Abstract
Subjective cognitive decline (SCD) is one of those significant concerns faced by older individuals. Though it is predominantly self-reported, it is not an event that should be overlooked, considering its significant association with cognitive disorders like Alzheimer’s disease, mild cognitive impairment, and so on. This makes it imperative to find ways to manage the event to enhance the cognitive performance of older adults and/or suppress the rate at which cognitive decline results in impairment. While multiple interventions have been used for SCD, multi-component non-pharmacological interventions are beginning to gain more attention among researchers. This is due to how such interventions have effectively contributed to improved cognitive performance across different outcome domains. Against this backdrop, this literature review has been conducted to explore the different multi-component non-pharmacological interventions utilized in managing SCD. Papers from databases such as PubMed, Scopus, and EBSCO were retrieved, with relevant data being extracted on the subject matter to address the objective of this review.
1. Introduction
Elderly population is increasing rapidly worldwide, and so is the number of older adults having age-related cognitive decline, as well as dementia. Ageing is associated with a decline in cognitive functions that are critical to independence, social engagement, and quality of life. This decline in cognitive function, if not checked, can advance to clinical cognitive decline and in turn can progress to dementia [1,2,3]. As the proportion of people aged over 65 years continues to expand, it is estimated that, by 2050, dementia could affect some 106.2 million people globally [4]. Age-related cognitive decline affects far more people than dementia [5]. Most dementias are caused by Alzheimer’s disease (AD), and there is no cure available for AD or other types of dementia [6]. Therefore, it is important to develop an intervention to reduce the number of dementias. It has been discovered that pathophysiology associated with AD starts ten years or more before objective cognitive impairment that may be assessed using standardized neuropsychological instruments [6,7]. The seven risk factors for dementia are diabetes, hypertension, obesity, smoking, depression, lower education, and physical inactivity. It is estimated that a 10–25% improvement in all seven risk factors could potentially prevent up to 1.1 to 3.0 million cases of AD worldwide [8]. Dementia prevention primarily focuses on reducing the risk factors and enhancing the lifestyle of the middle-aged population at very early stages before the onset of symptoms and secondarily by trying to reduce or halt the progression of a disease once the symptoms start to appear [8,9]. The potential early symptomatic manifestation of AD is subjective cognitive decline (SCD), a pre-clinical stage of AD. Researchers were motivated to turn their attention to the preclinical stage of AD when several earlier clinical trials of treatments in dementia or mild cognitive impairment (MCI) stages failed [10,11]. SCD is a cognitive state that lies between objective cognitive impairment and intact cognition [12].
Subjective cognitive decline (SCD) is the self-reported experience of worsening memory or cognition or more frequent confusion or memory loss. The distinction cannot be made by cognitive testing, because individuals with SCD and cognitively unimpaired individuals without SCD are, by definition, objectively unimpaired, and they both perform above the cut-off for impairment in cognitive tests [13]. Subjective cognitive decline (SCD) represents a significant concern among the ageing population, and it is more often than not self-reported, although it is also possible for an informant to report about another person having SCD based on specific observations [14]. Some of the most common symptoms of SCD are depression, anxiety, and cognitive complaints [14,15]. Furthermore, an individual with SCD may also experience a heightened level of stress, anger, and fear of dementia. The prevalence of SCD is around 18–55% globally [16], despite the unavailability of any concrete objective measure to ascertain the decline [17]. The prevalence figure comes even in the face of how the cognitive tests of the majority of the individuals’ nursing worries over SCD have often turned out within the normal (healthy) range [12]. Besides, it has been widely acknowledged that people with SCD have better access to cognitive reserve and also exhibit considerably preserved current cognitive function [18]. There is also a higher probability for older adults with SCD show neurodegeneration and other Alzheimer’s disease biomarkers than others without SCD [19,20,21,22]. As no disease-modifying treatment has been found to be effective in the treatment of cognitive impairment and Alzheimer’s disease, researchers are focusing on non-pharmacological treatment. The dementia research community has increasingly concentrated on multidomain therapies that address numerous risk variables at once in order to give a larger possibility of achieving observable improvements during study periods [23]. The emphasis is on close attention to the preclinical phases of cognitive disorders in a bid to ensure early detection that would further boost the chances of creating suitable prevention and interventions that adequately take care of such cognitive disorders [24].
1.1. Management of Subjective Cognitive Decline
The effective management of subjective cognitive decline is regarded as an incumbent need considering the rising elderly population across the globe, and the significant association between SCD and the heightened risks of pathological ageing [25]. Managing SCD does, however, come with specific challenges, as there have been contradicting outcomes regarding the effectiveness of certain non-pharmacological or even pharmacological interventions. The risk factors for SCD identified are older age, female sex, anemia, thyroid diseases, lack of physical exercises, living alone, minimal anxiety symptoms, and daytime dysfunction [26]. Interventions that could slow cognitive ageing or lower the risk of dementia are promised by the idea of cognitive reserve. Many recent studies have focused more intently on lifestyle characteristics, intellectually stimulating behaviors, and personality aspects, while the original observations focused on clearly measured variables like education or occupational accomplishment. Overall, they show that cognitive reserve is not a constant entity but can change throughout the course of a person’s lifespan depending on exposures and actions and that contributions to reserve originate from a variety of sources. It is encouraging to think that lifestyle adjustments made even later in life may provide protection against dementia or age-related cognitive impairment. Despite this intriguing possibility, careful research will be necessary to turn this concept into a workable solution. Such research would provide useful details both about the combination and timing of activities that may lead to maintaining or improving cognition and more successful ageing [18].
1.2. Multi-Component Intervention
It is believed that the probability of gaining more satisfactory outcomes on cognitive performance will be boosted as multitask activities are engaged [27]. Furthermore, there has been evidence that multi-component non-pharmacological interventions are more effective than single-component interventions, since the former will bring about positive impacts in more than one domain [28]. That said, the perceived economic advantages offered by non-pharmacological interventions for the management of SCD makes people see them as a good alternative, even though studies in this direction are hard to come by [29]. A review reported that multi-domain lifestyle intervention strategies are effective in the delay and/or prevention of cognitive impairment in healthy older individuals [30]. Nevertheless, people should start performing them regularly as early as midlife, so that they could have an impact on cognitive function in later life. The results confirm that diet/nutrition, cognitive training, and physical exercise interventions are particularly effective in this sense [30]. This opinion is currently supported by the European Dementia Prevention Initiative, an investigator-initiated initiative of several groups involved in ongoing dementia prevention trials in Europe [31]. Since 2017, this initiative has been spreading to other continents and the reduplication of European projects e.g., Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), have been undertaken in Asia, USA, and Australia [32].
The benefits of physical activity for brain health have been linked to increased cognitive performance, lowered anxiety and depression risk, better sleep, and higher quality of life [33]. Physical activity may also be a promising intervention in the cognition of included patients with dementia and moderate cognitive impairment (MCI), according to recent meta-analyses of randomized controlled studies [34,35]. This evidence was also supported by cross-sectional investigations, long-term observational studies, and prospective intervention trials [36,37]. While a large number of studies have examined biomarker data to identify the underlying processes by which physical activity (PA) safeguards the health of the brain in healthy individuals and animal models, a 6-month resistance training program improved the activation of three critical cortical areas, i.e., the right frontal pole, the right occipital-fusiform gyrus, and the right lingial gyrus, during an associative memory test in a Canadian RCT involving 86 female participants with MCI [38]. An Australian SMART (Study of Mental Activity and Resistance Training) trial, involving 100 older people with MCI and six months of progressive resistance training, was linked to enhanced global cognition, a slowed progression of white matter lesions on MRI, and improved global cognition [39]. Finally, a 6-month aerobic PA intervention improved global cognitive function as measured by the ADAS-Cog, physical fitness as measured by the 6-min walking test, and diastolic blood pressure compared to usual care plus education in a Canadian RCT with 70 older adults with mild vascular cognitive impairment [40].
Cognitive training has become one of the most popular non-pharmacological interventions, primarily due to its effectiveness [41,42]. However, some studies have shown this particular intervention to bring about no improvement in cognitive performance [43]. Specifically stating, cognitive training has been reported to result in a significant increase in the volume of gray matter in the brain, even as cortical volume expansion was also achieved. The probability of SCD patients showing improvement in verbal recall increased [44]. Lifestyle interventions hovering around nutrition, exercises, and one or more component(s), such as behavioral modification, counseling, and so on [45], have also been utilized as a viable multi-component intervention, and this has reportedly caused the rate of cognitive decline to be slowed down, thus allowing elderly individuals to attain some degree of independence [46,47].
The Mediterranean diet is one dietary pattern that has shown promise in recent studies (MeDi). The MeDi is a diet that emphasizes a primarily plant-based diet with a high intake of fruits, vegetables, nuts, and legumes; a moderately high intake of fish; a low intake of red meat; and the primary source of fat being extra virgin olive oil [48]. The MeDi has been proven to directly reduce the risk of dementia through decreased levels of amyloid plaques [49], brain atrophy [50], and structural connections, as well as indirectly through changing cardiovascular risk factors and brain atrophy [51]
A substantial body of literature is developing that supports the notion that frequent social activity may help to prevent or delay cognitive decline in old age. Social activity could also provide meaningful social roles and a sense of purpose in old age [52], which could have direct neurohormonal influences on the brain, including the reduction of the stress response. Finally, although we controlled for physical activity, social activity also requires a degree of physical activity above and beyond regular exercise and walking, which could enhance cardiopulmonary fitness, leading to vascular changes in the brain and cerebral oxygenation that might protect against neuropathology [53].
It follows that, despite the advances made in finding a tenable treatment or management measure for SCD, there is yet to be a consensus as to the most suitable management protocol. Continued efforts are being directed at finding the most suitable interventions to effectively manage subjective cognitive decline. Researchers have gone ahead and employed single-component interventions, as well as multicomponent ones, but there still appear to be contrasting outcomes that are attainable from such measures. However, the focus on multicomponent interventions for SCD is yet to be extensively considered, and this is where this present study finds justification. In essence, the researcher will conduct a literature review to identify and explore papers reporting the use of multicomponent interventions in managing SCD. No review has been done on the use of multi-component non-pharmacological interventions for subjective cognitive decline. The objective of this study is to discuss available non-pharmacological multi-component interventions in the improvement and management of subjective cognitive decline in older adults. This information will enable researchers and clinicians to develop a suitable strategy for effectively managing or delaying cognitive impairment.
2. Materials and Methods
The methodology of the study, with reference to the search strategy, the selection of papers, and the inclusion and exclusion criteria, will be discussed in this section.
2.1. Sources of Information
In this study, the researcher gathered data from papers extracted from PubMed, Scopus, and EBSCO databases. Searches were run on each of these databases using the appropriate keywords and search strategy, which will be discussed later in this section. Utilizing two or more databases gave room for the retrieval of enough materials to address the objective of the study [54].
2.2. Years of Publication
As far as this review is concerned, the researcher limited the years of publications used to 2012–2022, meaning a timeframe of 10 years was ensured. This allows the researcher to explore more current data for the subject matter being addressed. As a result, the review’s findings can be better integrated into further studies aimed at arriving at more effective interventions for subjective cognitive decline.
2.3. Search Strategy
The databases were searched using the search terms highlighted as follow: ‘memory’ or ‘subjective cognitive decline’ or ‘subjective cognitive impairment’ or ‘subjective memory complaint’ or ‘subjective memory decline’ or ‘subjective cognitive complaint’ or ‘cognitive complaint’ AND ‘Multi-component interventions’ or ‘multidomain intervention’ or ‘multidomain lifestyle intervention’ or ‘training’ or ‘intervention’ or ‘therapy’ or ‘treatments’ or ‘non-pharmacological’ or ‘multiple training modalities’ AND ‘physical exercise’ or ‘physical activity’ or ‘physical therapy’ or ‘occupational therapy’ or ‘cognitive behaviour therapy’ or ‘cognitive training’ or ‘cognitive stimulation’ or ‘memory training’ or ‘mind body intervention’ or ‘computer games’ or ‘lifestyle’ or ‘socialisation’ or ‘nutrient’ or ‘nursing’ or ‘psychological’ or ‘psychosocial’ or ‘complementary therapies’ or ‘yoga’ or ‘spirituality’ or ‘activities of daily living’ AND ‘quality of life’.
2.4. Inclusion and Exclusion Criteria
The articles included were (1) randomized controlled trials (RCTs), cohort studies, reviews, and systematic reviews; (2) studies published in English only between 2012 and 2022 (May); (3) subjects aged 45 years or older, without gender and race restrictions; (4) subjects having self-experienced decline in cognitive capacity (unrelated to an acute event) despite normal performance on standardized cognitive tests, and failure to meet the criteria for MCI or dementia; (5) subjects from health care, memory clinics, or community settings; and (6) intervention strategies of all types of non-pharmacological Interventions (physical, psychosocial, cognitive, lifestyle, mindfulness, nutrition, etc.), including multicomponent interventions.
Exclusion criteria were (1) interventions for patients with mild cognitive impairment or Alzheimer’s disease; (2) subjects with objective impairment in neuropsychological tests; (3) subjects without cognitive complaints; (4) history of stroke, systemic diseases, other central nervous system diseases (e.g., Parkinson’s disease, tumors, encephalitis, and epilepsy), major depression, psychosis, and medical causes that may cause cognitive impairment; and (5) pharmacological intervention studies.
2.5. Selection of Papers
A total of 1845 papers were recovered after the search was run through all the databases that were resorted to. This number was trimmed down to 655 papers after reviewing the titles and removing duplicates. The papers were further reduced to 70 papers after abstracts were evaluated, and it was found that some of the papers were duplicates, while some did not meet the inclusion criteria—there were papers in other languages (asides English) and others addressing other concerns. The 70 papers were then reviewed, with the reviewer taking out those that were on MCI, Alzheimer’s diseases, non-RCT publications, non-cohort studies, and editorials. This led to the selection of 12 papers that were extensively reviewed after they had fulfilled the inclusion criteria (Figure 1).
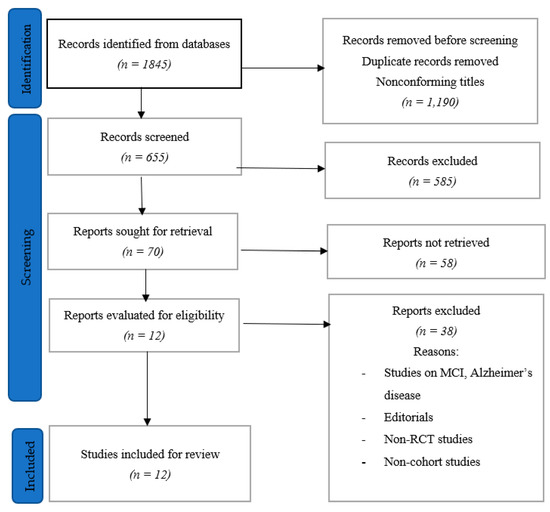
Figure 1.
Study selection process.
3. Results
3.1. Assessment of Outcomes
The variables include psychological well-being, objective cognitive performance, and metacognition. The sway towards metacognition, which basically borders on how people perceive their cognitive performance (Fleming & Dolan) [55], was informed by the underestimation [of cognitive performance] that often prevails in most cases of SCD (Metternich, Schmidtke, and Hull cited in Bhome et al.) [16,56]. It has also been found that individuals with SCD often show relatively poor metacognitive performance, and as such, the effects of SCD may be reduced as metacognition is enhanced. The prevalence of distress in cases of SCD makes psychological well-being highly important to measure when attempting to establish whether or not an intervention is effective [14]. Again, objective cognitive performance can also not be missed, as it has widely been used in several studies bordering on the management and treatment of SCD [39], and its (objective cognitive performance) adoption will help to create a good balance in the evaluation of the effectiveness of SCD intervention(s).
3.2. Study Yield
All except two of the studies reviewed for this study were randomized controlled trial, and the other two studies were quasi-experimental and prospective controlled studies. The cumulative population for all 12 studies was 2687 subjects, with the highest (study population) having 1680 subjects while the lowest had 40 subjects. As per the characteristics of the multicomponent nonpharmacological SCD interventions, five papers (Chan et al.; Boa Sorte Silva et al.; Zuniga et al.; Ramnath et al.; Hong et al.) [25,57,58,59,60] mentioned interventions bordering on exercises; education (Chan et al.; Kwok et al.; Frankenmolen et al.; Cohen-Mansfield et al.) [25,41,61,62] and the use of computer programs (Ramnath et al.; Frankenmolen et al.; Pereira-Morales et al.; Oh et al.) [59,61,63,64] were mentioned in four studies. Multicomponent interventions on lifestyle modifications (Chan et al.; Hong et al.; Andrieu et al.) [25,60,65] and dietary measures (Hong et al.; Chan et al.) [25,60] were also adopted in three and two studies, respectively. The summary of the papers that were reviewed is shown in Table 1.

Table 1.
Summary of study characteristics.
4. Discussion
The significance of multicomponent nonpharmacological interventions for managing subjective cognitive decline is well identified in some of the studies that were reviewed. The findings reported marked a reduction in subjective memory complaints by the participants [25,62,63,64,66]. This does not, however, necessarily mean that the interventions will ultimately lower the incidences of subjective memory impairment, which is more of a psychological problem [58,61]. As seen in one study, the multiple-modality intervention, along with mind-motor training, was found to significantly impact the subjects’ global cognitive functioning compared to the control group that also utilized similar multiple-modality intervention but without mind-motor training [57]. Likewise, the Dejian Mind–Body Intervention (DMBI) also resulted in significant improvements in immediate and delayed memory recall, as well as boosting the subjective feelings of physical and psychological health [25]. Again, the prospect of individuals having SCD being mindful of their state through educational courses and in turn improving their cognitive performances was also reflected in one study [62]. The study revealed how subjects improved in the aspects of executive function, attention, and visual–spatial function after taking courses that hover around different domains—memory, attention, cognition, and so on. The adoption and effectiveness of computer programs and/or games can also not be overlooked in the face of the positive impacts recorded in some of the studies wherein they were used.
Multiple-modality mind–motor training: As seen in one study, the multiple-modality intervention (involving aerobic exercise, resistance exercise, and stretching) along with mind–motor training (square-stepping exercise) was found to significantly impact the subjects’ global cognitive functioning compared to the control group that also utilized similar multiple-modality intervention but without mind–motor training. Both experimental and control groups showed slight improvements in concentration, reasoning, and planning [57]. The square-stepping exercise (SSE) [67] is a novel form of mind–motor training that has been associated with positive effects on global [68] and domain-specific cognitive functioning [68,69] in older adults.
Dejian Mind–Body Intervention (DMBI): Likewise, the Dejian Mind–Body Intervention (DMBI) also resulted in significant improvements in immediate and delayed memory recall, as well as boosting the subjective feelings of physical and psychological health. Dejian Mind–Body Intervention (DMBI) involves the Chan practice (whereby subjects were guided on self-awareness and self-control in relation to unrealistic goals); Nei Gong practice (that has to do with the teaching of mind–body exercises bordering on a series of breathing exercises and calm and gentle movements); and dietary modifications. Conventional Memory Intervention (CMI): Involves psychoeducation, mnemonic training, and progress monitoring [25].
Educative courses: The prospect of individuals having SCD being mindful of their state through educational courses and in turn improving their cognitive performances was also reflected in one study. Educative courses used were: the Health Promotion Course (HPC) involving teaching topics like health behaviors; dementia, and delirium; communication; cognitive activities to keep the mind fit; relationships; depression; life-long learning, etc; Cognitive Training Course (CTC): Hinges on the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) course. It focuses on memory, reasoning, and processing speed, but only the memory training aspect was employed in this study; and Participation-centred Course, which focuses on memory and cognitive and organizational strategies. All interventions showed significant improvements in global cognitive function score compared to what was obtainable for the baseline. However, the CTC group was observed to have a significantly better global cognition score than the other groups. Slight improvements in executive function, visual spatial function, and attention were found across all the groups. Loneliness and self-report on memory difficulties also declined with all interventions [62].
Computer programs and games: The adoption and effectiveness of computer programs and/or games can also not be overlooked in the face of the positive impacts recorded in some of the studies wherein they were used. For example, the SMART and Fit Brains models employed by Oh et al. were reported to have significantly improved working memory scores and reduced subjective memory complaints. [64]. In Ramnath et al.’s study, interactive video games (IVG) showed significant improvements in cognitive measures of processing speed and executive functioning in the IVG group compared to the CMI group [59]. In another study, the Integrated Psychostimulation Program (IPP), a combination of computerized cognitive training (CCT) and conventional cognitive training (like meta-memory activities, progressive relaxation exercises, group discussions, and pen and paper exercises) was done for 90 min for 4 days per week [63]. The overall results indicate that the IPP was more effective in the improvement of cognitive performance and symptoms of anxiety compared with the group that only received sessions with the CCT, although cognitive training using technologies showed favorable results.
Memory strategy training (MST): in a study, the effect of a memory strategy training, including psychoeducation, cognitive restructuring, and strategy training, in older adults with SMC was examined. For the active control memory training, the computer program was used. It was concluded that memory strategy training improves memory functioning in daily life situations. The combination of psycho-education, expectancy management, sharing experiences in a group, and training to use memory strategies in daily life situations is effective in older adults with subjective memory complaints and could be implemented in clinical practice [61]
Physical Activity: Physical activity is the key to ascertaining the level of effectiveness of a nonpharmacological intervention. Five studies utilized physical activity in different forms, such as aerobic activities, resistive activities, stretching activities, standing and balancing, walking, coordinated stepping, etc. Physical activity coupled with mental activity (multi-modality mind–motor) has shown to produce significant improvement in cognition [57]. In contrast, in a study, the aerobic exercises and resistance training employed for 12 months did not yield any significant improvement in subjective memory impairment but significantly improved happiness levels [58]. Interactive video gaming involving standing and balancing activities has yielded positive results [59]. In a study, Chinese Chan-based lifestyle intervention was experimented on, involving some sort of mind–body exercises along with self-control training and dietary modifications to have improved memory functioning [25]. In another study, cognitive intervention, along with lifestyle modifications (including balance training, stretching, and walking), resulted in improvement in functions such as phonemic total, memory delayed recall, and quality of life. Furthermore, anxiety and depression were observed to be significantly reduced for the same group [60].
Cognitive training program (ACTIVE): Another study reported that the use of ACTIVE cognitive training, which is for 12 weeks, was noted to have improved cognitive functions and that the result even lasted for a minimum of 9 months [41].
Omega 3 polyunsaturated fatty acid supplementation and a multidomain intervention: Effect of omega 3 polyunsaturated fatty acid supplementation and a multidomain intervention (physical activity, cognitive training, and nutritional advice), alone or in combination, compared with placebo, on cognitive decline was studied (The Multidomain Alzheimer Preventive Trial, MAPT). No significant effects on cognitive decline over 3 years in elderly people with memory complaints was seen. In their study, it has been suggested to create an effective multidomain intervention strategy to prevent or delay cognitive impairment in the target population, particularly in real-world settings [65].
Cognitive intervention and lifestyle modifications: Interventions utilized a pencil-and-paper method to stimulate cognitive functions (like memory, executive, attention, visuospatial and language functions), physical exercises (including balance training, stretching, and walking), and lifestyle modification, along with home-based lifestyle modifications bordering on regular exercises, low alcohol consumption, smoking cessation, increasing cognitive activities, increasing social activities, and consuming a good diet for brain function, as well as controlling hyperlipidemia and hypertension. The subjects in both groups also received education on the prevention and risk factors of dementia and also on how to go about making lifestyle modifications. Functions such as phonemic total, memory delayed recall, and quality of life were significantly improved for the group that had cognitive intervention along with exercises and lifestyle modifications. Furthermore, anxiety and depression were observed to be significantly reduced for the same group [60].
Cognitive, functional, and psychoeducation program: The Rehacop is a cognitive rehabilitation program, theoretically based on strategies of rehabilitation (restoration, compensation, and optimization). The Rehacop uses a bottom-up approach. Top-down training with the ADL module is used later to help with the generalization of gains to the participant’s life. It is a 5-month intervention allowing either an individual or group approach. The results showed direct effects on neurocognition, as well as far-transfer effects on apathy, QoL, and subjective complaints after the intervention. The effect size was small for neurocognition, medium for apathy and QoL, and large for participants’ subjective complaints [66].
The effect of multicomponent nonpharmacological interventions on the quality of life of subjects with SCD was directly mentioned in three studies [59,60,66]. It is impossible to leave out how the significant reduction in anxiety, depression, and perceived stress as reported will contribute to overall wellbeing, which could translate to improved quality of life [58,62,63]. Another critical aspect that seems to be scarcely addressed when selecting multicomponent nonpharmacological interventions has to do with the length of time used in giving the intervention. According to a study, the increase in the use of management strategies is often the most significant predictor for subject memory complaint improvement [61]. This could mean that the more an SCD subject engages an effective intervention, the higher the prospect of actualizing a positive result.
This study has tried to collect all up-to-date evidence of non-pharmacological multi-component interventions for SCD in older adults. The amount of available information is insufficient to draw firm conclusions regarding the type and combination of multi-component interventions to be used for SCD in older adults. Larger trials utilizing multi-component interventions may be conducted for concrete results on the type of interventions beneficial for preventing or delaying cognitive decline. Most importantly, the development of an assessment scale for SCD is much required. Future studies could assess the extent to which intervention characteristics, such as the number, length, and frequency of sessions, and patient characteristics (such as demographics, education. daily functioning, and social activity), have an impact on the outcomes.
5. Conclusions
Subjective cognitive decline remains a concern that individuals will always look to beat as they near the latter part of their existence, and based on the findings from this review, it is apparent that a much more significant result can be attained by adopting multicomponent interventions. However, while different studies have shown promises of not only helping people reduce the reportage of SCD but also improving cognitive performances or delaying cognitive decline, at least there is still more work to be done in order to arrive at multicomponent nonpharmacological interventions that are widely accepted and highly effective.
Author Contributions
Conceptualization, M.M and P.K.; methodology, M.M and P.K.; writing—original draft preparation, M.M.; writing—review and editing, P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
References
- Caracciolo, B.; Palmer, K.; Monastero, R.; Winblad, B.; Bäckman, L.; Fratiglioni, L. Occurrence of cognitive impairment and dementia in the community: A 9-year-long prospective study. Neurology 2008, 70, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.C.; Xue, Q.-L.; Zhou, J.; Fried, L.P. Executive decline and dysfunction precedes declines in memory: The Women’s Health and Aging Study II. J. Gerontol. A-Biol. 2009, 64, 110–117. [Google Scholar] [CrossRef]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.L.; Langa, K.M.; McCammon, R.J.; Fisher, G.G.; Potter, G.G.; Burke, J.R.; Steffens, D.C.; Foster, N.L.; Giordani, B.; Unverzagt, F.W.; et al. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann. Neurol. 2011, 70, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; der Flier, W.M.V. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement 2016, 12, 292–323. [Google Scholar] [CrossRef]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Fratiglioni, L.; Winblad, B.; von Strauss, E. Prevention of Alzheimer’s disease and dementia. Major findings from the Kungsholmen Project. Physiol. Behav. 2007, 92, 98–104. [Google Scholar] [CrossRef]
- Honig, L.S.; Vellas, B.; Woodward, M.; Boada, M.; Bullock, R.; Borrie, M.; Hager, K.; Andreasen, N.; Scarpini, E.; Liu-Seifert, H.; et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N. Engl. J. Med. 2018, 378, 321–330. [Google Scholar] [CrossRef]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.S.; et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Subjective Cognitive Decline Initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Gomez, O.R.; Saykin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef]
- Molinuevo, J.L.; Rabin, L.A.; Amariglio, R.; Buckley, R.; Dubois, B.; Ellis, K.A.; Ewers, M.; Hampel, H.; Kloppel, S.; Rami, L.; et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s Dement. 2017, 13, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, M.D.; Alves, L.; Bugalho, P. From subjective cognitive complaints to dementia: Who is at risk?—A systematic review. Am. J. Alzheimer’s Dis. Other Demen. 2016, 2016, 31, 105–114. [Google Scholar] [CrossRef]
- Bhome, R.; Berry, A.J.; Huntley, J.D.; Howard, R.J. Interventions for subjective cognitive decline: Systematic review and meta-analysis. BMJ Open 2018, 8, e0216102018. [Google Scholar] [CrossRef]
- Colijn, M.A.; Grossberg, G.T. Amyloid and Tau Biomarkers in Subjective Cognitive Impairment. J. Alzheimer’s Dis. 2015, 47, 1–8. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef]
- Peter, J.; Scheef, L.; Abdulkadir, A.; Boecker, H.; Heneka, M.; Wagner, M.; Alzheimer’s Disease Neuroimaging Initiative. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimer’s Dement. 2014, 10, 99–108. [Google Scholar] [CrossRef]
- Meiberth, D.; Scheef, L.; Wolfsgruber, S.; Boecker, H.; Block, W.; Träber, F.; Jessen, F. Cortical thinning in individuals with subjective memory impairment. J. Alzheimer’s Dis. 2015, 45, 139–1346. [Google Scholar] [CrossRef]
- Stone, J.; Pal, S.; Blackburn, D.; Reuber, M.; Thekkumpurath, P.; Carson, A. Functional (psychogenic) cognitive disorders: A perspective from the neurology clinic. J. Alzheimer’s Dis. 2015, 48, S5–S17. [Google Scholar] [CrossRef]
- Valech, N.; Mollica, M.A.; Olives, J.; Tort, A.; Fortea, J.; Lleo, A.; Belen, S.S.; Molinuevo, J.L.; Rami, L. Informants’ perception of subjective cognitive decline helps to discriminate preclinical Alzheimer’s disease from normal aging. J. Alzheimer’s Dis. 2015, 48, S87–S98. [Google Scholar] [CrossRef] [PubMed]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levalahti, E.; Ahtiluoto, S.; Antikainen, R.; Backman, L.; Hanninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, B.; Tolppanen, A.-M.; Kivipelto, M.; Soininen, H. Future directions in Alzheimer’s disease from risk factors to prevention. Biochem. Pharmacol. 2014, 88, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Cheung, W.K.; Yeung, M.K.; Woo, J.; Kwok, T.; Shum, D.H.K.; Yu, R.; Cheung, M. A Chinese Chan-based Mind-Body Intervention Improves Memory of Older Adults. Front. Aging Neurosci. 2017, 9, 190. [Google Scholar] [CrossRef]
- Wen, C.; Hu, H.; Ou, Y.-N.; Bi, Y.-L.; Ma, Y.-H.; Tan, L.; Yu, J.-T. Risk factors for subjective cognitive decline: The CABLE study. Transl. Psychiatry 2021, 11, 576. [Google Scholar] [CrossRef]
- Suzuki, T.; Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Lee, S.; Park, H. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: A randomized controlled trial. BMC Neurol. 2012, 12, 128. [Google Scholar]
- Ozbe, D.; Graessel, E.; Donath, C.; Pendergrass, A. Immediate intervention effects of standardized multicomponent group interventions on people with cognitive impairment: A systematic review. J. Alzheimer’s Dis. 2019, 67, 653–670. [Google Scholar] [CrossRef]
- Davis, J.C.; Bryan, S.; Marra, C.A.; Hsiung, G.-Y.R.; Liu-Ambrose, T. Challenges with cost-utility analyses of behavioral interventions among older adults at risk for dementia. Br. J. Sport Med. 2015, 49, 1343–1347. [Google Scholar] [CrossRef]
- Toman, J.; Klímová, B.; Vališ, M. Multidomain Lifestyle Intervention Strategies for the Delay of Cognitive Impairment in Healthy Aging. Nutrients 2018, 10, 1560. [Google Scholar] [CrossRef]
- Richard, E.; Charante, E.P.M.; Blom, M.P.H.; Coley, N.; Barbera, M.; Groep, A.; Meiller, Y.; Mangialasche, F.; Beishuizen, C.B.; Jongstra, S.; et al. Healthy ageing through internet counselling in the elderly (HATICE): A multinational, randomized controlled trial. Lancet Digit Health. 2019, 18, e424–e434. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; ALZFORUM. FINGER Studies. Available online: https://www.alzforum.org/news/conference-coverage/new-dementia-trials-test-lifestyle (accessed on 3 November 2022).
- Zheng, G.; Xia, R.; Zhou, W.; Tao, J.; Chen, L. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: A systematic review and meta—Analysis of randomised controlled trials. Br. J. Sport. Med. 2016, 50, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Liang, J.; Xu, Y.; Wang, Y. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: A meta-analysis. BMC Geriatr. 2019, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Groot, C.; Hooghiemstra, A.M.; Raijmakers, P.G.H.M.; van Berckel, B.N.M.; Schettens, P.; Scherder, E.J.A.; van der Flier, W.M.; Ossenkoppele, R. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res. Rev. 2016, 25, 13–23. [Google Scholar] [CrossRef]
- Engeroff, T.; Ingmann, T.; Banzer, W. Physical activity throughout the adult life span and domain-specific cognitive function in old age: A systematic review of cross-sectional and longitudinal data. Sport. Med. 2018, 48, 1405–1436. [Google Scholar] [CrossRef]
- Sofi, F.; Valecchi, D.; Bacci, D.; Abbate, R.; Gensini, G.F.; Casini, A.; Macchi, C. Physical activity and risk of cognitive decline: A meta-analysis of prospective studies. J. Intern. Med. 2011, 269, 107–117. [Google Scholar] [CrossRef]
- Nagamatsu, L.S.; Handy, T.C.; Hsu, C.L.; Voss, M.; Liu-Ambrose, T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch. Intern. Med. 2012, 172, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Suo, C.; Singh, M.F.; Gates, N.; Wen, W.; Sachdev, P.; Brodaty, H.; Saigal, N.; Wilson, G.C.; Meiklejohn, J.; Singh, N.; et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry 2016, 21, 164. [Google Scholar]
- Liu-Ambrose, T.; Best, J.R.; Davis, J.C.; Eng, J.C.; Lee, P.E.; Jacova, C.; Boyd, L.A.; Brasher, P.E.; Munkacsy, M.; Cheung, W.; et al. Aerobic exercise and vascular cognitive impairment: A randomized controlled trial. Neurology 2016, 87, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Kwok, T.C.; Bai, X.; Li, J.C.; Ho, F.K.; Lee, T.M. Effectiveness of cognitive training in Chinese older people with subjective cognitive complaints: A randomized placebo-controlled trial. Int. J. Geriatr. Psychiatry 2013, 28, 208–215. [Google Scholar] [CrossRef]
- Hyer, L.; Scott, C.; Lyles, J.; Dhabliwala, J.; McKenzie, L. Memory intervention: The value of a clinical holistic program for older adults with memory impairments. Aging Ment. Health 2014, 18, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Santos-Modesitt, W.; Poelke, G.; Kramer, A.F.; Castro, C.; Middleton, L.E.; Yaffe, K. The Mental Activity and eXercise (MAX) trial: A randomized controlled trial to enhance cognitive function in older adults. JAMA Intern. Med. 2013, 173, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Engvig, A.; Fjell, A.M.; Westlye, L.T.; Skaane, N.V.; Dale, A.M.; Holland, D.; Due-Tonnessen, P.; Sundseth, O.; Walhovd, K.B. Effects of cognitive training on gray matter volumes in memory clinic patients with subjective memory impairment. J. Alzheimer’s Dis. JAD 2014, 41, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Giustini, D.; Kramer, B.M.R. Comparing the coverage, recall, and precision of searches for 120 systematic reviews in Embase, MEDLINE, and Google Scholar: A prospective study. Syst Rev. 2016, 5, 39. [Google Scholar] [CrossRef]
- Clark, F.; Jackson, J.; Carlson, M.; Chou, C.; Cherry, B.J.; Jordan-Marsh, M.; Knight, B.G.; Mandel, D.; Blanchard, J.; Granger, A.D.; et al. Effectiveness of a lifestyle intervention in promoting the well-being of independently living older people: Results of the well elderly 2 randomised controlled trial. J. Epidemiol. Commun. Health 2012, 66, 782–790. [Google Scholar] [CrossRef]
- Starr, K.N.P.; McDonald, S.R.; Bales, C.W. Obesity and physical frailty in older adults: A scoping review of lifestyle intervention trials. J. Am. Med. Dir. Assoc. 2014, 15, 240–250. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean diet; a literature review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Berti, V.; Walters, M.; Sterling, J.; Quinn, C.G.; Logue, M.; Andrews, R.; Matthews, D.C.; Osorio, R.S.; Pupi, A.; Vallabhajosula, S.; et al. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology 2018, 90, e1789–e1798. [Google Scholar] [CrossRef]
- Luciano, M.; Corley, J.; Cox, S.R.; Hernandez, M.C.V.; Craig, L.C.A.; Dickie, D.A.; Karama, S.; McNeill, G.M.; Bastin, M.E.; Wardlaw, J.M.; et al. Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology 2017, 88, 449–455. [Google Scholar] [CrossRef]
- Pelletier, A.; Barul, C.; Féart, C.; Helmer, C.; Bernard, C.; Periot, O.; Dilharreguy, B.; Dartigues, J.F.; Allard, M.; Gateau, P.B.; et al. Mediterranean diet and preserved brain structural connectivity in older subjects. Alzheimer’s Dement. 2015, 11, 1023–1031. [Google Scholar] [CrossRef]
- Berkman, L.F.; Glass, T. Social integration, social networks, social support and health. In Social Epidemiology; Berkman, L.F., Kawachi, I., Eds.; Oxford University Press: New York, NY, USA, 2000; pp. 158–162. [Google Scholar]
- Fratiglioni, L.; Paillard-Borg, S.; Winblad, B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004, 3, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Hartling, L.; Featherstone, R.; Nuspl, M.; Shave, K.; Dryden, D.M.; Vandermeer, B. The contribution of databases to the results of systematic reviews: A crosssectional study. BMC Med. Res. Methodol. 2016, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M.; Dolan, R.J. The neural basis of metacognitive ability. Philos. Trans. R Soc. Lond. B Biol. Sci. 2012, 367, 1338–1349. [Google Scholar] [CrossRef]
- Metternich, B.; Schmidtke, K.; Härter, M.; Dykierek, P.; Hull, M. Development and evaluation of a group therapy for functional memory and attention disorder. Psychother. Psychosom. Med. Psychol. 2010, 60, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Boa Sorte Silva, N.C.; Gill, D.P.; Owen, A.M.; Liu-Ambrose, T.; Hachinski, V.; Shigematsu, R.; Petrella, R.J. Cognitive changes following multiple-modality exercise and mind-motor training in older adults with subjective cognitive complaints: The M4 study. PLoS ONE 2018, 13, e0196356. [Google Scholar] [CrossRef]
- Zuniga, K.E.; MacKenzie, M.; Kramer, A.; McAuley, E. Subjective Memory Impairment and Well-Being in Community-Dwelling Older Adults. Psychogeriatrics 2016, 16, 20–26. [Google Scholar] [CrossRef]
- Ramnath, U.; Rauch, L.; Lambert, E.V.; Kolbe-Alexander, T. Efficacy of interactive video gaming in older adults with memory complaints: A cluster-randomized ercise intervention. PLoS ONE 2021, 16, e0252016. [Google Scholar] [CrossRef]
- Hong, Y.J.; Lee, J.-H.; Choi, E.J.; Han, N.; Kim, J.E.; Park, S.-H.; Kim, H.J.; Kang, D.W. Efficacies of Cognitive Interventions in the Elderly with Subjective Cognitive Decline: A Prospective, Three-Arm, Controlled Trial. J. Clin. Neurol. 2020, 16, 304–313. [Google Scholar] [CrossRef]
- Frankenmolen, N.L.; Overdorp, E.J.; Fasotti, L.; Claassen, J.A.H.R.; Kessels, R.P.C.; Oosterman, J.M. Memory Strategy Training in Older Adults with Subjective Memory Complaints: A Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2018, 24, 1110–1120. [Google Scholar] [CrossRef]
- Cohen-Mansfield, J.; Cohen, R.; Buettner, L.; Eyal, N.; Jakobovits, H.; Rebok, G.; Rotenberg-Shpigelman, S.; Sternberg, S. Interventions for older persons reporting memory difficulties: A randomized controlled pilot study. Int. J. Geriatr. Psychiatry 2015, 30, 478–486. [Google Scholar] [CrossRef]
- Pereira-Morales, A.J.; Cruz-Salinas, A.F.; Aponte, J.; Pereira-Manrique, F. Efficacy of a computer-based cognitive training program in older people with subjective memory complaints: A randomized study. Int. J. Neurosci. 2018, 128, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Seo, S.; Lee, J.H.; Song, M.J.; Shin, M.-S. Effects of smartphone-based memory training for older adults with subjective memory complaints: A randomized controlled trial. Aging Ment. Health 2018, 22, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.N.; Dantoine, T.; Dartigues, J.F.; et al. Effect of long-term omega-3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Murillo, G.; Ibarretxe-Bilbao, N.; Pena, J.; Ojeda, N. Effects of Cognitive Rehabilitation on Cognition, Apathy, Quality of Life, and Subjective Complaints in the Elderly: A Randomized Controlled Trial. Am. J. Geriatr. Psychiatry 2020, 28, 518−529. [Google Scholar] [CrossRef]
- Shigematsu, R.; Okura, T.; Nakagaichi, M.; Tanaka, K.; Sakai, T.; Kitazumi, S.; Rantanen, T. Square-Stepping Exercise and Fall Risk Factors in Older Adults: A Single-Blind, Randomized Controlled Trial. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 76–82. [Google Scholar] [CrossRef]
- Teixeira, C.V.L.; Gobbi, S.; Pereira, J.R.; Vital, T.M.; Hernandéz, S.S.S.; Shigematsu, R.; Gobbi, L.T. Effects of squarestepping exercise on cognitive functions of older people. Psychogeriatrics 2013, 13, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, R. Effects of exercise program requiring attention, memory and imitation on cognitive function in elderly persons: A non-randomized pilot study. J. Gerontol. Geriatr. Res. 2014, 3, 1–6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).