Questionable Validity of Creatinine-Based eGFR in Elderly Patients but Cystatin C Is Helpful in First-Line Diagnostics
Abstract
:1. Introduction
- Are kidney disease patients correctly identified using the recommended first-line diagnostic test?
- 2.
- Are patients diagnosed using the recommended first-line diagnostic test assigned to the correct GFR stage?
2. Materials and Methods
Calculation of the eGFR
3. Results
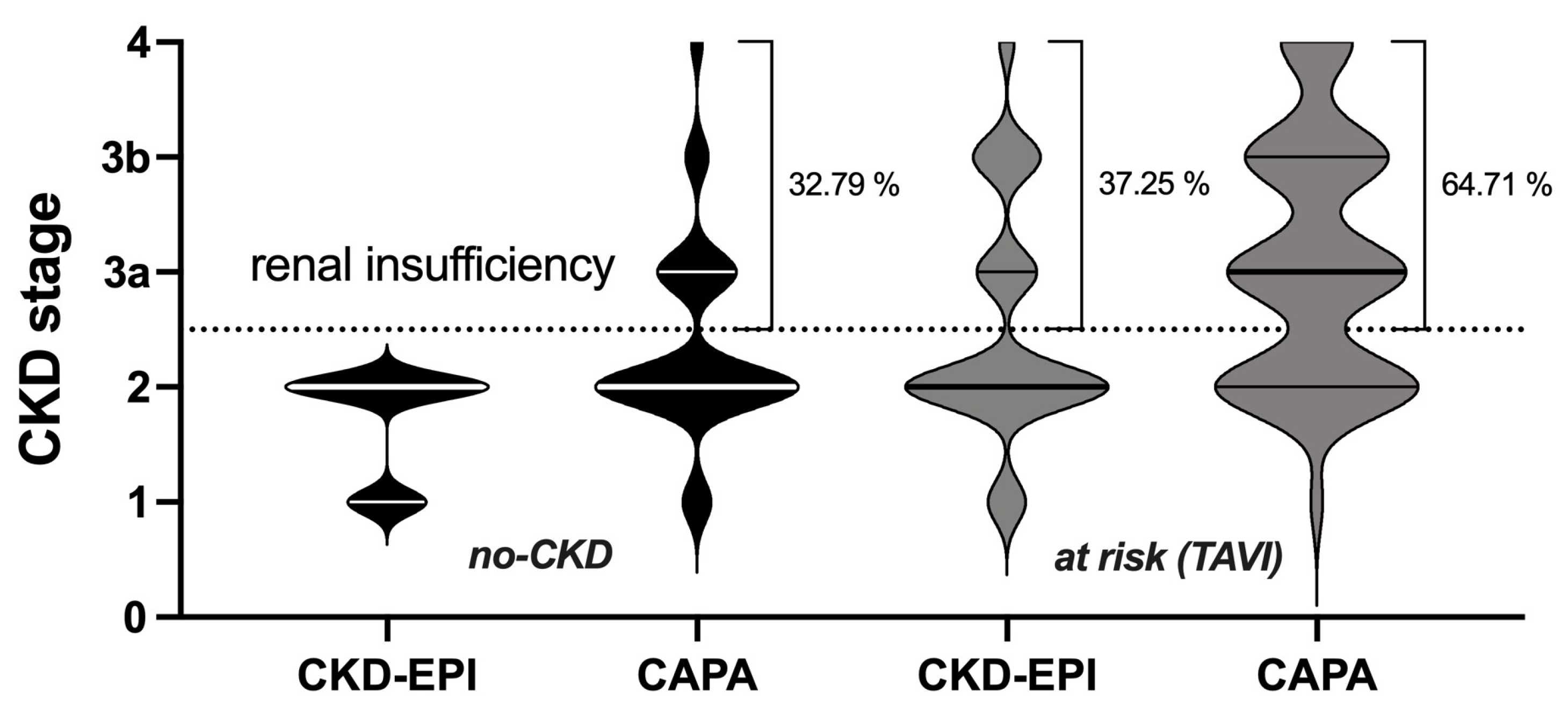
3.1. Cystatin C-Based eGFR (CAPA) Is Linearly Correlated with but Almost Always Lower Than Creatinine-Based eGFR (CKD-EPI)
3.2. Potential Impact of eGFR (CAPA) on CKD Stage and Clinical Classification of Kidney Disease
3.3. Sensitivity of eGFR (CKD-EPI) and eGFR (CAPA) for Kidney-Relevant Clinical Diagnoses
3.4. Correlation of Serum Urea and eGFR
4. Discussion
4.1. Sensitivity of First-Line Diagnostic for Kidney Dysfunction
4.2. Correct Assessment of CKD Stage
4.3. What Is the Ultimate Filtration Marker?
4.4. Limitations of Our Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAPA | Caucasian, Asian, pediatric, and adult |
| CKD | Chronic kidney disease |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| CVD | Cardiovascular disease |
| eGFR | Estimated glomerular filtration rate |
| GFR | Glomerular filtration rate |
| ICD-10 | International Statistical Classification of Diseases and Related Health Problems |
| IFFC | International Federation of Clinical Chemistry and Laboratory Medicine |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| TAVI | Transcatheter aortic valve implantation |
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.-Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003, 42, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Goel, K.; Kolte, D.; Khera, S.; Villablanca, P.A.; Aronow, W.S.; Bortnick, A.E.; Slovut, D.P.; Taub, C.C.; Kizer, J.R.; et al. Association of Chronic Kidney Disease With In-Hospital Outcomes of Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 2050–2060. [Google Scholar] [CrossRef]
- Thourani, V.H.; Forcillo, J.; Beohar, N.; Doshi, D.; Parvataneni, R.; Ayele, G.M.; Kirtane, A.J.; Babaliaros, V.; Kodali, S.; Devireddy, C.; et al. Impact of Preoperative Chronic Kidney Disease in 2531 High-Risk and Inoperable Patients Undergoing Transcatheter Aortic Valve Replacement in the PARTNER Trial. Ann. Thorac. Surg. 2016, 102, 1172–1180. [Google Scholar] [CrossRef]
- Kuwabara, K.; Zen, K.; Yashige, M.; Takamatsu, K.; Ito, N.; Kadoya, Y.; Yamano, M.; Yamano, T.; Nakamura, T.; Yaku, H.; et al. Cystatin C in risk prediction after transcatheter aortic valve replacement: A retrospective analysis. ESC Heart Fail. 2022, 9, 2601–2609. [Google Scholar] [CrossRef]
- Raman, M.; Middleton, R.J.; Kalra, P.A.; Green, D. Estimating renal function in old people: An in-depth review. Int. Urol. Nephrol. 2017, 49, 1979–1988. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Delanaye, P.; Ebert, N.; Melsom, T.; Gaspari, F.; Mariat, C.; Cavalier, E.; Björk, J.; Christensson, A.; Nyman, U.; Porrini, E.; et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: A review. Part 1: How to measure glomerular filtration rate with iohexol? Clin. Kidney J. 2016, 9, 682–699. [Google Scholar] [CrossRef]
- Delanaye, P.; Melsom, T.; Ebert, N.; Bäck, S.-E.; Mariat, C.; Cavalier, E.; Björk, J.; Christensson, A.; Nyman, U.; Porrini, E.; et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: A review. Part 2: Why to measure glomerular filtration rate with iohexol? Clin. Kidney J. 2016, 9, 700–704. [Google Scholar] [CrossRef]
- Thomas, L. Labor und Diagnose Indikation und Bewertung von Laborbefunden für Die medizinische Diagnostik 1; Th-Books-Verlagsgesellschaft: Frankfurt/Main, Germany, 2012. [Google Scholar]
- Shemesh, O.; Golbetz, H.; Kriss, J.P.; Myers, B.D. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985, 28, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Thakkar, H.; Edwards, R.G.; Wilkie, M.; White, T.; Grubb, A.O.; Price, C.P. Serum cystatin C measured by automated immunoassay: A more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995, 47, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Luis-Lima, S.; Escamilla-Cabrera, B.; Negrín-Mena, N.; Estupiñán, S.; Delgado-Mallén, P.; Marrero-Miranda, D.; González-Rinne, A.; Miquel-Rodríguez, R.; Cobo-Caso, M.; Hernández-Guerra, M.; et al. Chronic kidney disease staging with cystatin C or creatinine-based formulas: Flipping the coin. Nephrol. Dial. Transplant. 2019, 34, 287–294. [Google Scholar] [CrossRef]
- Svensson-Färbom, P.; Ohlson Andersson, M.; Almgren, P.; Hedblad, B.; Engström, G.; Persson, M.; Christensson, A.; Melander, O. Cystatin C identifies cardiovascular risk better than creatinine-based estimates of glomerular filtration in middle-aged individuals without a history of cardiovascular disease. J. Intern. Med. 2014, 275, 506–521. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Matsushita, K.; Ärnlöv, J.; Inker, L.A.; Katz, R.; Polkinghorne, K.R.; Rothenbacher, D.; Sarnak, M.J.; Astor, B.C.; Coresh, J.; et al. Cystatin C versus creatinine in determining risk based on kidney function. N. Engl. J. Med. 2013, 369, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Hensey, M.; Murdoch, D.J.; Sathananthan, J.; Wood, D.A.; Webb, J.G. Impact of Chronic Kidney Disease on Decision Making and Management in Transcatheter Aortic Valve Interventions. Can. J. Cardiol. 2019, 35, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Grubb, A.; Blirup-Jensen, S.; Lindström, V.; Schmidt, C.; Althaus, H.; Zegers, I. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin. Chem. Lab. Med. 2010, 48, 1619–1621. [Google Scholar] [CrossRef]
- Phinney, C.S.; Murphy, K.E.; Welch, M.J.; Ellerbe, P.M.; Long, S.E.; Pratt, K.W.; Schiller, S.B.; Sniegoski, L.T.; Rearick, M.S.; Vetter, T.W.; et al. Definitive method certification of clinical analytes in lyophilized human serum: NIST Standard Reference Material (SRM) 909b. Fresenius J. Anal. Chem. 1998, 361, 71–80. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Grubb, A.; Horio, M.; Hansson, L.-O.; Björk, J.; Nyman, U.; Flodin, M.; Larsson, A.; Bökenkamp, A.; Yasuda, Y.; Blufpand, H.; et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin. Chem. 2014, 60, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Potok, O.A.; Katz, R.; Bansal, N.; Siscovick, D.S.; Odden, M.C.; Ix, J.H.; Shlipak, M.G.; Rifkin, D.E. The Difference Between Cystatin C- and Creatinine-Based Estimated GFR and Incident Frailty: An Analysis of the Cardiovascular Health Study (CHS). Am. J. Kidney Dis. 2020, 76, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.L.; Verhave, J.C.; Spiegelman, D.; Hillege, H.L.; de Zeeuw, D.; Curhan, G.C.; de Jong, P.E. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004, 65, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Glassock, R.J.; Rule, A.D. Optimally predicting mortality with kidney function markers is not the same as optimally determining how kidney function predicts mortality. Nephrol. Dial. Transplant. 2017, 32, 585–587. [Google Scholar] [CrossRef]
- Shardlow, A.; McIntyre, N.J.; Fraser, S.D.S.; Roderick, P.; Raftery, J.; Fluck, R.J.; McIntyre, C.W.; Taal, M.W. The clinical utility and cost impact of cystatin C measurement in the diagnosis and management of chronic kidney disease: A primary care cohort study. PLoS Med. 2017, 14, e1002400. [Google Scholar] [CrossRef]
- Beunders, R.; van Groenendael, R.; Leijte, G.P.; Kox, M.; Pickkers, P. Proenkephalin Compared to Conventional Methods to Assess Kidney Function in Critically Ill Sepsis Patients. Shock 2020, 54, 308–314. [Google Scholar] [CrossRef]
- von Groote, T.; Albert, F.; Meersch, M.; Koch, R.; Porschen, C.; Hartmann, O.; Bergmann, D.; Pickkers, P.; Zarbock, A. Proenkephalin A 119–159 predicts early and successful liberation from renal replacement therapy in critically ill patients with acute kidney injury: A post hoc analysis of the ELAIN trial. Crit. Care 2022, 26, 333. [Google Scholar] [CrossRef]
- (DGKL) DGfrNDDGfrKCuLeV. Interdisziplinäre S2k-Leitlinie: Rationelle Labordiagnostik zur Abklärung Akuter Nierenschädigungen und Progredienter Nierenerkrankungen: Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e. V. (AWMF). 2021. Available online: https://register.awmf.org/assets/guidelines/115-001l_S2k_Rationelle_Labordiagnostik_Abklärung_Nierenschädigungen_Nierenerkrankungen_2021-09_01.pdf (accessed on 9 January 2023).
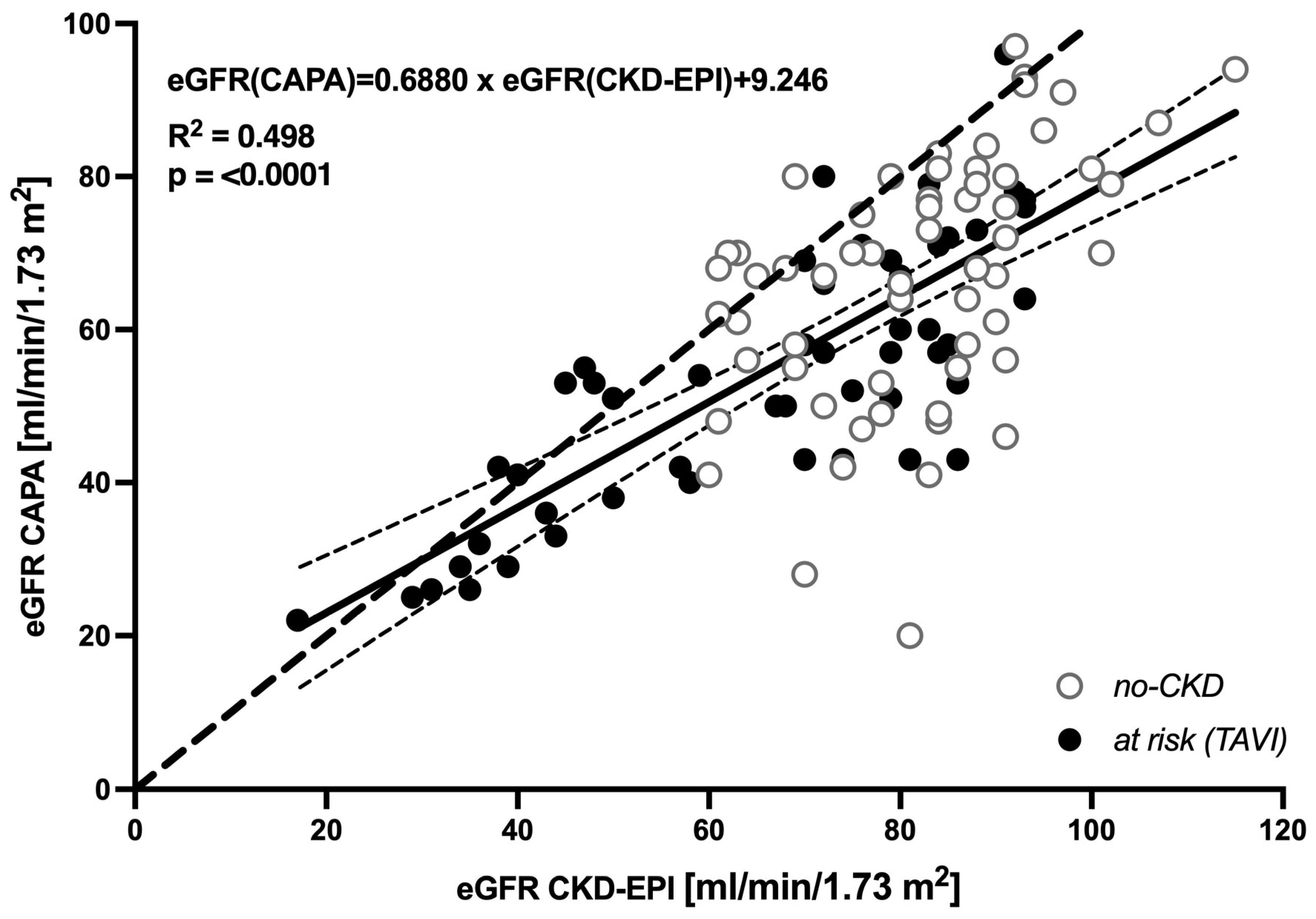
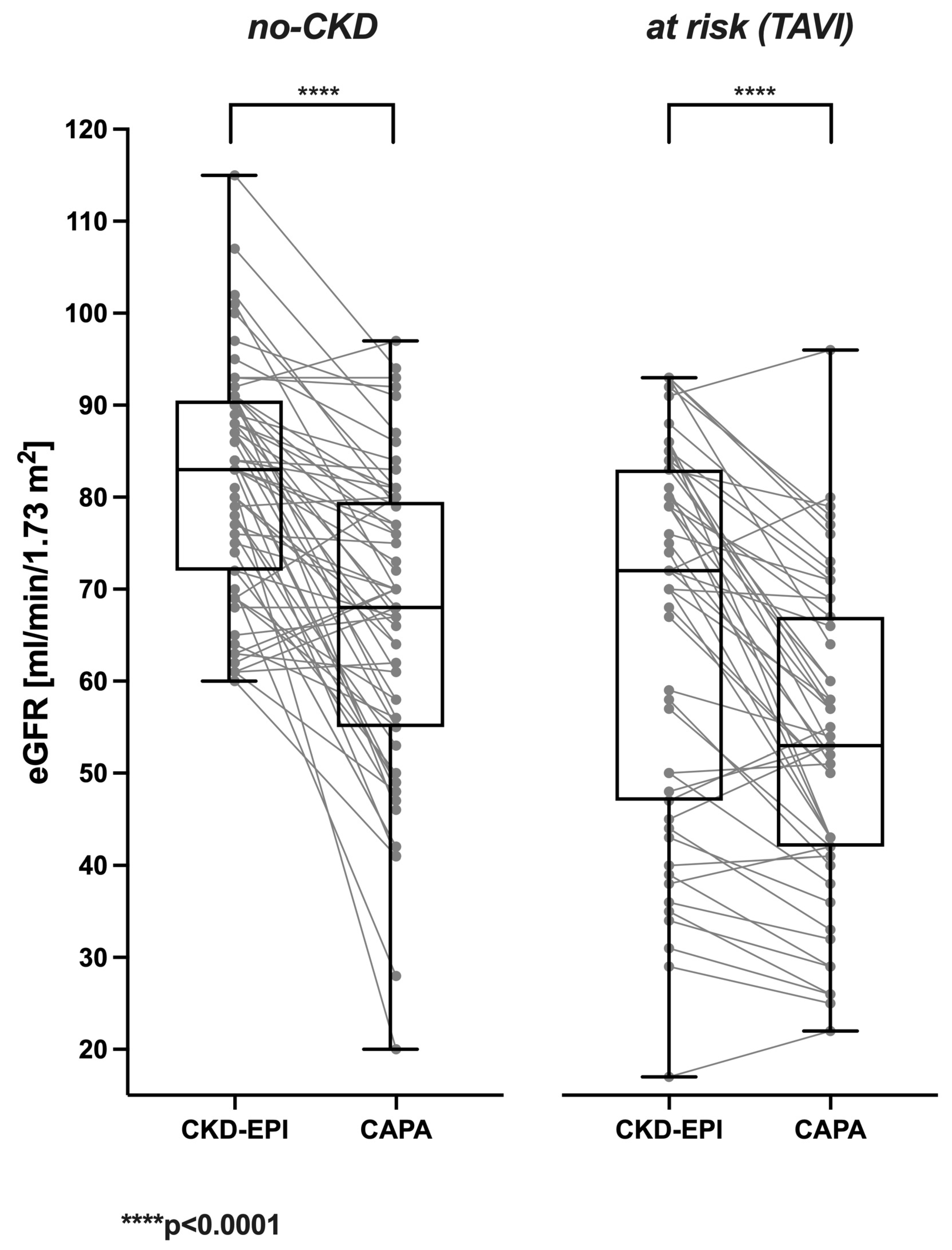
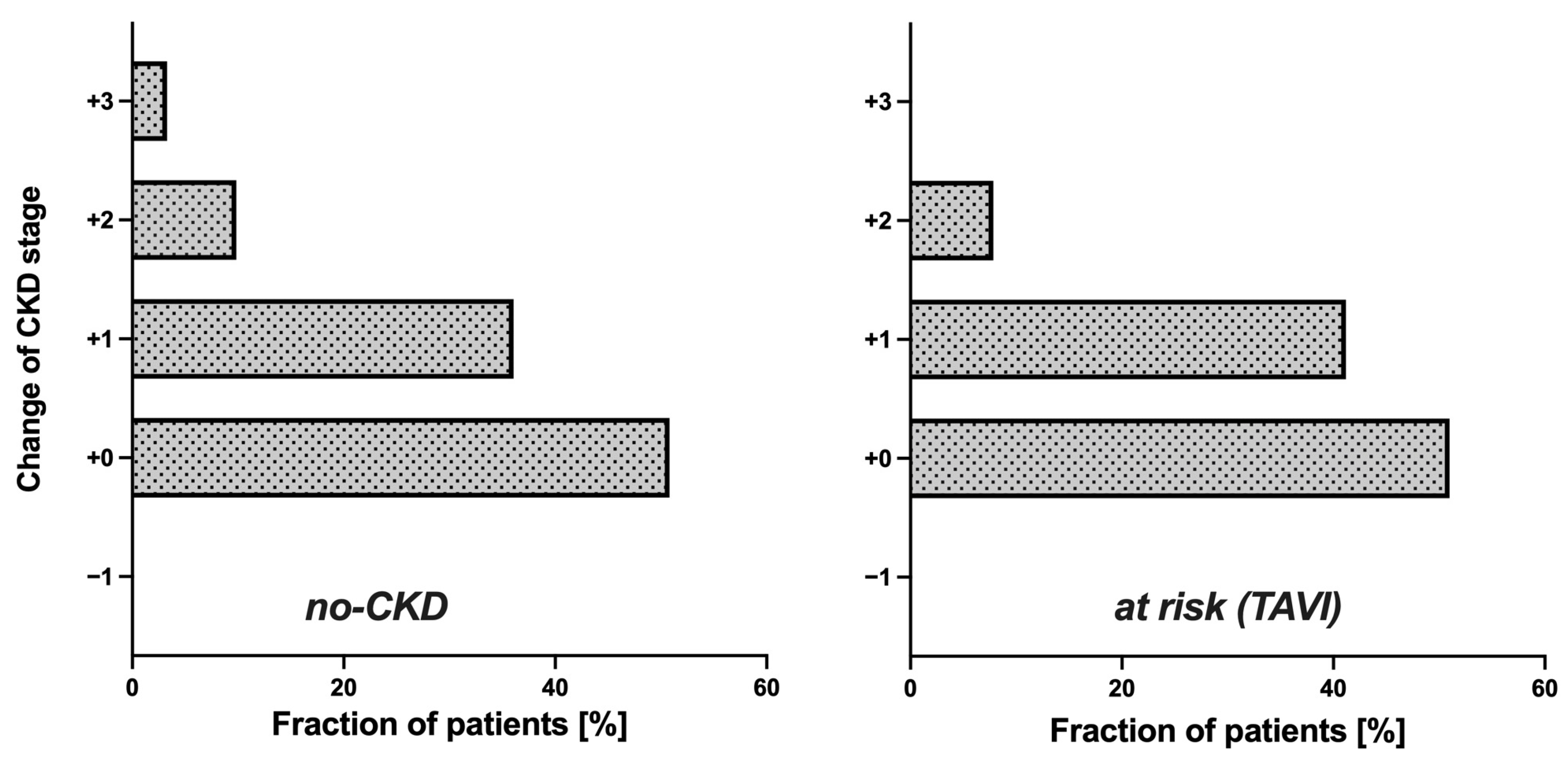
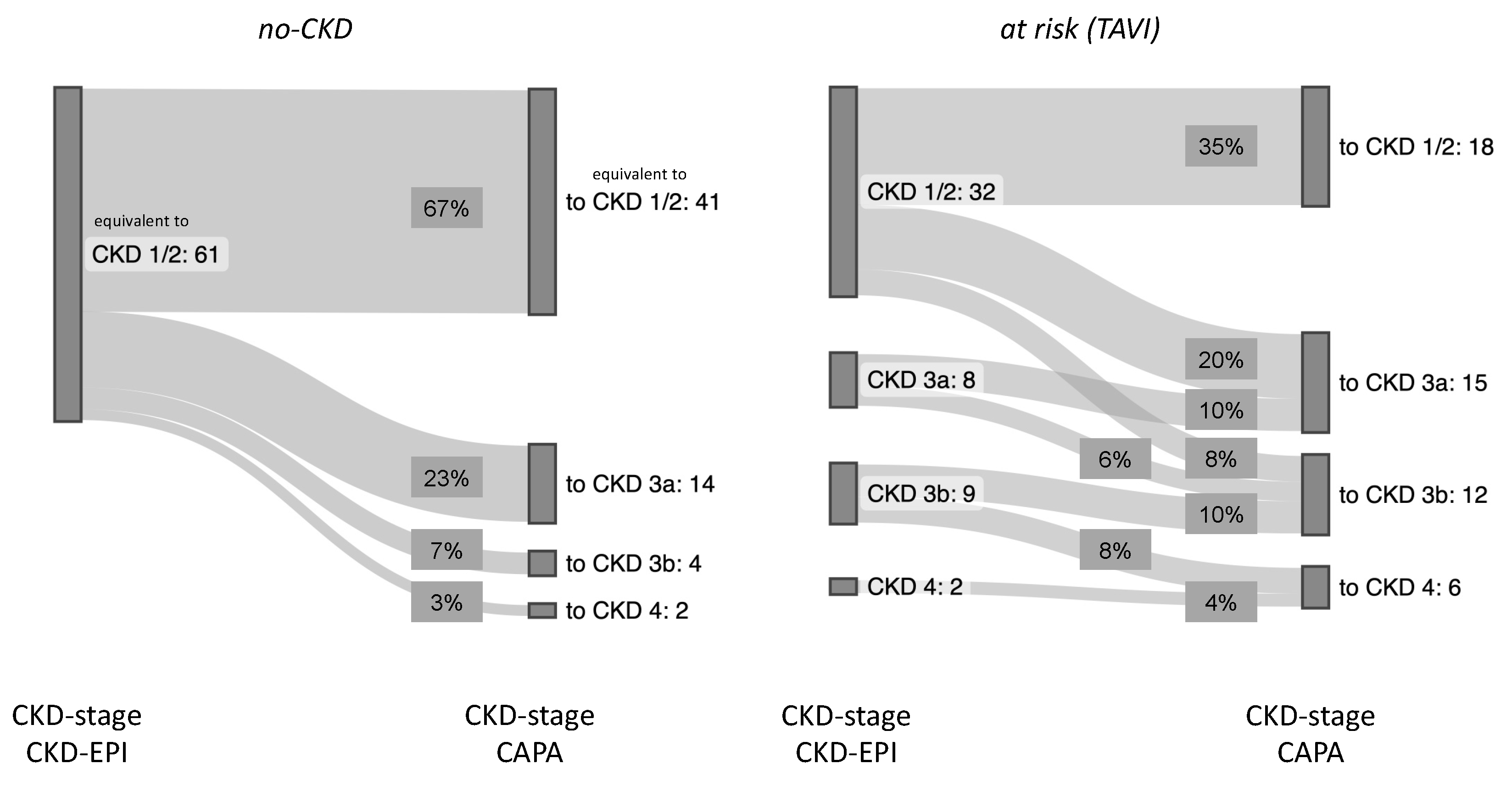

| Reclassification (eGFR—CAPA) | N (%) | Etiologies 1 (Number of Patients) |
|---|---|---|
| Remained equivalent CKD stage 1/2 | 3 (7) | Renal disease: Acute or chronic kidney disease (1) Neoplasia (kidney) or loss of kidney (2) |
| 38 (93) | Nonrenal disease: Arthropathy (1) Autoimmune disease (2) Cardiovascular disease (1) Disease of the bile ducts (1) Disease of the eyes (2) Hematological disease (2) Infectious disease (1) Neoplasia/dysplasia (nonrenal) (7) Neurological disease (4) Rheumatic disease (13) Unknown/others (4) | |
| Reclassified CKD stage 3a, 3b or 4 | 6 (30) | Renal disease: Acute or chronic kidney disease (3) Kidney transplant (1) Neoplasia (kidney) or loss of kidney (2) |
| 14 (70) | Nonrenal disease: Autoimmune disease (2) Disease of the eyes (1) Dysphagia (1) Flanks/abdominal pain (2) Gait and mobility disorders (1) Infectious disease (1) Neoplasia/dysplasia (nonrenal) (3) Rheumatic disease (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geißer, D.; Hetzel, L.; Westenfeld, R.; Boege, F. Questionable Validity of Creatinine-Based eGFR in Elderly Patients but Cystatin C Is Helpful in First-Line Diagnostics. Geriatrics 2023, 8, 120. https://doi.org/10.3390/geriatrics8060120
Geißer D, Hetzel L, Westenfeld R, Boege F. Questionable Validity of Creatinine-Based eGFR in Elderly Patients but Cystatin C Is Helpful in First-Line Diagnostics. Geriatrics. 2023; 8(6):120. https://doi.org/10.3390/geriatrics8060120
Chicago/Turabian StyleGeißer, Dario, Lina Hetzel, Ralf Westenfeld, and Fritz Boege. 2023. "Questionable Validity of Creatinine-Based eGFR in Elderly Patients but Cystatin C Is Helpful in First-Line Diagnostics" Geriatrics 8, no. 6: 120. https://doi.org/10.3390/geriatrics8060120
APA StyleGeißer, D., Hetzel, L., Westenfeld, R., & Boege, F. (2023). Questionable Validity of Creatinine-Based eGFR in Elderly Patients but Cystatin C Is Helpful in First-Line Diagnostics. Geriatrics, 8(6), 120. https://doi.org/10.3390/geriatrics8060120





