Circulating Regulatory B-Lymphocytes in Patients with Acute Myocardial Infarction: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
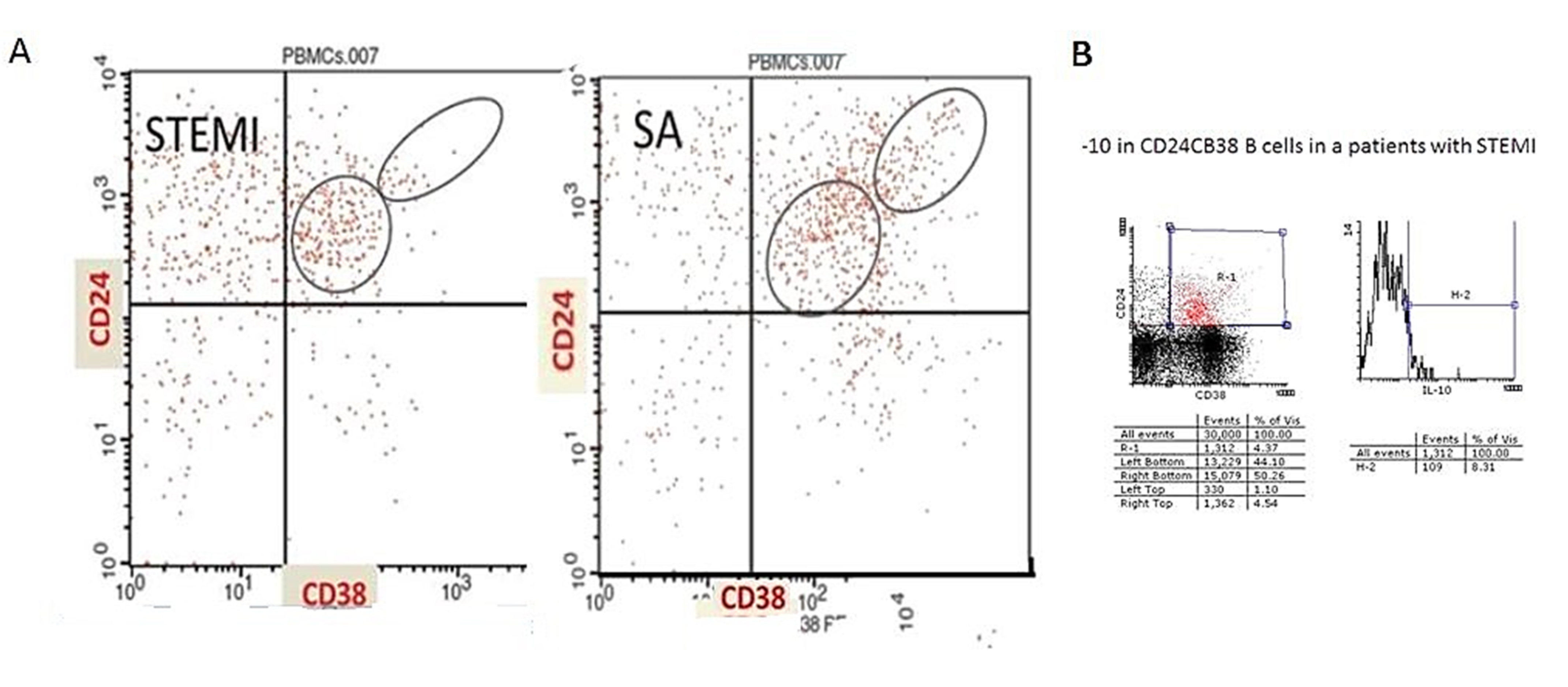
2.1. Quantification of Regulatory B Cells by Flow Cytometry
- Mouse anti-human CD24 (eBioscience; 17-0247-41), + mouse anti human CD38 (eBioscience; 12-0388-41) + mouse anti-human CD20 (eBioscience; 11-0209-41).
- Mouse anti-human CD24 + mouse anti human CD27 (eBioscience; 12-0279-41) + mouse anti-human CD20.
2.2. Intracellular IL10 Staining
2.3. Serum IL-10 Assessment
2.4. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MI | myocardial infarction |
| SAP | stable angina pectoris |
| B-regs | B-regulatory lymphocytes |
| CAD | coronary artery disease |
| WBC | white blood cells |
| LVEF | left ventricular ejection fraction |
References
- Badimon, L.; Padro, T.; Vilahur, G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Packard, R.R.; Lichtman, A.H.; Libby, P. Innate and adaptive immunity in atherosclerosis. Semin. Immunopathol. 2009, 31, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Stemme, S.; Hansson, G.K. Evidence for a local immune response in atherosclerosis. Cd4+ t cells infiltrate lesions of apolipoprotein-e-deficient mice. Am. J. Pathol. 1996, 149, 359–366. [Google Scholar] [PubMed]
- Major, A.S.; Fazio, S.; Linton, M.F. B-lymphocyte deficiency increases atherosclerosis in ldl receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1892–1898. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Herbin, O.; Bouaziz, J.D.; Binder, C.J.; Uyttenhove, C.; Laurans, L.; Taleb, S.; Van Vre, E.; Esposito, B.; Vilar, J.; et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 2010, 207, 1579–1587. [Google Scholar] [CrossRef]
- Kyaw, T.; Tay, C.; Khan, A.; Dumouchel, V.; Cao, A.; To, K.; Kehry, M.; Dunn, R.; Agrotis, A.; Tipping, P.; et al. Conventional b2 b cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J. Immunol. 2010, 185, 4410–4419. [Google Scholar] [CrossRef]
- Martin, F.; Kearney, J.F. B1 cells: Similarities and differences with other b cell subsets. Curr. Opin. Immunol. 2001, 13, 195–201. [Google Scholar] [CrossRef]
- Esplin, B.L.; Welner, R.S.; Zhang, Q.; Borghesi, L.A.; Kincade, P.W. A differentiation pathway for b1 cells in adult bone marrow. Proc. Natl. Acad. Sci. USA 2009, 106, 5773–5778. [Google Scholar] [CrossRef]
- George, J.; Afek, A.; Gilburd, B.; Levkovitz, H.; Shaish, A.; Goldberg, I.; Kopolovic, Y.; Wick, G.; Shoenfeld, Y.; Harats, D. Hyperimmunization of apo-e-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis 1998, 138, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Caligiuri, G.; Hamsten, A.; Lefvert, A.K.; Hansson, G.K. Ldl immunization induces t-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.J.; Malik, T.H.; Ehrenstein, M.R.; Boyle, J.J.; Botto, M.; Haskard, D.O. Immunoglobulin m is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2009, 120, 417–426. [Google Scholar] [CrossRef]
- Karvonen, J.; Paivansalo, M.; Kesaniemi, Y.A.; Horkko, S. Immunoglobulin m type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation 2003, 108, 2107–2112. [Google Scholar] [CrossRef]
- Ravandi, A.; Boekholdt, S.M.; Mallat, Z.; Talmud, P.J.; Kastelein, J.J.; Wareham, N.J.; Miller, E.R.; Benessiano, J.; Tedgui, A.; Witztum, J.L.; et al. Relationship of igg and igm autoantibodies and immune complexes to oxidized ldl with markers of oxidation and inflammation and cardiovascular events: Results from the epic-norfolk study. J. Lipid Res. 2011, 52, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, A.; Bhan, A.K. A case for regulatory b cells. J. Immunol. 2006, 176, 705–710. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Mizoguchi, E.; Smith, R.N.; Preffer, F.I.; Bhan, A.K. Suppressive role of b cells in chronic colitis of t cell receptor alpha mutant mice. J. Exp. Med. 1997, 186, 1749–1756. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Mizoguchi, E.; Takedatsu, H.; Blumberg, R.S.; Bhan, A.K. Chronic intestinal inflammatory condition generates il-10-producing regulatory b cell subset characterized by cd1d upregulation. Immunity 2002, 16, 219–230. [Google Scholar] [CrossRef]
- Katz, S.I.; Parker, D.; Turk, J.L. B-cell suppression of delayed hypersensitivity reactions. Nature 1974, 251, 550–551. [Google Scholar] [CrossRef]
- Wolf, S.D.; Dittel, B.N.; Hardardottir, F.; Janeway, C.A., Jr. Experimental autoimmune encephalomyelitis induction in genetically b cell-deficient mice. J. Exp. Med. 1996, 184, 2271–2278. [Google Scholar] [CrossRef]
- Fillatreau, S.; Sweenie, C.H.; McGeachy, M.J.; Gray, D.; Anderton, S.M. B cells regulate autoimmunity by provision of il-10. Nat. Immunol. 2002, 3, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Bouaziz, J.D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A regulatory b cell subset with a unique cd1dhicd5+ phenotype controls t cell-dependent inflammatory responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Vadasz, Z.; Toubi, E. The many faces of b regulatory cells. Isr. Med. Assoc. J. 2014, 16, 631–633. [Google Scholar] [PubMed]
- Rosser, E.C.; Mauri, C. Regulatory b cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef]
- Rincon-Arevalo, H.; Sanchez-Parra, C.C.; Castano, D.; Yassin, L.; Vasquez, G. Regulatory b cells and mechanisms. Int. Rev. Immunol. 2016, 35, 156–176. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.A.; Norena, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. Cd19(+)cd24(hi)cd38(hi) b cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Chang, R.; Go, N.; Hastings, R.; Haughton, G.; Howard, M. Ly-1 b (b-1) cells are the main source of b cell-derived interleukin 10. Eur. J. Immunol. 1992, 22, 711–717. [Google Scholar] [CrossRef]
- Spencer, N.F.; Daynes, R.A. Il-12 directly stimulates expression of il-10 by cd5+ b cells and il-6 by both cd5+ and cd5- b cells: Possible involvement in age-associated cytokine dysregulation. Int. Immunol. 1997, 9, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.G.; Chavez-Rueda, K.A.; Eddaoudi, A.; Meyer-Bahlburg, A.; Rawlings, D.J.; Ehrenstein, M.R.; Mauri, C. Novel suppressive function of transitional 2 b cells in experimental arthritis. J. Immunol. 2007, 178, 7868–7878. [Google Scholar] [CrossRef] [PubMed]
- Duddy, M.E.; Alter, A.; Bar-Or, A. Distinct profiles of human b cell effector cytokines: A role in immune regulation? J. Immunol. 2004, 172, 3422–3427. [Google Scholar] [CrossRef]
- Sage, A.P.; Nus, M.; Baker, L.L.; Finigan, A.J.; Masters, L.M.; Mallat, Z. Regulatory b cell-specific interleukin-10 is dispensable for atherosclerosis development in mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Strom, A.C.; Cross, A.J.; Cole, J.E.; Blair, P.A.; Leib, C.; Goddard, M.E.; Rosser, E.C.; Park, I.; Hultgardh Nilsson, A.; Nilsson, J.; et al. B regulatory cells are increased in hypercholesterolaemic mice and protect from lesion development via il-10. Thromb. Haemost. 2015, 114, 835–847. [Google Scholar] [PubMed]
- Liu, Y.; Duan, W.R.; Liu, S.; Liu, T.; Chang, Y.J.; Fan, X.M. Correlation of cd19+cd24hicd38hi b cells in coronary artery disease with severity of atherosclerosis. Chin. Med. J. 2020, 133, 1257–1258. [Google Scholar] [CrossRef] [PubMed]
- Gjurich, B.N.; Taghavie-Moghadam, P.L.; Ley, K.; Galkina, E.V. L-selectin deficiency decreases aortic b1a and breg subsets and promotes atherosclerosis. Thromb. Haemost. 2014, 112, 803–811. [Google Scholar] [CrossRef]
- Matsumoto, M.; Baba, A.; Yokota, T.; Nishikawa, H.; Ohkawa, Y.; Kayama, H.; Kallies, A.; Nutt, S.L.; Sakaguchi, S.; Takeda, K.; et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 2014, 41, 1040–1051. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Kelly, C.R.; Weisz, G.; Maehara, A.; Mintz, G.S.; Mehran, R.; Lansky, A.J.; Parise, H.; de Bruyne, B.; Serruys, P.W.; Stone, G.W. Relation of c-reactive protein levels to instability of untreated vulnerable coronary plaques (from the prospect study). Am. J. Cardiol. 2014, 114, 376–383. [Google Scholar] [CrossRef]
- Shindo, A.; Tanemura, H.; Yata, K.; Hamada, K.; Shibata, M.; Umeda, Y.; Asakura, F.; Toma, N.; Sakaida, H.; Fujisawa, T.; et al. Inflammatory biomarkers in atherosclerosis: Pentraxin 3 can become a novel marker of plaque vulnerability. PLoS ONE 2014, 9, e100045. [Google Scholar] [CrossRef]
- van Dijk, R.A.; Duinisveld, A.J.; Schaapherder, A.F.; Mulder-Stapel, A.; Hamming, J.F.; Kuiper, J.; de Boer, O.J.; van der Wal, A.C.; Kolodgie, F.D.; Virmani, R.; et al. A change in inflammatory footprint precedes plaque instability: A systematic evaluation of cellular aspects of the adaptive immune response in human atherosclerosis. J. Am. Heart Assoc. 2015, 4, e001403. [Google Scholar] [CrossRef]
- Kessel, A.; Haj, T.; Peri, R.; Snir, A.; Melamed, D.; Sabo, E.; Toubi, E. Human cd19(+)cd25(high) b regulatory cells suppress proliferation of cd4(+) t cells and enhance foxp3 and ctla-4 expression in t-regulatory cells. Autoimmun. Rev. 2012, 11, 670–677. [Google Scholar] [CrossRef]
- Klingenberg, R.; Brokopp, C.E.; Grives, A.; Courtier, A.; Jaguszewski, M.; Pasqual, N.; Vlaskou Badra, E.; Lewandowski, A.; Gaemperli, O.; Hoerstrup, S.P.; et al. Clonal restriction and predominance of regulatory t cells in coronary thrombi of patients with acute coronary syndromes. Eur. Heart J. 2015, 36, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Liuzzo, G.; Kopecky, S.L.; Frye, R.L.; O’Fallon, W.M.; Maseri, A.; Goronzy, J.J.; Weyand, C.M. Perturbation of the t-cell repertoire in patients with unstable angina. Circulation 1999, 100, 2135–2139. [Google Scholar] [CrossRef] [PubMed]
- Liuzzo, G.; Vallejo, A.N.; Kopecky, S.L.; Frye, R.L.; Holmes, D.R.; Goronzy, J.J.; Weyand, C.M. Molecular fingerprint of interferon-gamma signaling in unstable angina. Circulation 2001, 103, 1509–1514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- George, J.; Schwartzenberg, S.; Medvedovsky, D.; Jonas, M.; Charach, G.; Afek, A.; Shamiss, A. Regulatory t cells and il-10 levels are reduced in patients with vulnerable coronary plaques. Atherosclerosis 2012, 222, 519–523. [Google Scholar] [CrossRef] [PubMed]
- George, J. Mechanisms of disease: The evolving role of regulatory t cells in atherosclerosis. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 531–540. [Google Scholar] [CrossRef]
- Mor, A.; Luboshits, G.; Planer, D.; Keren, G.; George, J. Altered status of cd4(+)cd25(+) regulatory t cells in patients with acute coronary syndromes. Eur. Heart j. 2006, 27, 2530–2537. [Google Scholar] [CrossRef]
- Porsch, F.; Mallat, Z.; Binder, C.J. Humoral immunity in atherosclerosis and myocardial infarction: From b cells to antibodies. Cardiovasc. Res. 2021, 117, 2544–2562. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Shao, J.; Lin, L.; Jiang, M.; Wang, L.; Lu, X.; Zhang, H.; Chen, Y.; Zhang, R. Immune and inflammation in acute coronary syndrome: Molecular mechanisms and therapeutic implications. J. Immunol. Res. 2020, 2020, 4904217. [Google Scholar] [CrossRef]
- Lagraauw, H.M.; Wezel, A.; van der Velden, D.; Kuiper, J.; Bot, I. Stress-induced mast cell activation contributes to atherosclerotic plaque destabilization. Sci. Rep. 2019, 9, 2134. [Google Scholar] [CrossRef]
- Mangge, H.; Pruller, F.; Schnedl, W.; Renner, W.; Almer, G. Beyond macrophages and t cells: B cells and immunoglobulins determine the fate of the atherosclerotic plaque. Int. J. Mol. Sci. 2020, 21, 4082. [Google Scholar] [CrossRef]
- Ma, S.D.; Mussbacher, M.; Galkina, E.V. Functional role of b cells in atherosclerosis. Cells 2021, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Catalan, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillon, J.C.; Aravena, O. Immunosuppressive mechanisms of regulatory b cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef] [PubMed]
- Ponnuswamy, P.; Joffre, J.; Herbin, O.; Esposito, B.; Laurans, L.; Binder, C.J.; Tedder, T.F.; Zeboudj, L.; Loyer, X.; Giraud, A.; et al. Angiotensin ii synergizes with baff to promote atheroprotective regulatory b cells. Sci. Rep. 2017, 7, 4111. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T. Regulatory and effector b cells: Friends or foes? J. Dermatol. Sci. 2019, 93, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.M.; Hanes, M.R.; Marshall, J.S. Mast cell modulation of b cell responses: An under-appreciated partnership in host defence. Front. Immunol. 2021, 12, 718499. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, M.B.; Lee, D.; Min, K.Y.; Koo, J.; Kim, H.W.; Park, Y.H.; Kim, S.J.; Ikutani, M.; Takaki, S.; et al. The regulatory b cell-mediated peripheral tolerance maintained by mast cell il-5 suppresses oxazolone-induced contact hypersensitivity. Sci. Adv. 2019, 5, eaav8152. [Google Scholar] [CrossRef]
- Jansen, K.; Cevhertas, L.; Ma, S.; Satitsuksanoa, P.; Akdis, M.; van de Veen, W. Regulatory b cells, a to z. Allergy 2021, 76, 2699–2715. [Google Scholar] [CrossRef]
- Niccoli, G.; Montone, R.A.; Sabato, V.; Crea, F. Role of allergic inflammatory cells in coronary artery disease. Circulation 2018, 138, 1736–1748. [Google Scholar] [CrossRef]
- Niccoli, G.; Calvieri, C.; Flego, D.; Scalone, G.; Imaeva, A.; Sabato, V.; Schiavino, D.; Liuzzo, G.; Crea, F. Allergic inflammation is associated with coronary instability and a worse clinical outcome after acute myocardial infarction. Circ. Cardiovasc. Interv. 2015, 8, e002554. [Google Scholar] [CrossRef]
- Anguera, I.; Miranda-Guardiola, F.; Bosch, X.; Filella, X.; Sitges, M.; Marin, J.L.; Betriu, A.; Sanz, G. Elevation of serum levels of the anti-inflammatory cytokine interleukin-10 and decreased risk of coronary events in patients with unstable angina. Am. Heart J. 2002, 144, 811–817. [Google Scholar] [CrossRef]
- Falcao, R.A.; Christopher, S.; Oddi, C.; Reznikov, L.; Grizzard, J.D.; Abouzaki, N.A.; Varma, A.; Van Tassell, B.W.; Dinarello, C.A.; Abbate, A. Interleukin-10 in patients with st-segment elevation myocardial infarction. Int. J. Cardiol. 2014, 172, e6–e8. [Google Scholar] [CrossRef] [PubMed]
- Malarstig, A.; Eriksson, P.; Hamsten, A.; Lindahl, B.; Wallentin, L.; Siegbahn, A. Raised interleukin-10 is an indicator of poor outcome and enhanced systemic inflammation in patients with acute coronary syndrome. Heart 2008, 94, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Dittel, B.N. Mechanisms of regulatory b cell function in autoimmune and inflammatory diseases beyond il-10. J. Clin. Med. 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef]
- Mo, F.; Luo, Y.; Yan, Y.; Li, J.; Lai, S.; Wu, W. Are activated b cells involved in the process of myocardial fibrosis after acute myocardial infarction? An in vivo experiment. BMC Cardiovasc. Disord. 2021, 21, 5. [Google Scholar] [CrossRef]
- Ma, X.L.; Lin, Q.Y.; Wang, L.; Xie, X.; Zhang, Y.L.; Li, H.H. Rituximab prevents and reverses cardiac remodeling by depressing b cell function in mice. Biomed. Pharmacother. 2019, 114, 108804. [Google Scholar] [CrossRef]
- Jiao, J.; He, S.; Wang, Y.; Lu, Y.; Gu, M.; Li, D.; Tang, T.; Nie, S.; Zhang, M.; Lv, B.; et al. Regulatory b cells improve ventricular remodeling after myocardial infarction by modulating monocyte migration. Basic Res. Cardiol. 2021, 116, 46. [Google Scholar] [CrossRef]
| STEMI n = 29 | SAP n = 18 | p (STEMI vs. SAP) | |
|---|---|---|---|
| Age | 61 ± 10 | 65 ± 7 | 0.3 |
| Male gender | 26 | 17 | 0.9 |
| Hypertension | 15 | 13 | 0.28 |
| Diabetes mellitus | 11 | 6 | 0.9 |
| Hyperlipidemia | 21 | 17 | 0.124 |
| Troponin (ng/mL) | 23.79 ± 24.27 | ||
| Number of vessels >50% stenosis (1/2/3 vessel disease (%) | 28/26/46 | 17/50/33 | 0.128 |
| History of MI n (%) | 9(31) | 9 (50) | 0.32 |
| History of revascularization | 2(7) | 5(27) | 0.09 |
| STEMI n = 29 | SAP n = 18 | p (STEMI vs. SAP) | |
|---|---|---|---|
| CD20+(%) | 6.96 ± 2.6 | 6.79 ± 3.3 | 0.6 |
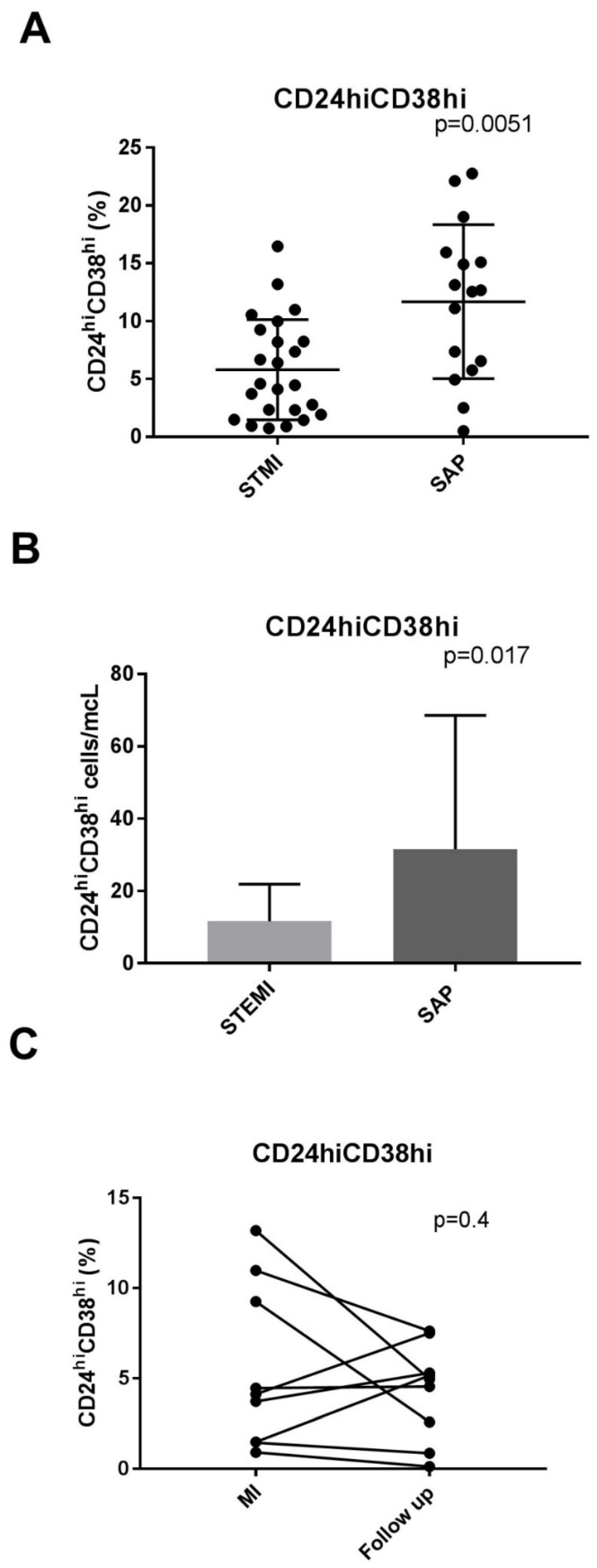
| CD24hiCD38hi(%) | 5.7 ± 4% | 11.6 ± 6% | 0.0051 |
| CD24hiCD38hi \(cells/mcL) | 8 (2–16) | 18 (7–44) | 0.017 |
| CD24intCD38int(%) | 34 ± 13 | 37 ± 20 | 0.68 |
| CD24+CD27+(%) | 20.8 ± 10.7 | 23.7 ± 6.1 a | 0.4 |
| CD24+CD27+ (cells/mcL) | 33 (18–53) | 43 (27–100) | 0.3 |
| IL-10 | 30.67 ± 12.7 |
| Acute Phase MI | Convalescent Phase | p-Value | |
|---|---|---|---|
| CD20+(%) | 7.98 ± 2.9 | 8.14 ± 2.86 | 0.465 |
| CD24highCd38high (%) | 5.7 ± 4 | 4.3 ± 0.9 | 0.408 |
| CD24intCd38int (%) | 26 ± 14 | 32 ± 12 | 0.23 |
| CD24+CD27+ (%) | 25.8 ± 9.34 | 21.7 ± 10.18 | 0.0493 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volodarsky, I.; Shimoni, S.; Haberman, D.; Mirkin, V.; Fabrikant, Y.; Yoskovich Mashriki, T.; Zalik, A.; George, J. Circulating Regulatory B-Lymphocytes in Patients with Acute Myocardial Infarction: A Pilot Study. J. Cardiovasc. Dev. Dis. 2023, 10, 2. https://doi.org/10.3390/jcdd10010002
Volodarsky I, Shimoni S, Haberman D, Mirkin V, Fabrikant Y, Yoskovich Mashriki T, Zalik A, George J. Circulating Regulatory B-Lymphocytes in Patients with Acute Myocardial Infarction: A Pilot Study. Journal of Cardiovascular Development and Disease. 2023; 10(1):2. https://doi.org/10.3390/jcdd10010002
Chicago/Turabian StyleVolodarsky, Igor, Sara Shimoni, Dan Haberman, Vita Mirkin, Yakov Fabrikant, Tal Yoskovich Mashriki, Adi Zalik, and Jacob George. 2023. "Circulating Regulatory B-Lymphocytes in Patients with Acute Myocardial Infarction: A Pilot Study" Journal of Cardiovascular Development and Disease 10, no. 1: 2. https://doi.org/10.3390/jcdd10010002
APA StyleVolodarsky, I., Shimoni, S., Haberman, D., Mirkin, V., Fabrikant, Y., Yoskovich Mashriki, T., Zalik, A., & George, J. (2023). Circulating Regulatory B-Lymphocytes in Patients with Acute Myocardial Infarction: A Pilot Study. Journal of Cardiovascular Development and Disease, 10(1), 2. https://doi.org/10.3390/jcdd10010002






