. Calcific aortic valve disease: Cellular origins of valve calcification. Arter. Thromb. Vasc. Biol. 2011, 31, 2777–2778. [Google Scholar] [CrossRef]
- Stephens, E.H.; Saltarrelli, J.G.; Baggett, L.S.; Nandi, I.; Kuo, J.J.; Davis, A.R.; Olmsted-Davis, E.A.; Reardon, M.J.; Morrisett, J.D.; Grande-Allen, K.J. Differential proteoglycan and hyaluronan distribution in calcified aortic valves. Cardiovasc. Pathol. 2011, 20, 334–342. [Google Scholar] [CrossRef]
- Gupta, V.; Barzilla, J.E.; Mendez, J.S.; Stephens, E.H.; Lee, E.L.; Collard, C.D.; Laucirica, R.; Weigel, P.H.; Grande-Allen, K.J. Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves. Cardiovasc. Pathol. 2009, 18, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.B.; Norris, R.A.; Thompson, R.P.; Argraves, W.S.; Fairey, S.E.; Reyes, L.; Hoffman, S.; Markwald, R.R.; Mjaatvedt, C.H. Versican proteolysis mediates myocardial regression during outflow tract development. Dev. Dyn. 2007, 236, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Goddard, L.M.; Duchemin, A.L.; Ramalingan, H.; Wu, B.; Chen, M.; Bamezai, S.; Yang, J.; Li, L.; Morley, M.P.; Wang, T.; et al. Hemodynamic Forces Sculpt Developing Heart Valves through a KLF2-WNT9B Paracrine Signaling Axis. Dev. Cell 2017, 43, 274–289.e275. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Zhao, Y.D.; Courtman, D.W.; Stewart, D.J. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 2000, 101, 2345–2348. [Google Scholar] [CrossRef]
- Bosse, K.; Hans, C.P.; Zhao, N.; Koenig, S.N.; Huang, N.; Guggilam, A.; LaHaye, S.; Tao, G.; Lucchesi, P.A.; Lincoln, J.; et al. Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease. J. Mol. Cell Cardiol. 2013, 60, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.N.; Bosse, K.M.; Nadorlik, H.A.; Lilly, B.; Garg, V. Evidence of Aortopathy in Mice with Haploinsufficiency of Notch1 in Nos3-Null Background. J. Cardiovasc. Dev. Dis. 2015, 2, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, A.L.; Vignes, H.; Vermot, J. Mechanically activated piezo channels modulate outflow tract valve development through the Yap1 and Klf2-Notch signaling axis. Elife 2019, 8, e44706. [Google Scholar] [CrossRef]
- Kern, C.B.; Twal, W.O.; Mjaatvedt, C.H.; Fairey, S.E.; Toole, B.P.; Iruela-Arispe, M.L.; Argraves, W.S. Proteolytic cleavage of versican during cardiac cushion morphogenesis. Dev. Dyn. 2006, 235, 2238–2247. [Google Scholar] [CrossRef]
- Barakat, A.I.; Lieu, D.K.; Gojova, A. Secrets of the code: Do vascular endothelial cells use ion channels to decipher complex flow signals? Biomaterials 2006, 27, 671–678. [Google Scholar] [CrossRef]
- Ohno, M.; Cooke, J.P.; Dzau, V.J.; Gibbons, G.H. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J. Clin. Investig. 1995, 95, 1363–1369. [Google Scholar] [CrossRef]
- De, R.; Zemel, A.; Safran, S.A. Do cells sense stress or strain? Measurement of cellular orientation can provide a clue. Biophys. J. 2008, 94, L29–L31. [Google Scholar] [CrossRef]
- Livne, A.; Bouchbinder, E.; Geiger, B. Cell reorientation under cyclic stretching. Nat. Commun. 2014, 5, 3938. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; de Groot, R.; Scilabra, S.D.; Kwok, H.F.; Santamaria, S. Editorial: ADAM, ADAMTS and Astacin Proteases: Challenges and Breakthroughs in the -Omics Era. Front. Mol. Biosci. 2021, 8, 780242. [Google Scholar] [CrossRef] [PubMed]
- Barallobre-Barreiro, J.; Radovits, T.; Fava, M.; Mayr, U.; Lin, W.Y.; Ermolaeva, E.; Martinez-Lopez, D.; Lindberg, E.L.; Duregotti, E.; Daroczi, L.; et al. Extracellular Matrix in Heart Failure: Role of ADAMTS5 in Proteoglycan Remodeling. Circulation 2021, 144, 2021–2034. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupuis, L.E.; Evins, S.E.; Abell, M.C.; Blakley, M.E.; Horkey, S.L.; Barth, J.L.; Kern, C.B. Increased Proteoglycanases in Pulmonary Valves after Birth Correlate with Extracellular Matrix Maturation and Valve Sculpting. J. Cardiovasc. Dev. Dis. 2023, 10, 27. https://doi.org/10.3390/jcdd10010027
Dupuis LE, Evins SE, Abell MC, Blakley ME, Horkey SL, Barth JL, Kern CB. Increased Proteoglycanases in Pulmonary Valves after Birth Correlate with Extracellular Matrix Maturation and Valve Sculpting. Journal of Cardiovascular Development and Disease. 2023; 10(1):27. https://doi.org/10.3390/jcdd10010027
Chicago/Turabian StyleDupuis, Loren E., Sarah E. Evins, Morgan C. Abell, Morgan E. Blakley, Samuel L. Horkey, Jeremy L. Barth, and Christine B. Kern. 2023. "Increased Proteoglycanases in Pulmonary Valves after Birth Correlate with Extracellular Matrix Maturation and Valve Sculpting" Journal of Cardiovascular Development and Disease 10, no. 1: 27. https://doi.org/10.3390/jcdd10010027
APA StyleDupuis, L. E., Evins, S. E., Abell, M. C., Blakley, M. E., Horkey, S. L., Barth, J. L., & Kern, C. B. (2023). Increased Proteoglycanases in Pulmonary Valves after Birth Correlate with Extracellular Matrix Maturation and Valve Sculpting. Journal of Cardiovascular Development and Disease, 10(1), 27. https://doi.org/10.3390/jcdd10010027





