Abstract
Age-related loss of lower extremity muscle strength is pronounced in individuals with chronic kidney disease (CKD). In contrast, an increase in intrarenal flow pulsatility results in initial age-related changes in renal hemodynamics, leading to the development of CKD. To date, it remains unclear whether lower extremity muscle strength determines elevated renal flow pulsatility. This study aimed to determine the association of lower extremity muscle strength and function with intrarenal hemodynamics in individuals with and without CKD. One hundred seventy-six individuals without CKD (aged 63 ± 9 years) and 101 individuals with CKD (aged 66 ± 8 years) were included in this study. Using Doppler ultrasound, the renal resistive index (RI) was measured as a parameter of renal hemodynamics. Knee extensor muscle strength (KES), gait speed (GS), and the 30 s chair stand test (30s-CST) were used to measure lower extremity muscle strength and function. Multivariate analyses showed that GS and 30s-CST scores were independent determinants of renal RI, whereas the KES score was not associated with renal RI in individuals with and without CKD. In the two-way analysis of covariance, renal RI was the highest in individuals with CKD who had lower KES, GS, and 30s-CST scores. Reduced lower extremity muscle strength and function are independent determinants of elevated renal flow pulsatility in individuals with and without CKD.
1. Introduction
The kidneys are high-flow, low-resistance organs that receive approximately 20–25% of the cardiac output at rest [1]. Furthermore, the kidneys have numerous short arteries branching from major arteries, referred to as “strain vessels” [2]. While these kidney characteristics are essential for maintaining normal glomerular filtration, they also render them vulnerable to the mechanical stress of arterial pulsatility [3]. Continuous exposure of renal microcirculation to high flow pulsatility can lead to renal dysfunction and damage [4,5]. Therefore, close monitoring of the pulsatile components in the kidneys may contribute to the early detection of progressive renal dysfunction and damage in clinical settings.
Renal duplex ultrasonography has been used for noninvasive evaluation of blood flow pulsatility in the kidneys [6]. Previous studies have demonstrated that the renal resistive index (RI) derived from the pulsatile flow-velocity waveform is associated with several histological and biochemical findings of renal microvascular damage, such as peritubular capillary loss [7]. Moreover, high renal RI is associated with cardiovascular events and mortality in patients with hypertension, diabetes, and chronic kidney disease (CKD) [8,9,10,11,12]. Thus, the number of studies highlighting the clinical significance of renal RI is increasing. However, evidence regarding the determinants of renal RI values remains insufficient. Elucidating the determinants of renal RI is crucial when designing intervention strategies targeting renal flow pulsatility.
Our previous study reported that advancing age is associated with a progressive elevation of renal RI, which manifests during middle age and accelerates later in life [13]. Additionally, it has been demonstrated that renal RI is significantly influenced by extrarenal hemodynamic factors, such as central pulse pressure and stiffness [14]. In addition to these physiological determinants, we have reported a significant correlation between renal RI and handgrip strength in middle-aged and older individuals with normal kidney function [15]. In this study, the reduced handgrip strength was related to the elevated renal RI, independent of age and central hemodynamics, suggesting that reduced muscular strength may be a determinant of increased renal RI [15]. It has been shown that reduced muscle strength and function were associated with an increased risk of cardiovascular disease (CVD), such as coronary heart disease and stroke [16]. Reduced muscle strength and function can emerge due to multi-factorial mechanisms such as inflammation, oxidative stress, and metabolic disorders, all of which can also lead to vascular dysfunction [16]. We consider that these previous findings can extrapolate into the association of muscle strength and function with renal hemodynamics. However, it is worth noting that prior research only assessed handgrip strength, mainly reflecting upper limb strength, as a measure of muscular strength. It is essential to investigate the relationship between various measures of muscular strength, including lower extremity muscle strength, and renal RI to assert that muscular strength is a determinant of renal RI. Furthermore, there is room for investigation regarding whether a similar relationship is observed in a population with CKD. Therefore, the present study aimed to investigate the cross-sectional association of lower extremity muscle strength and function with renal flow pulsatility (i.e., renal RI) in individuals with and without CKD. We hypothesized that individuals with CKD who have decreased lower extremity muscle strength and function would have high renal flow pulsatility.
2. Materials and Methods
2.1. Participants
In this study, 333 adults were recruited through local newspaper advertisements, flyers, and the Department of Nephrology of the University of Tsukuba Hospital. The exclusion criteria were age < 45 years (n = 23), arrhythmia (n = 5), and unilateral nephrectomy (n = 4). Participants who ate or smoked on the morning of the measurement day (n = 5) or had missing data (n = 19) were also excluded from the analysis. The final sample included 277 participants comprising 176 individuals without CKD and 101 individuals with CKD who were diagnosed according to standard criteria [17]: estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 and/or urinary albumin creatinine ratio (ACR) ≥ 30 mg/g (Figure 1). Although the diagnosis of CKD usually requires that these conditions persist for at least 3 months, in the current study, eGFR and urinary ACR were measured at a single time point according to a previous report [18]. The participants were required to avoid engaging in vigorous exercise and ingesting alcohol and caffeine 24 h before the measurement. All measurements, except for the lower extremity muscle strength and function tests, were performed in the morning after 12 h of overnight fasting. This study was approved by the Ethics Committee of the University of Tsukuba Hospital (approval no. H30-161). The study conformed to the principles outlined in the Declaration of Helsinki, and written informed consent was obtained from all participants.
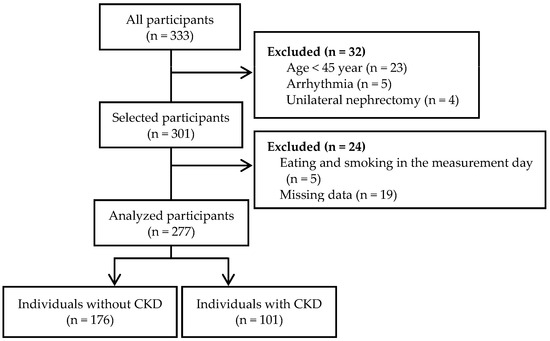
Figure 1.
Flow diagram.
2.2. Measurements
2.2.1. Renal Doppler Ultrasound
Renal blood flow velocity (BFV) in the intrarenal segmental arteries of each kidney was measured using Doppler ultrasonography with a 3.5 MHz convex array probe (Noblus C25, Hitachi Aloka Medical Ltd., Tokyo, Japan). All renal BFV measurements were performed with the focal zone set to the depth of the target artery, and the probe insonation angle to the target artery was set to less than 60°. Because breathing changes the position of the kidneys, the participants were instructed to hold their breath for 10–15 s during renal BFV measurements. Renal BFV was measured thrice (three segmental arteries in each kidney). Renal RI was calculated from renal flow pulsatility indices using the following equation: (peak systolic BFV—end-diastolic BFV)/peak systolic BFV [19]. The average of three measurements for each kidney (more than six times in total) was used for statistical analysis. The renal BFV measurement was performed by a well-trained examiner who understands the international standard procedure at a single center. This reduced the inter-operator error in the renal flow pulsatility assessment. Additionally, we presented the number of participants who had a renal RI over 0.70 because 0.70 was established as a cutoff value of renal RI to predict future clinical events [8,10].
2.2.2. Lower Extremity Muscle Strength and Function
We evaluated three indices that have been reported to be useful in assessing lower extremity muscle strength and function [20,21,22,23]. Knee extensor muscle strength (KES) and the 30 s chair stand test (30s-CST) were evaluated for lower extremity muscle strength, and gait speed (GS) was evaluated for lower extremity function. KES was measured using a handheld dynamometer (μTas; Anima, Tokyo, Japan). The handheld dynamometer was fixed to the leg, directly opposing the leg motion. Measurements were obtained from subjects sitting on the bed with their legs perpendicular to the floor and knees flexed approximately 90°. The participants gradually increased force to maximum extensions and then maintained a static state for approximately 3 s, during which the maximum force was noted. Isometric knee extension strength is strongly influenced by body mass; therefore, the values were divided by body mass [24,25]. The averages of the left and right maximum values were used for analysis [26]. GS was calculated for each participant using distance in meters and time in seconds. The walking distance was 10 m, and the participants were instructed to walk as fast as possible from a standing start position [27]. Further, 30s-CST was measured using a chair with a height of 40 cm. The participants repeated the action, rose to a complete stand, and returned to their initial seated position. The number of complete stands performed within 30 s was used for analysis [28,29].
2.2.3. Biochemical Measurements
Blood samples were collected from antecubital veins. Serum samples were used to measure high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglyceride, fasting blood glucose, creatinine, and cystatin C levels. Hemoglobin A1c (HbA1c) levels were measured in whole blood samples. Two eGFR values were calculated from serum creatinine and cystatin C levels [30,31], and the values were averaged to improve the accuracy of these estimates [32]. Urinary albumin and creatinine concentrations were measured in spot urine samples. The urinary albumin concentration was corrected for the urinary creatinine concentration to adjust for the effect of urine specific gravity.
2.2.4. Covariates
Anthropometric measurements were performed, and body mass index (BMI) was calculated from height and weight in kg/m2. Brachial systolic blood pressure (SBP), brachial diastolic blood pressure (DBP), heart rate, and pulse wave velocity (PWV) were measured using a semiautomated vascular testing device (Form PWV/ABI, Colin Medical Technology, Aichi, Japan). Carotid arterial pressure waveform was measured using applanation tonometry sensors (TU-100; Colin Medical Technology, Aichi, Japan). The pressure waveform data were fed into SphygmoCor software version 8.2 (AtCor Medical, Sydney, Australia), and a generalized transfer function was applied to estimate the aortic pulse pressure [33]. The carotid-femoral PWV, indicating aortic stiffness, was calculated by dividing the distance between the two arterial sites (carotid and femoral) where the tonometry sensors were attached by the arterial pulse transit time [34]. The levels of physical activity (metabolic equivalents [METs]∙hour/week) in daily life were assessed using the International Physical Activity Questionnaire Short Version [35]. In this study, the underlying conditions were diagnosed when one of the following criteria was met: hypertension (brachial SBP: ≥140 mmHg, brachial DBP: ≥90 mmHg, and/or antihypertensive medication use), diabetes mellitus (fasting blood glucose: ≥126 mg/dL, HbA1c: ≥6.5%, and/or hypoglycemic medication use), and dyslipidemia (HDL cholesterol: <40 mg/dL, LDL cholesterol: ≥140 mg/dL, triglyceride: ≥150 mg/dL, and/or use of antidyslipidemic medication).
2.3. Statistical Analysis
Data are expressed as mean ± standard deviation, median with interquartile range, or frequencies and percentages, unless otherwise indicated. Statistical analyses were performed using SPSS Statistics software 28.0 (IBM Japan Ltd., Tokyo, Japan). Statistical significance was set at p < 0.05. The group differences between individuals with and without CKD were examined using the unpaired t-test, Mann–Whitney U test, or chi-square test. Univariate linear associations were assessed using Pearson’s correlation coefficients. Multiple linear regression analysis was used to estimate the regression coefficients (β) for the association of lower extremity muscle strength and function with renal RI in all participants, individuals without CKD, and individuals with CKD, separately. All models were adjusted for age, sex, BMI, eGFR, urinary ACR, comorbidities (hypertension, diabetes mellitus, and dyslipidemia), physical activity level, heart rate, aortic pulse pressure, and carotid-femoral PWV. Two-way analysis of covariance (ANCOVA) was performed to examine the interaction between lower extremity muscle strength and function measures (KES, GS, and 30s-CST) and CKD. Participants were allocated to one of four categories based on the median split of each lower extremity muscle strength measure and the presence of CKD. Covariates used in the multiple linear regression analysis were adjusted. Post hoc comparisons were corrected using Bonferroni’s method.
3. Results
Table 1 shows the characteristics of all participants. The mean age of all participants, those without CKD, and those with CKD were 64 ± 9 years, 63 ± 9 years, and 66 ± 8 years, respectively. There were no differences in height, BMI, KES score, physical activity levels, or prevalence of dyslipidemia between individuals with and without CKD. The proportion of women, eGFR values, GS, and 30s-CST scores were lower in individuals with CKD than in those without CKD. In contrast, age, body mass, urinary ACR, heart rate, brachial SBP, brachial DBP, mean arterial pressure, aortic pulse pressure, carotid-femoral PWV, renal RI, prevalence rates of hypertension and diabetes mellitus, and rate of medication use were higher in individuals with CKD than in those without CKD.

Table 1.
Characteristics of the participants.
The renal RI for individuals without and with CKD by GFR stage is shown in Table 2. Among participants with eGFR stage 1, significant differences in renal RI were shown between those without CKD and those with CKD (p = 0.032). In participants with eGFR stage 2, renal RI did not differ significantly between those without CKD and those with CKD (p = 0.161).

Table 2.
Renal RI in individuals with and without CKD by GFR stage.
As presented in Figure 2, renal RI was negatively correlated with KES, GS, and 30s-CST scores in all participants and individuals with CKD; however, in those without CKD, renal RI was negatively correlated with GS and 30s-CST scores but not significantly with the KES score. In all participants, renal RI positively correlated with age, BMI, urinary ACR, brachial SBP, aortic pulse pressure, and carotid-femoral PWV and negatively correlated with eGFR. In contrast, renal RI showed no significant correlation with heart rate, brachial DBP, mean arterial pressure, or physical activity levels. In adults without CKD, renal RI positively correlated with age, urinary ACR, brachial SBP, aortic pulse pressure, and carotid-femoral PWV and negatively correlated with eGFR (Table 3).
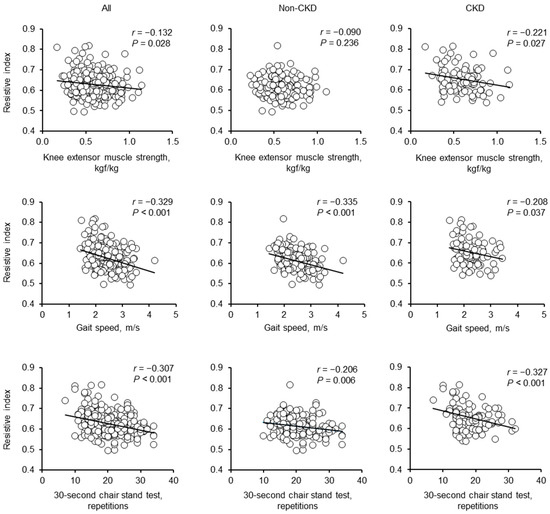
Figure 2.
Univariate correlations between the renal RI and lower extremity muscle strength in individuals without CKD, individuals with CKD, and all participants.

Table 3.
Simple correlation matrix for renal RI.
In contrast, renal RI showed no significant correlation with BMI, heart rate, brachial DBP, mean arterial pressure, or physical activity level. In individuals with CKD, renal RI was positively correlated with age, BMI, urinary ACR, aortic pulse pressure, and carotid-femoral PWV and negatively correlated with eGFR and brachial DBP. In contrast, renal RI showed no significant correlation with heart rate, brachial SBP, mean arterial pressure, or physical activity levels.
The results of the multiple linear regression analyses of renal RI are presented in Table 4 (all participants), Table 5 (individuals without CKD), and Table 6 (individuals with CKD). KES score was not significantly associated with renal RI after adjusting for covariates such as age and sex in all participants, adults without CKD, and patients with CKD (β = −0.003, p = 0.952; β = −0.097, p = 0.129; β = −0.093, p = 0.235, respectively). In contrast, GS and 30s-CST scores remained independent determinants of renal RI, even after adjusting for covariates.

Table 4.
Multivariable-adjusted linear regression models for renal RI in all participants.

Table 5.
Multivariable-adjusted linear regression models for renal RI in individuals without CKD.

Table 6.
Multivariable-adjusted linear regression models for renal RI in individuals with CKD.
A two-way ANCOVA was used to compare renal RI according to CKD status and lower extremity muscle strength status, with adjustment for the same covariates as in the multiple linear regression models (Figure 3). The two-way ANCOVA showed an interaction effect for KES score and CKD status (Figure 3A), but not for GS and 30s-CST scores (Figure 3B,C). However, some results showed significant main effects for CKD status and/or each lower extremity muscle strength measure. Specifically, individuals with CKD who had a lower KES score had a higher renal RI than those with CKD who had a higher KES score and those without CKD who had a lower KES score. Individuals with CKD who had a lower GS score had a higher renal RI than those without CKD who had a lower GS score. Moreover, individuals with CKD who had a higher GS score had a higher renal RI than those without CKD who had a higher GS score. Individuals with CKD who had a lower 30s-CST score had a higher renal RI than those with CKD who had a higher 30s-CST score and those without CKD who had a lower 30s-CST score.
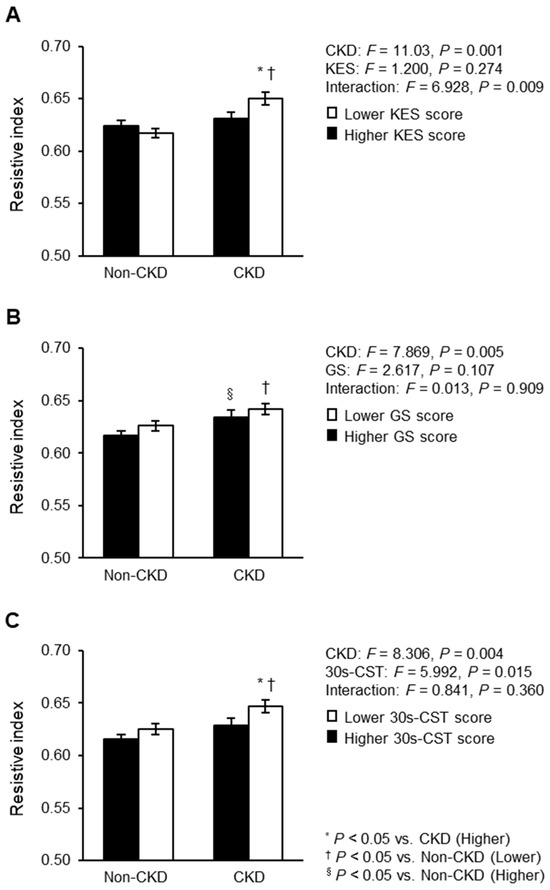
Figure 3.
Renal RI compared between CKD status and lower extremity muscle strength: (A) KES; (B) GS; (C) 30s-CST. Mean ± standard error. These associations were adjusted for age, sex, BMI, comorbidities (hypertension, diabetes mellitus, and dyslipidemia), carotid-femoral PWV, aortic pulse pressure, heart rate, and physical activity. RI, resistive index; CKD, chronic kidney disease; CKD, chronic kidney disease; BMI, body mass index; PWV, pulse wave velocity; KES, knee extensor muscle strength; GS, gait speed; 30s-CST, 30 s chair stand test. F statistics value was evaluated by two-way analysis of covariance. * p < 0.05 vs. CKD group with high lower extremity muscle strength and function; † p < 0.05 vs. non-CKD group with low lower extremity muscle strength and function; § p < 0.05 vs. non-CKD group with high lower extremity muscle strength and function.
4. Discussion
This cross-sectional study examined the association of lower extremity muscle strength and function with renal flow pulsatility in individuals with and without CKD. GS and 30s-CST scores showed independent negative correlations with renal RI. Individuals with CKD who had lower KES, GS, and 30s-CST scores had the highest renal RI, compared with other participants. These findings suggest that lower extremity muscle strength and function may be potential determinants of renal flow pulsatility in individuals with and without CKD.
It has been well-established that age and central hemodynamics are closely related to renal flow pulsatility [13,14]. Age, aortic pulse pressure, and carotid-femoral PWV were positively correlated with renal RI in the present study (Table 3). Therefore, we performed multiple linear regression analyses to adjust for potential covariates of renal flow pulsatility, including age and central hemodynamic parameters (Table 4, Table 5 and Table 6). The results showed that the KES score was not significantly associated with renal RI. In contrast, GS and 30s-CST scores were significantly associated with renal RI after adjusting for covariates in all participants, those without CKD, and those with CKD. Among the central hemodynamic indices, aortic pulse pressure was shown to be independently associated with renal RI. In a previous study, among the central hemodynamic indices, aortic pulse pressure was shown to be strongly related to renal RI [14]. Therefore, in the multiple regression analysis of this study, we considered that renal RI was significantly associated with aortic pulse pressure rather than hypertension or carotid-femoral PWV. These results suggest mechanistic links between renal flow pulsatility, age, aortic pulse pressure, and lower extremity muscle strength and function.
The increase in renal flow pulsatility with aging and arteriosclerosis confirmed before renal function decline is caused by decreased eGFR. Therefore, individuals with CKD have a higher renal RI than those without CKD [36]. In addition, inspired by our previous study showing that participants with low handgrip strength have high renal RI [15], we hypothesized that individuals with CKD who have decreased lower extremity muscular strength and function would have a higher renal flow pulsatility than those without CKD. Therefore, a two-way ANCOVA was performed to examine the effects of CKD status, lower extremity muscle strength level, and their interaction on renal flow pulsatility. Although a statistically significant interaction was observed only for KES, individuals with CKD who had reduced lower extremity muscle strength and function consistently had high renal RI. Renal RI was comparable between the decreased and increased lower extremity muscle strength groups in individuals without CKD; however, among individuals with CKD, those who had low KES or 30s-CST scores had a higher renal RI than did those who had high KES or 30s-CST scores. These results indicate that renal flow pulsatility may be more strongly affected by lower extremity muscle weakness in individuals with CKD than in those without.
Sarcopenia, defined as loss of muscle mass and decreased muscle strength, is widely known to be associated with vascular damage. This association involves multifactorial mechanisms such as chronic inflammation, oxidative stress, nutrition, and insulin regulation [37,38,39,40]. Furthermore, it has been shown that myokines secreted by muscle may be associated with the inhibition of vascular aging (e.g., arterial stiffness) [41,42]. Hanatani et al. reported that skeletal muscle growth in skeletal muscle-specific Akt1 transgenic mice suppressed renal injury, and these changes were accompanied by increased endothelial nitric oxide synthase (eNOS) phosphorylation in the kidneys [43]. Therefore, muscle mass loss is thought to contribute to increased renal flow pulsatility by decreasing eNOS signaling in the kidneys. Since the lower extremity muscles comprise the majority of the entire body’s muscles (approximately 60–70%), the mechanism reported above may be partially involved in the association of lower extremity muscle strength and function with renal flow pulsatility. However, it should be noted that the KES score was not significantly associated with renal RI in the multiple regression analyses. The inconsistent results may depend on residual confounding such as nutrition and inflammatory status, non-linear relationship of renal RI with KES, and limited sample size. Future studies should confirm the negative association between KES and renal RI while overcoming these methodological weaknesses.
Our previous study showed that participants with low handgrip strength have high renal RI [15]. This study suggests that reduced lower extremity muscle strength and function determines high renal flow pulsatility. Based on the results of this study, it is difficult to conclude which muscle strength is more important in renal RI. However, a previous study of participants with stage 2–4 CKD reported that measures of lower extremity performance were more than 30% lower than predicted, but handgrip strength was relatively preserved [44]. In addition, among the physical functions, impaired lower extremity performance is common in patients with CKD and has been reported to be strongly associated with all-cause mortality [44]. Therefore, we consider lower extremity muscle strength and function to be more related to renal flow pulsatility compared with upper extremity muscle strength.
Lower extremity muscle strength and function are essential in maintaining activities of daily living (ADL) and quality of life (QOL), and these declines are among the symptoms of sarcopenia [21]. Considering the results of this study, strategies to maintain lower extremity muscle strength may be effective in preserving not only ADL and QOL, but also low renal flow pulsatility. The effectiveness of resistance training in maintaining lower extremity muscle strength has been reported in several studies of individuals with and without CKD [45,46]. In a 12-week resistance training intervention study, the control group reported decreased renal function, whereas the resistance training group reported slight improvements in renal function [47]. Future studies are needed to determine whether an intervention to maintain lower extremity muscle strength (e.g., resistance training) is effective in preventing increased renal flow pulsatility.
This study has several limitations. First, we could not identify causal links between renal flow pulsatility and lower extremity muscle strength and function because of the cross-sectional study design. Second, the sample size was relatively small, and there was a difference in the number of participants between individuals with and without CKD. Third, measurement errors may have occurred because the trunk, lower back, and other factors were not adequately fixed in the measurement of KES. However, handheld dynamometers are widely used as a relatively simple and easy way to quantitatively measure lower extremity muscle strength [48]. Fourth, this study did not perform the skeletal muscle mass assessments (e.g., the dual-energy X-ray absorptiometry method). It should be noted that the confirmed negative associations of renal RI with GS and 30s-CST were not adjusted for skeletal muscle mass. Fifth, physical activity was evaluated using a questionnaire rather than an accelerometer, which can be evaluated objectively. Finally, we cannot deny the presence of residual confounding such as nutrition and inflammatory status [37,38,39,40]. Future longitudinal or interventional studies are needed to increase the number of participants and further examine the association of lower extremity muscle strength and function with renal flow pulsatility.
5. Conclusions
The present study showed that GS and 30s-CST scores were significantly associated with renal RI in individuals with and without CKD. Furthermore, renal RI was particularly elevated in individuals with CKD who had reduced lower extremity muscle strength and function. The findings of this study have raised the possibility that maintenance of lower extremity muscle strength and function may be important in preventing elevated renal flow pulsatility, particularly in individuals with CKD.
Author Contributions
Conceptualization, N.N., K.K. and S.M. (Seiji Maeda); formal analysis, N.N., S.M. (Shoya Mori) and M.M.; investigation, N.N., S.M. (Shoya Mori) and M.M.; writing—original draft preparation, N.N.; writing—review and editing, K.K., S.M. (Shoya Mori), M.M., T.S., M.K.-o., C.S., K.Y. and S.M. (Seiji Maeda); supervision, S.M. (Seiji Maeda); project administration, K.K. and S.M. (Seiji Maeda); funding acquisition, S.M. (Seiji Maeda). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by a Grant-in-Aid for Scientific Research KAKENHI from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (grant number 19H03995) and the MEXT Leading Initiative for Excellent Young Researchers Grant Number JPMXS0320200234. N.N. received a Grant-in-Aid for Research Fellowships from Japan Science and Technology (JPMJSP2124).
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University of Tsukuba Hospital (approval no. H30-161).
Informed Consent Statement
Informed consent was obtained from all the participants involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data were not publicly available due to privacy concerns.
Acknowledgments
We thank the study participants for their effort and time contributing to the study. Also, the authors are grateful to the members of our laboratory (University of Tsukuba) and Michiru Hotta (University of Tsukuba) for their technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Laughlin, M.H.; Davis, M.J.; Secher, N.H.; van Lieshout, J.J.; Arce-Esquivel, A.A.; Simmons, G.H.; Bender, S.B.; Padilla, J.; Bache, R.J.; Merkus, D.; et al. Peripheral circulation. Compr. Physiol. 2012, 2, 321–447. [Google Scholar]
- Ito, S.; Nagasawa, T.; Abe, M.; Mori, T. Strain vessel hypothesis: A viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens. Res. 2009, 32, 115–121. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Hashimoto, J. Mechanical factors in arterial aging: A clinical perspective. J. Am. Coll. Cardiol. 2007, 50, 1–13. [Google Scholar] [CrossRef]
- Martin, J.E.; Sheaff, M.T. Renal ageing. J. Pathol. 2007, 211, 198–205. [Google Scholar] [CrossRef]
- Zhou, X.J.; Rakheja, D.; Yu, X.; Saxena, R.; Vaziri, N.D.; Silva, F.G. The aging kidney. Kidney Int. 2008, 74, 710–720. [Google Scholar] [CrossRef]
- O’Neill, W.C. Renal resistive index: A case of mistaken identity. Hypertension 2014, 64, 915–917. [Google Scholar] [CrossRef]
- Ikee, R.; Kobayashi, S.; Hemmi, N.; Imakiire, T.; Kikuchi, Y.; Moriya, H.; Suzuki, S.; Miura, S. Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am. J. Kidney Dis. 2005, 46, 603–609. [Google Scholar] [CrossRef]
- Parolini, C.; Noce, A.; Staffolani, E.; Giarrizzo, G.F.; Costanzi, S.; Splendiani, G. Renal resistive index and long-term outcome in chronic nephropathies. Radiology 2009, 252, 888–896. [Google Scholar] [CrossRef]
- Doi, Y.; Iwashima, Y.; Yoshihara, F.; Kamide, K.; Hayashi, S.; Kubota, Y.; Nakamura, S.; Horio, T.; Kawano, Y. Renal resistive index and cardiovascular and renal outcomes in essential hypertension. Hypertension 2012, 60, 770–777. [Google Scholar] [CrossRef]
- Toledo, C.; Thomas, G.; Schold, J.D.; Arrigain, S.; Gornik, H.L.; Nally, J.V.; Navaneethan, S.D. Renal resistive index and mortality in chronic kidney disease. Hypertension 2015, 66, 382–388. [Google Scholar] [CrossRef]
- Boddi, M.; Bonizzoli, M.; Chiostri, M.; Begliomini, D.; Molinaro, A.; Tadini Buoninsegni, L.; Gensini, G.F.; Peris, A. Renal Resistive Index and mortality in critical patients with acute kidney injury. Eur. J. Clin. Investig. 2016, 46, 242–251. [Google Scholar] [CrossRef]
- Afsar, B.; Elsurer, R. Increased renal resistive index in type 2 diabetes: Clinical relevance, mechanisms and future directions. Diabetes Metab. Syndr. 2017, 11, 291–296. [Google Scholar] [CrossRef]
- Kosaki, K.; Tarumi, T.; Sugawara, J.; Tanahashi, K.; Kumagai, H.; Matsui, M.; Sugaya, T.; Osuka, Y.; Tanaka, K.; Kuro-o, M.; et al. Renal hemodynamics across the adult lifespan: Relevance of flow pulsatility to chronic kidney disease. Exp. Gerontol. 2021, 152, 111459. [Google Scholar] [CrossRef]
- Hashimoto, J.; Ito, S. Central pulse pressure and aortic stiffness determine renal hemodynamics: Pathophysiological implication for microalbuminuria in hypertension. Hypertension 2011, 58, 839–846. [Google Scholar] [CrossRef]
- Kosaki, K.; Kamijo-Ikemori, A.; Sugaya, T.; Tanahashi, K.; Kumagai, H.; Sawano, Y.; Osuka, Y.; Tanaka, K.; Kimura, K.; Shibagaki, Y.; et al. Association between muscular strength and intrarenal vascular resistance in middle-aged and older individuals. Exp. Gerontol. 2017, 91, 72–78. [Google Scholar] [CrossRef]
- Artero, E.G.; Lee, D.C.; Lavie, C.J.; España-Romero, V.; Sui, X.; Church, T.S.; Blair, S.N. Effects of muscular strength on cardiovascular risk factors and prognosis. J. Cardiopulm. Rehabil. Prev. 2012, 32, 351–358. [Google Scholar] [CrossRef]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef]
- Woodard, T.; Sigurdsson, S.; Gotal, J.D.; Torjesen, A.A.; Inker, L.A.; Aspelund, T.; Eiriksdottir, G.; Gudnason, V.; Harris, T.B.; Launer, L.J.; et al. Mediation analysis of aortic stiffness and renal microvascular function. J. Am. Soc. Nephrol. 2015, 26, 1181–1187. [Google Scholar] [CrossRef]
- Platt, J.F.; Ellis, J.H.; Rubin, J.M.; DiPietro, M.A.; Sedman, A.B. Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: Correlation of resistive index with biopsy findings. Am. J. Roentgenol. 1990, 154, 1223–1227. [Google Scholar] [CrossRef]
- Bohannon, R.W. Comfortable and maximum walking speed of adults aged 20–79 years: Reference values and determinants. Age Ageing 1997, 26, 15–19. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Alcazar, J.; Kamper, R.S.; Aagaard, P.; Haddock, B.; Prescott, E.; Ara, I.; Suetta, C. Relation between leg extension power and 30-s sit-to-stand muscle power in older adults: Validation and translation to functional performance. Sci. Rep. 2020, 10, 16337. [Google Scholar] [CrossRef]
- Kis, O.; Buch, A.; Eldor, R.; Rubin, A.; Dunsky, A.; Stern, N.; Moran, D.S. Should knee extension strength testing be implemented as a screening test for identifying probable and confirmed sarcopenia in older T2DM patients? Eur. Rev. Aging Phys. Act. 2022, 19, 5. [Google Scholar] [CrossRef]
- Hiraki, K.; Yasuda, T.; Hotta, C.; Izawa, K.P.; Morio, Y.; Watanabe, S.; Sakurada, T.; Shibagaki, Y.; Kimura, K. Decreased physical function in pre-dialysis patients with chronic kidney disease. Clin. Exp. Nephrol. 2013, 17, 225–231. [Google Scholar] [CrossRef]
- Matsuzawa, R.; Matsunaga, A.; Wang, G.; Yamamoto, S.; Kutsuna, T.; Ishii, A.; Abe, Y.; Yoneki, K.; Yoshida, A.; Takahira, N. Relationship between lower extremity muscle strength and all-cause mortality in Japanese patients undergoing dialysis. Phys. Ther. 2014, 94, 947–956. [Google Scholar] [CrossRef]
- Katoh, M.; Yamasaki, H. Comparison of reliability of isometric leg muscle strength measurements made using a hand-held dynamometer with and without a restraining belt. J. Phys. Ther. Sci. 2009, 21, 37–42. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Jones, C.; Rikli, R.; Beam, W. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef]
- Yoshioka, M.; Kosaki, K.; Matsui, M.; Takahashi, K.; Shibata, A.; Oka, K.; Kuro-o, M.; Saito, C.; Yamagata, K.; Maeda, S. Physical activity, sedentary behavior, and skeletal muscle strength in patients with chronic kidney disease: An isotemporal substitution approach. Phys. Ther. 2021, 101, pzab101. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Horio, M.; Imai, E.; Yasuda, Y.; Watanabe, T.; Matsuo, S.; Collaborators Developing the Japanese Equation for Estimated GFR. GFR estimation using standardized serum cystatin C in Japan. Am. J. Kidney Dis. 2013, 61, 197–203. [Google Scholar] [CrossRef]
- Horio, M.; Imai, E.; Yasuda, Y.; Watanabe, T.; Matsuo, S. Performance of GFR equations in Japanese subjects. Clin. Exp. Nephrol. 2013, 17, 352–358. [Google Scholar] [CrossRef]
- Sugawara, J.; Hayashi, K.; Tanaka, H. Distal shift of arterial pressure wave reflection sites with aging. Hypertension 2010, 56, 920–925. [Google Scholar] [CrossRef]
- Sugawara, J.; Hayashi, K.; Yokoi, T.; Tanaka, H. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc. Imaging 2008, 1, 739–748. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Calabia, J.; Torguet, P.; Garcia, I.; Martin, N.; Mate, G.; Marin, A.; Molina, C.; Valles, M. The relationship between renal resistive index, arterial stiffness, and atherosclerotic burden: The link between macrocirculation and microcirculation. J. Clin. Hypertens. 2014, 16, 186–191. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, S.R.; Choi, M.J.; Kim, S.G.; Lee, Y.K.; Noh, J.W.; Kim, H.J.; Song, Y.R. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin. Nutr. 2014, 33, 64–68. [Google Scholar] [CrossRef]
- Tabibi, H.; As’habi, A.; Najafi, I.; Hedayati, M. Prevalence of dynapenic obesity and sarcopenic obesity and their associations with cardiovascular disease risk factors in peritoneal dialysis patients. Kidney Res. Clin. Pract. 2018, 37, 404–413. [Google Scholar] [CrossRef]
- Tap, L.; Kirkham, F.A.; Mattace-Raso, F.; Joly, L.; Rajkumar, C.; Benetos, A. Unraveling the links underlying arterial stiffness, bone demineralization, and muscle loss. Hypertension 2020, 76, 629–639. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Klich-Rączka, A.; Skalska, A.; Gryglewska, B.; Grodzicki, T.; Gąsowski, J. Pulse wave velocity and sarcopenia in older persons—A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 6477. [Google Scholar] [CrossRef]
- Fujie, S.; Sato, K.; Miyamoto-Mikami, E.; Hasegawa, N.; Fujita, S.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Reduction of arterial stiffness by exercise training is associated with increasing plasma apelin level in middle-aged and older adults. PLoS ONE 2014, 9, e93545. [Google Scholar] [CrossRef]
- Inoue, K.; Fujie, S.; Hasegawa, N.; Horii, N.; Uchida, M.; Iemitsu, K.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Aerobic exercise training-induced irisin secretion is associated with the reduction of arterial stiffness via nitric oxide production in adults with obesity. Appl. Physiol. Nutr. Metab. 2020, 45, 715–722. [Google Scholar] [CrossRef]
- Hanatani, S.; Izumiya, Y.; Araki, S.; Rokutanda, T.; Kimura, Y.; Walsh, K.; Ogawa, H. Akt1-mediated fast/glycolytic skeletal muscle growth attenuates renal damage in experimental kidney disease. J. Am. Soc. Nephrol. 2014, 25, 2800–2811. [Google Scholar] [CrossRef]
- Roshanravan, B.; Robinson-Cohen, C.; Patel, K.V.; Ayers, E.; Littman, A.J.; de Boer, I.H.; Ikizler, T.A.; Himmelfarb, J.; Katzel, L.I.; Kestenbaum, B.; et al. Association between physical performance and all-cause mortality in CKD. J. Am. Soc. Nephrol. 2013, 24, 822–830. [Google Scholar] [CrossRef]
- Watson, E.L.; Greening, N.J.; Viana, J.L.; Aulakh, J.; Bodicoat, D.H.; Barratt, J.; Feehally, J.; Smith, A.C. Progressive resistance exercise training in CKD: A feasibility study. Am. J. Kidney Dis. 2015, 66, 249–257. [Google Scholar] [CrossRef]
- Straight, C.R.; Lindheimer, J.B.; Brady, A.O.; Dishman, R.K.; Evans, E.M. Effects of resistance training on lower-extremity muscle power in middle-aged and older adults: A systematic review and meta-analysis of randomized controlled trials. Sports Med. 2016, 46, 353–364. [Google Scholar] [CrossRef]
- Castaneda, C.; Gordon, P.L.; Uhlin, K.L.; Levey, A.S.; Kehayias, J.J.; Dwyer, J.T.; Fielding, R.A.; Roubenoff, R.; Singh, M.F. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Ann. Intern. Med. 2001, 135, 965–976. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamada, S.; Inamura, A.; Omori, Y.; Kirimoto, H.; Sugimura, S.; Miyamoto, M. Reliability and validity of measurements of knee extension strength obtained from nursing home residents with dementia. Am. J. Phys. Med. Rehabil. 2009, 88, 924–933. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).