Age-Related Differences in the Functional Demand Placed on the Lumbar Spine during Walking in Healthy Older versus Younger Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Three-Dimensional Motion Analysis
2.3. Calculating Functional Demand
2.4. Statistical Analysis
3. Results
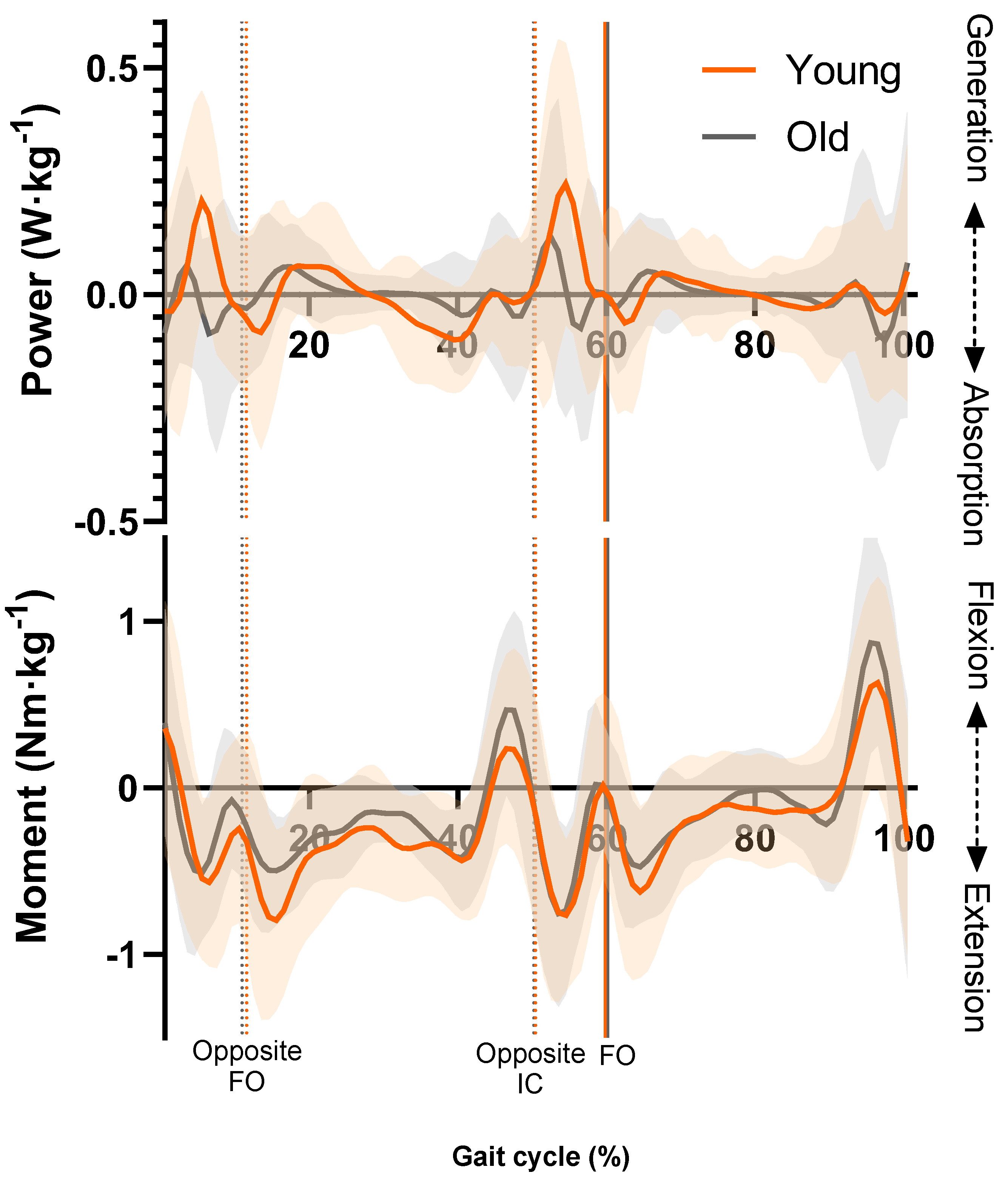
3.1. Lumbar Spine Moments and Power
3.2. Functional Demand
4. Discussion
4.1. Clinical and Practical Applications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Watts, E.L.; Matthews, C.E.; Freeman, J.R.; Gorzelitz, J.S.; Hong, H.G.; Liao, L.M.; McClain, K.M.; Saint-Maurice, P.F.; Shiroma, E.J.; Moore, S.C. Association of Leisure Time Physical Activity Types and Risks of All-Cause, Cardiovascular, and Cancer Mortality among Older Adults. JAMA Netw. Open 2022, 5, E2228510. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Rios, D.A.; Edelberg, H.K. Gait Variability and Fall Risk in Community-Living Older Adults: A 1-Year Prospective Study. Arch. Phys. Med. Rehabil. 2001, 82, 1050–1056. [Google Scholar] [CrossRef]
- Abreu, H.C.d.A.; Reiners, A.A.O.; Azevedo, R.C.d.S.; da Silva, A.M.C.; Abreu, D.R.d.O.M.; de Oliveira, A.D. Incidence and Predicting Factors of Falls of Older Inpatients. Rev. Saude Publica 2015, 49, 1–8. [Google Scholar] [CrossRef]
- Verghese, J.; LeValley, A.; Hall, C.B.; Katz, M.J.; Ambrose, A.F.; Lipton, R.B. Epidemiology of Gait Disorders in Community-Residing Older Adults. J. Am. Geriatr. Soc. 2006, 54, 255–261. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Beck Jepsen, D.; Robinson, K.; Ogliari, G.; Montero-Odasso, M.; Kamkar, N.; Ryg, J.; Freiberger, E.; Tahir, M. Predicting Falls in Older Adults: An Umbrella Review of Instruments Assessing Gait, Balance, and Functional Mobility. BMC Geriatr. 2022, 22, 615. [Google Scholar] [CrossRef]
- Stephan, Y.; Sutin, A.R.; Terracciano, A. “Feeling Younger, Walking Faster”: Subjective Age and Walking Speed in Older Adults. Age 2015, 37, 86. [Google Scholar] [CrossRef] [PubMed]
- DeVita, P.; Hortobagyi, T. Age Causes a Redistribution of Joint Torques and Powers during Gait. J. Appl. Physiol. 2000, 88, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Reeves, N.D.; Spanjaard, M.; Mohagheghi, A.A.; Baltzopoulos, V.; Maganaris, C.N. The Demands of Stair Descent Relative to Maximum Capacities in Elderly and Young Adults. J. Electromyogr. Kinesiol. 2008, 18, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Samuel, D.; Rowe, P.; Hood, V.; Nicol, A. The Biomechanical Functional Demand Placed on Knee and Hip Muscles of Older Adults during Stair Ascent and Descent. Gait Posture 2011, 34, 239–244. [Google Scholar] [CrossRef]
- Samuel, D.; Rowe, P.; Nicol, A. The Functional Demand (FD) Placed on the Knee and Hip of Older Adults during Everyday Activities. Arch. Gerontol. Geriatr. 2013, 57, 192–197. [Google Scholar] [CrossRef][Green Version]
- Winter, D.A.; Patla, A.E.; Frank, J.S.; Walt, S.E. Biomechanical Walking Pattern Changes in the Fit and Healthy Elderly. Phys. Ther. 1990, 70, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.J.; Winter, D.A. Kinetic Analysis of the Lower Limbs during Walking: What Information Can Be Gained from a Three-Dimensional Model? J. Biomech. 1995, 28, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Silder, A.; Heiderscheit, B.; Thelen, D.G. Active and Passive Contributions to Joint Kinetics during Walking in Older Adults. J. Biomech. 2008, 41, 1520–1527. [Google Scholar] [CrossRef]
- Karthikbabu, S.; Solomon, J.M.; Manikandan, N.; Rao, B.K.; Chakrapani, M.; Nayak, A. Role of Trunk Rehabilitation on Trunk Control, Balance and Gait in Patients with Chronic Stroke: A Pre-Post Design. Neurosci. Med. 2011, 2, 61–67. [Google Scholar] [CrossRef]
- Cromwell, R.L.; Aadland-Monahan, T.K.; Nelson, A.T.; Stern-Sylvestre, S.M.; Seder, B. Sagittal Plane Analysis of Head, Neck, and Trunk Kinematics and Electromyographic Activity during Locomotion. J. Orthop. Sports Phys. Ther. 2001, 31, 255–262. [Google Scholar] [CrossRef]
- Ceccato, J.C.; de Sèze, M.; Azevedo, C.; Cazalets, J.R. Comparison of Trunk Activity during Gait Initiation and Walking in Humans. PLoS ONE 2009, 4, e8193. [Google Scholar] [CrossRef]
- Carmo, A.A.; Kleiner, A.F.R.; Lobo da Costa, P.H.; Barros, R.M.L. Three-Dimensional Kinematic Analysis of Upper and Lower Limb Motion during Gait of Post-Stroke Patients. Braz. J. Med. Biol. Res. 2012, 45, 537–545. [Google Scholar] [CrossRef]
- Hicks, G.E.; Simonsick, E.M.; Harris, T.B.; Newman, A.B.; Weiner, D.K.; Nevitt, M.A.; Tylavsky, F.A. Trunk Muscle Composition as a Predictor of Reduced Functional Capacity in the Health, Aging and Body Composition Study: The Moderating Role of Back Pain. J Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Cholewicki, J.; Panjabi, M.M.; Khachatryan, A. Stabilizing Function of Trunk Flexor-Extensor Muscles around a Neutral Spine Posture. Spine 1997, 22, 2207–2212. [Google Scholar] [CrossRef]
- Callaghan, J.P.; Patla, A.E.; McGill, S.M. Low Back Three-Dimensional Joint Forces, Kinematics, and Kinetics during Walking. Clin. Biomech. 1999, 14, 203–216. [Google Scholar] [CrossRef]
- McGibbon, C.A.; Krebs, D.E. Age-Related Changes in Lower Trunk Coordination and Energy Transfer during Gait. J. Neurophysiol. 2001, 85, 1923–1931. [Google Scholar] [CrossRef]
- Leteneur, S.; Gillet, C.; Sadeghi, H.; Allard, P.; Barbier, F. Effect of Trunk Inclination on Lower Limb Joint and Lumbar Moments in Able Men during the Stance Phase of Gait. Clin. Biomech. 2009, 24, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, B.D.; Wolf, E.J. Three-Dimensional Joint Reaction Forces and Moments at the Low Back during over-Ground Walking in Persons with Unilateral Lower-Extremity Amputation. Clin. Biomech. 2014, 29, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Armada-da-Silva, P.; Pool-Goudaazward, A.; Moniz-Pereira, V.; Veloso, A.P. Three Dimensional Multi-Segmental Trunk Kinematics and Kinetics during Gait: Test-Retest Reliability and Minimal Detectable Change. Gait Posture 2016, 46, 18–25. [Google Scholar] [CrossRef]
- Dallaway, A.; Duncan, M.; Griffen, C.; Tallis, J.; Renshaw, D.; Hattersley, J. Age-Related Differences in Trunk Kinematics and Interplanar Decoupling with the Pelvis during Gait in Healthy Older versus Younger Men. J. Clin. Med. 2023, 12, 2951. [Google Scholar] [CrossRef] [PubMed]
- Dallaway, A.; Hattersley, J.; Tallis, J.; Renshaw, D.; Griffen, C.; Duncan, M. Age-Related Changes in Concentric and Eccentric Isokinetic Peak Torque of the Trunk Muscles in Healthy Older Versus Younger Men. J. Aging Phys. Act. 2021, 29, 941–951. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.M.; Irrgang, J.J. A Comparison of a Modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys. Ther. 2001, 81, 776–788. [Google Scholar] [CrossRef]
- Vicon Plug-in Gait. Product Guide—Foundation Notes. Available online: https://help.vicon.com/space/Nexus216/11607059/Plug-in+Gait+Reference+Guide (accessed on 10 June 2020).
- Sinclair, J.; Edmundson, C.J.; Brooks, D.; Hobbs, S.J.; Taylor, P.J. The Influence of Footwear Kinetic, Kinematic and Electromyographical Parameters on the Energy Requirements of Steady State Running. Mov. Sport. Sci. 2013, 80, 39–49. [Google Scholar] [CrossRef]
- Christophy, M.; Senan, N.A.F.; Lotz, J.C.; O’Reilly, O.M. A Musculoskeletal Model for the Lumbar Spine. Biomech. Model. Mechanobiol. 2012, 11, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.G.; Bouxsein, M.L.; Anderson, D.E. Development and Validation of a Musculoskeletal Model of the Fully Articulated Thoracolumbar Spine and Rib Cage. J. Biomech. Eng. 2015, 137, 0810031. [Google Scholar] [CrossRef] [PubMed]
- de Zee, M.; Hansen, L.; Wong, C.; Rasmussen, J.; Simonsen, E.B. A Generic Detailed Rigid-Body Lumbar Spine Model. J. Biomech. 2007, 40, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Raabe, M.E.; Chaudhari, A.M.W. An Investigation of Jogging Biomechanics Using the Full-Body Lumbar Spine Model: Model Development and Validation. J. Biomech. 2016, 49, 1238–1243. [Google Scholar] [CrossRef]
- Shourijeh, M.S.; Mehrabi, N.; McPhee, J. Forward Static Optimization in Dynamic Simulation of Human Musculoskeletal Systems: A Proof-of-Concept Study. J. Comput. Nonlinear Dyn. 2017, 12, 051005. [Google Scholar] [CrossRef]
- Derrick, T.R.; van den Bogert, A.J.; Cereatti, A.; Dumas, R.; Fantozzi, S.; Leardini, A. ISB Recommendations on the Reporting of Intersegmental Forces and Moments during Human Motion Analysis. J. Biomech. 2020, 99, 109533. [Google Scholar] [CrossRef]
- Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E.; Penny, W.D. Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: London, UK, 2007; ISBN 9780123725608. [Google Scholar]
- Le Huec, J.C.; Richards, J.; Tsoupras, A.; Price, R.; Léglise, A.; Faundez, A.A. The Mechanism in Junctional Failure of Thoraco-Lumbar Fusions. Part I: Biomechanical Analysis of Mechanisms Responsible of Vertebral Overstress and Description of the Cervical Inclination Angle (CIA). Eur. Spine J. 2018, 27, 129–138. [Google Scholar] [CrossRef]
- Sartor, C.; Alderink, G.; Greenwald, H.; Elders, L. Critical Kinematic Events Occurring in the Trunk during Walking. Hum. Mov. Sci. 1999, 18, 669–679. [Google Scholar] [CrossRef]
- Schloemer, S.A.; Thompson, J.A.; Silder, A.; Thelen, D.G.; Siston, R.A. Age-Related Differences in Gait Kinematics, Kinetics, and Muscle Function: A Principal Component Analysis. Ann. Biomed. Eng. 2017, 45, 695–710. [Google Scholar] [CrossRef]
- Estrázulas, J.A.; Estrázulas, J.A.; de Jesus, K.; de Jesus, K.; da Silva, R.A.; Libardoni dos Santos, J.O. Evaluation Isometric and Isokinetic of Trunk Flexor and Extensor Muscles with Isokinetic Dynamometer: A Systematic Review. Phys. Ther. Sport 2020, 45, 93–102. [Google Scholar] [CrossRef]
- Guilhem, G.; Giroux, C.; Couturier, A.; Maffiuletti, N.A. Validity of Trunk Extensor and Flexor Torque Measurements Using Isokinetic Dynamometry. J. Electromyogr. Kinesiol. 2014, 24, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ferrada, W.; Chirosa-Rios, L.; Rodriguez-Perea, A.; Jerez-Mayorga, D.; Chirosa-Rios, I. Isokinetic Trunk Strength in Acute Low Back Pain Patients Compared to Healthy Subjects: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 2576. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ferrada, W.; Chirosa-Rios, L.; Martinez-Garcia, D.; Rodríguez-Perea, Á.; Jerez-Mayorga, D. Reliability of Trunk Strength Measurements with an Isokinetic Dynamometer in Non-Specific Low Back Pain Patients: A Systematic Review. J. Back Musculoskelet. Rehabil. 2022, 35, 937–948. [Google Scholar] [CrossRef]
- Zouita Ben Moussa, A.; Zouita, S.; Ben Salah, F.; Behm, D.; Chaouachi, A. Isokinetric Trunk Strength, Validity, Reliability, Normative Data and Relation to Physical Performance and Low Back Pain: A Review of the Literature. Int. J. Sports Phys. Ther. 2020, 15, 160–174. [Google Scholar] [CrossRef]
- Chung, C.Y.; Park, M.S.; Lee, S.H.; Kong, S.J.; Lee, K.M. Kinematic Aspects of Trunk Motion and Gender Effect in Normal Adults. J. Neuroeng. Rehabil. 2010, 7, 9. [Google Scholar] [CrossRef]
- Titus, A.W.; Hillier, S.; Louw, Q.A.; Inglis-Jassiem, G. An Analysis of Trunk Kinematics and Gait Parameters in People with Stroke. Afr. J. Disabil. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Konz, R.J.; Fatone, S.; Stine, R.L.; Ganju, A.; Gard, S.A.; Ondra, S.L. A Kinematic Model to Assess Spinal Motion during Walking. Spine 2006, 31, E898–E906. [Google Scholar] [CrossRef]
- Gutierrez, E.M.; Bartonek, Å.; Haglund-Åkerlind, Y.; Saraste, H. Centre of Mass Motion during Gait in Persons with Myelomeningocele. Gait Posture 2003, 18, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zou, J.; Lu, P.; Hu, J.; Cai, Y.; Xiao, C.; Li, G.; Zeng, Q.; Zheng, M.; Huang, G.Z. Analysis of Lumbar Spine Loading during Walking in Patients with Chronic Low Back Pain and Healthy Controls: An OpenSim-Based Study. Front. Bioeng. Biotechnol. 2024, 12, 1377767. [Google Scholar] [CrossRef]
- Smith, J.A.; Stabbert, H.; Bagwell, J.J.; Teng, H.-L.; Wade, V.; Lee, S.-P. Do People with Low Back Pain Walk Differently? A Systematic Review and Meta-Analysis. J. Sport Health Sci. 2022, 11, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Kent-Braun, J.A. Skeletal Muscle Fatigue in Old Age: Whose Advantage? Exerc. Sport Sci. Rev. 2009, 37, 3–9. [Google Scholar] [CrossRef]
- Sundberg, C.W.; Kuplic, A.; Hassanlouei, H.; Hunter, S.K. Mechanisms for the Age-Related Increase in Fatigability of the Knee Extensors in Old and Very Old Adults. J. Appl. Physiol. 2018, 125, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Allman, B.L.; Rice, C.L. Neuromuscular Fatigue and Aging: Central and Peripheral Factors. Muscle Nerve 2002, 25, 785–796. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Younger Group (n = 12) | Older Group (n = 12) | Independent t-Test | Cohen’s d |
|---|---|---|---|---|
| Participant characteristics | ||||
| Age (years) | 24.7 ± 3.1 | 67.3 ± 6.0 | t(22) = −21.8, p < 0.001 | 8.92 |
| Height (m) | 1.78 ± 0.1 | 1.74 ± 0.1 | t(22) = 1.2, p = 0.23 | 0.40 |
| Mass (kg) | 76.4 ± 11.2 | 79.2 ± 10.8 | t(22) = -0.6, p = 0.55 | 0.25 |
| BMI (kg.m−2) | 24.1 ± 2.2 | 26.0 ± 2.7 | t(22) = −1.9, p = 0.07 | 0.77 |
| ODQ-m (%) | 2.2 ± 2.3 | 2.2 ± 3.5 | t(22) = 0.0, p = 1.00 | 0.00 |
| MVPA (hours per day) | 6.6 ± 1.4 | 6.3 ± 1.5 | t(22) = 0.5, p = 0.60 | 0.21 |
| Spatiotemporal data | ||||
| Walking Speed (m·s−1) | 1.45 ± 0.19 | 1.33 ± 0.16 | t(22) = 1.70, p = 0.10 | 0.69 |
| Cadence (steps·min−1) | 113.9 ± 5.8 | 113.0 ± 7.2 | t(22) = 0.34, p = 0.74 | 0.14 |
| Step Length (m) | 0.76 ± 0.08 | 0.71 ± 0.06 | t(22) = 1.85, p = 0.08 | 0.76 |
| Normalised Step Length | 0.43 ± 0.05 | 0.41 ± 0.03 | t(19.7) = 1.43, p = 0.17 | 0.59 |
| Step Width (m) | 0.14 ± 0.03 | 0.16 ± 0.03 | t(22) = −1.22, p = 0.23 | 0.50 |
| Neuromuscular Action and Gait Cycle Phase | Younger Group (Nm·kg−1) | Older Group (Nm·kg−1) | Independent t-Test | Cohen’s d |
|---|---|---|---|---|
| Extensor moment—LR | 0.78 ± 0.39 | 0.74 ± 0.26 | t(22) = 0.31, p = 0.76 | 0.13 |
| Extensor moment—MS * | 0.96 ± 0.45 | 0.62 ± 0.24 | t(22) = 2.28, p = 0.032 | 0.93 |
| Flexor moment—TS | 0.63 ± 0.27 | 0.70 ± 0.41 | t(22) = −0.50, p = 0.62 | 0.20 |
| Extensor moment—PSw | 0.97 ± 0.34 | 0.99 ± 0.25 | t(22) = −0.15, p = 0.89 | 0.06 |
| Extensor moment—ISw | 0.73 ± 0.29 | 0.63 ± 0.20 | t(22) = 1.01, p = 0.33 | 0.41 |
| Flexor moment—TSw * | 0.74 ± 0.34 | 1.05 ± 0.37 | t(22) = −2.16, p = 0.042 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dallaway, A.; Duncan, M.; Griffen, C.; Renshaw, D.; Tallis, J.; Hattersley, J. Age-Related Differences in the Functional Demand Placed on the Lumbar Spine during Walking in Healthy Older versus Younger Men. Geriatrics 2024, 9, 108. https://doi.org/10.3390/geriatrics9050108
Dallaway A, Duncan M, Griffen C, Renshaw D, Tallis J, Hattersley J. Age-Related Differences in the Functional Demand Placed on the Lumbar Spine during Walking in Healthy Older versus Younger Men. Geriatrics. 2024; 9(5):108. https://doi.org/10.3390/geriatrics9050108
Chicago/Turabian StyleDallaway, Alexander, Michael Duncan, Corbin Griffen, Derek Renshaw, Jason Tallis, and John Hattersley. 2024. "Age-Related Differences in the Functional Demand Placed on the Lumbar Spine during Walking in Healthy Older versus Younger Men" Geriatrics 9, no. 5: 108. https://doi.org/10.3390/geriatrics9050108
APA StyleDallaway, A., Duncan, M., Griffen, C., Renshaw, D., Tallis, J., & Hattersley, J. (2024). Age-Related Differences in the Functional Demand Placed on the Lumbar Spine during Walking in Healthy Older versus Younger Men. Geriatrics, 9(5), 108. https://doi.org/10.3390/geriatrics9050108







