The Prognostic and Discriminatory Utility of the Clinical Frailty Scale and Modified Frailty Index Compared to Age
Abstract
:1. Introduction
Objectives
2. Methods
2.1. Study Design
2.2. Ethics and Data Availability
2.3. Population
2.4. Prognostic Factors
2.5. Outcomes
2.6. Statistical Analyses
3. Results
3.1. Day 28 Mortality
3.2. Prolonged Admission
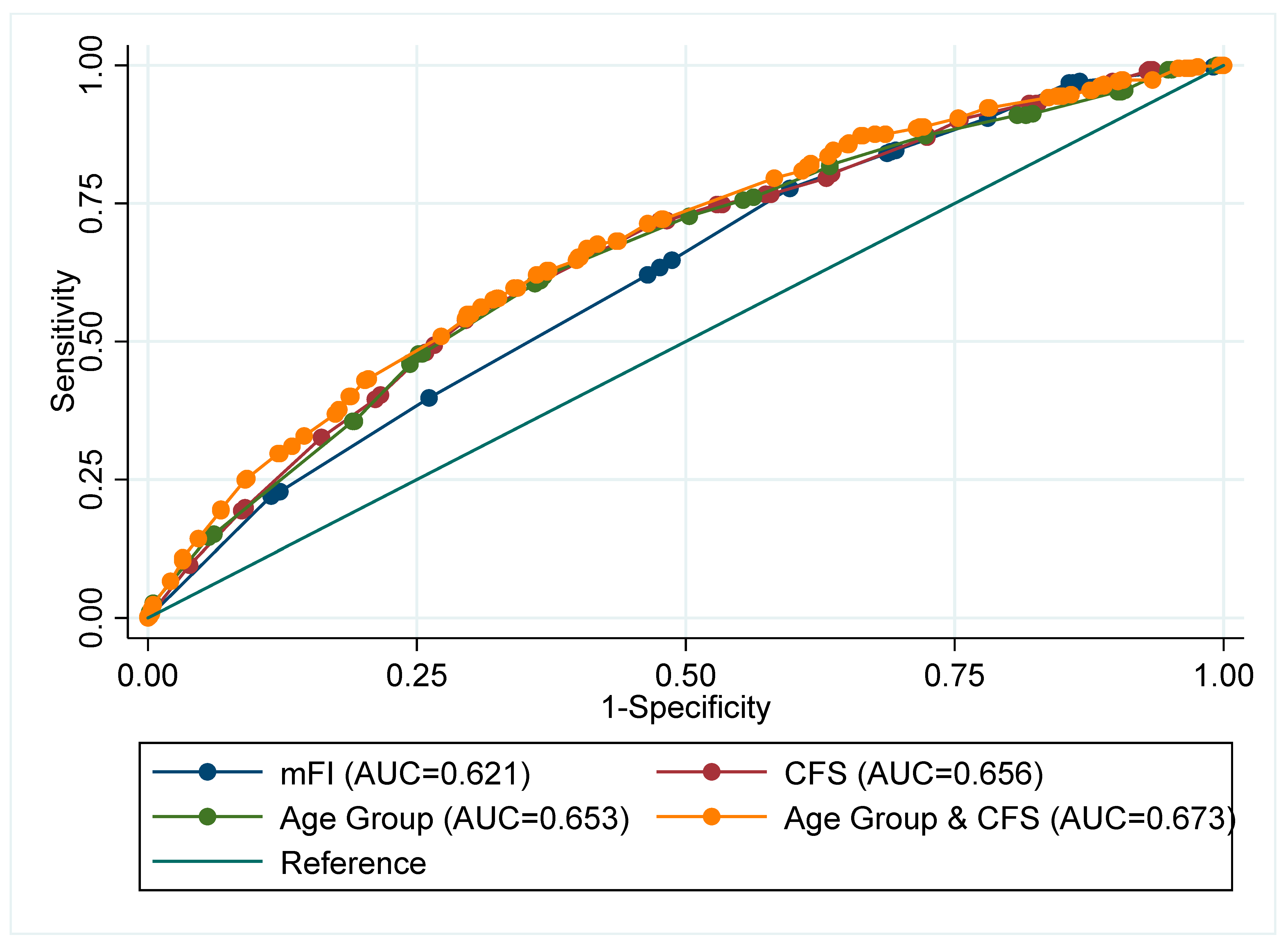
3.3. Discrimination
3.4. Validation Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, K.; Mitnitski, A. Frailty in Relation to the Accumulation of Deficits. J. Gerontol. Ser. A 2007, 62, 722–727. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World 2001, 1, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Theou, O.; Rockwood, M.R.; Mitnitski, A.; Rockwood, K. Disability and co-morbidity in relation to frailty: How much do they overlap? Arch. Gerontol. Geriatr. 2012, 55, e1–e8. [Google Scholar] [CrossRef]
- Subramaniam, S.; Aalberg, J.J.; Soriano, R.P.; Divino, C.M. New 5-Factor Modified Frailty Index Using American College of Surgeons NSQIP Data. J. Am. Coll. Surg. 2018, 226, 173–181.e8. [Google Scholar] [CrossRef]
- Chimukangara, M.; Helm, M.C.; Frelich, M.J.; Bosler, M.E.; Rein, L.E.; Szabo, A.; Gould, J.C. A 5-item frailty index based on NSQIP data correlates with outcomes following paraesophageal hernia repair. Surg. Endosc. 2016, 31, 2509–2519. [Google Scholar] [CrossRef]
- Broad, A.; Carter, B.; Mckelvie, S.; Hewitt, J. The Convergent Validity of the electronic Frailty Index (eFI) with the Clinical Frailty Scale (CFS). Geriatrics 2020, 5, 88. [Google Scholar] [CrossRef]
- Marincowitz, C.; Turner, V.; Allgar, V.L.; Bellwood, J.; Wheeler, A.; Hale, M.; Callaghan, H.; Clegg, A.; Sheldon, T.A. Can patient frailty be estimated from inpatient records? A prospective cohort study. Adv. Geriatr. Med. Res. 2020, 2, e200004. [Google Scholar]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020, 20, 393. [Google Scholar] [CrossRef]
- Quinn, T.J.; Mooijaart, S.P.; Gallacher, K.; Burton, J.K. Acute care assessment of older adults living with frailty. BMJ 2019, 364, l13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewitt, J.; Carter, B.; Vilches-Moraga, A.; Quinn, T.J.; Braude, P.; Verduri, A.; Pearce, F.; Stechman, M.; Short, R.; Guaraldi, G.; et al. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. Lancet Public Health 2020, 5, 444–451. [Google Scholar] [CrossRef]
- Osuafor, C.N.; Davidson, C.; Mackett, A.J.; Goujon, M.; Van Der Poel, L.; Taylor, V.; Preller, J.; Goudie, R.J.B.; Keevil, V.L. Clinical Features, Inpatient Trajectories and Frailty in Older Inpatients with COVID-19: A Retrospective Observational Study. Geriatrics 2021, 6, 11. [Google Scholar] [CrossRef]
- Geriatric Medicine Research Collaborative; Covid Collaborative; Welch, C. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: Results of an international multi-centre study. Age Ageing 2021, 50, 617–630. [Google Scholar] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806. [Google Scholar] [CrossRef] [Green Version]
- Price, A.; Barlow-Pay, F.; Duffy, S.; Pearce, L.; Vilches-Moraga, A.; Moug, S.; Quinn, T.; Stechman, M.; Braude, P.; Mitchell, E.; et al. Study protocol for the COPE study: COVID-19 in Older PEople: The influence of frailty and multimorbidity on survival. A multicentre, European observational study. BMJ Open 2020, 10, e040569. [Google Scholar] [CrossRef]
- Pulok, M.H.; Theou, O.; van der Valk, A.M.; Rockwood, K. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing 2020, 49, afaa089. [Google Scholar] [CrossRef]
- Davies, J.; Whitlock, J.; Gutmanis, I.; Kane, S.-L. Inter-Rater Reliability of the Retrospectively Assigned Clinical Frailty Scale Score in a Geriatric Outreach Population. Can. Geriatr. J. 2018, 21, 1–5. [Google Scholar] [CrossRef]
- Stringer, D.; Braude, P.; Myint, P.K.; Evans, L.; Collins, J.T.; Verduri, A.; Quinn, T.J.; Vilches-Moraga, A.; Stechman, M.J.; Carter, B.; et al. The role of C-reactive protein as a prognostic marker in COVID-19. Int. J. Epidemiol. 2021, 50, 420–429. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 837–845. [Google Scholar] [CrossRef]
- Gilbert, T.; Neuburger, J.; Kraindler, J.; Keeble, E.; Smith, P.; Ariti, C.; Arora, S.; Street, A.; Parker, S.; Roberts, H.C.; et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet 2018, 391, 1775–1782. [Google Scholar] [CrossRef] [Green Version]
- Velanovich, V.; Antoine, H.; Swartz, A.; Peters, D.; Rubinfieldl, I. Accumulating deficits model of frailty and postoperative mortality and morbidity: Its application to a national database. J. Surg. Res. 2013, 183, 104–110. [Google Scholar] [CrossRef]
- Aguayo, G.A.; Donneau, A.-F.; Vaillant, M.; Schritz, A.; Franco, O.; Stranges, S.; Malisoux, L.; Guillaume, M.; Witte, D. Agreement Between 35 Published Frailty Scores in the General Population. Am. J. Epidemiology 2017, 186, 420–434. [Google Scholar] [CrossRef]
- Ellis, G.; Gardner, M.; Tsiachristas, A.; Langhorne, P.; Burke, O.; Harwood, R.H.; Conroy, S.P.; Kircher, T.; Somme, D.; Saltvedt, I.; et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst. Rev. 2017, 9, CD006211. [Google Scholar] [CrossRef] [Green Version]
- Braude, P.; Short, R.; Bouamra, O.; Shipway, D.; Lecky, F.; Carlton, E.; Benger, J.; Gordon, A.; Carter, B. A national study of 23 major trauma centres to investigate the effect of a geriatrician assessment on clinical outcomes in older people admitted with serious injury in England (FiTR 2): A multicentre observational cohort study. Lancet Healthy Longev. 2022, 3, e549–e557. [Google Scholar] [CrossRef]
- Riley, R.D.; Hayden, J.A.; Steyerberg, E.W.; Moons, K.G.; Abrams, K.; Kyzas, P.A.; MMalats, N.; Briggs, A.; Schroter, S.; PROGRESS Group. Prognosis Research Strategy (PROGRESS) 2: Prognostic Factor Research. PLOS Med. 2013, 10, e1001380. [Google Scholar] [CrossRef] [Green Version]
- Quinn, T.J.; Dawson, J.; Lees, J.S.; Chang, T.P.; Walters, M.R.; Lees, K.R. Time spent at home poststroke: "home-time" a meaningful and robust outcome measure for stroke trials. Stroke 2008, 39, 231–233. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, T.; Amanda, L.; Schnier, C.; Rannikmäe, K.; Bush, K.; Brayne, C.; Quinn, T.J.; Sudlow, C.; UK Biobank Neurodegenerative Outcomes Group; Dementias Platform UK. Identifying dementia cases with routinely collected health data: A systematic review. Alzheimers Dement 2018, 14, 1038–1051. [Google Scholar] [CrossRef]
- Burton, J.K.; Marwick, C.A.; Galloway, J.; Hall, C.; Nind, T.; Reynish, E.L.; Guthrie, B. Identifying care-hone residents in routine healthcare datasets:a diagnostic test accuracy study of five methods. Age Ageing 2019, 48, 114–121. [Google Scholar] [CrossRef] [Green Version]
| Day 28 Mortality | |||
|---|---|---|---|
| Dead | Alive | Total | |
| Sites | n = 377 (37.8%) | n = 621 (62.2%) | n = 998 (%) |
| Age | |||
| 65–69 yrs | 23 (19.8) | 93 (80.2) | 116 (11.6) |
| 70–74 yrs | 58 (33.9) | 113 (66.1) | 171 (17.1) |
| 75–79 yrs | 81 (39.1) | 126 (58.3) | 207 (20.7) |
| 80–84 yrs | 90 (41.7) | 92 (59.7) | 216 (21.6) |
| 85–89 yrs | 62 (40.3) | 92 (59.7 | 154 (15.4) |
| 90 yrs or older | 63 (47.0) | 71 (53.0) | 134 (13.4) |
| Female | 152 (33.6) | 301 (66.5) | 453 (45.4) |
| Current smokers | 17 (32.1) | 36 (67.8) | 53 (5.3) |
| Diabetes | 123 (38.9) | 193 (61.8) | 316 (31.7) |
| Hypertension | 55 (38.7) | 87 (61.3) | 142 (14.2) |
| Hypertension (on treatment) | 157 (37.0) | 268 (63.1) | 425 (42.6) |
| Coronary Artery Disease | 123 (44.7) | 152 (55.3) | 275 (27.6) |
| Elevated CRP (>40) | 294 (44.1) | 372 (55.9) | 666 (66.7) |
| eGFR ≥ 60 | 167 (31.8) | 359 (68.3) | 526 (52.7) |
| COPD | 90 (45.2) | 109 (54.8) | 199 (19.9) |
| Heart Failure | 84 (49.7) | 85 (50.3) | 169 (16.9) |
| Modified Frailty Index Items (mFI) | |||
| 0 | 95 (37.0) | 162 (63.0) | 257 (25.8) |
| 1 | 80 (31.4) | 175 (68.6) | 255 (25.6) |
| 2 | 119 (37.3) | 200 (62.7) | 319 (32.0) |
| 3 | 68 (50.4) | 67 (49.6) | 135 (13.5) |
| 4 | 10 (40.0) | 15 (60.0) | 25 (2.5) |
| 5 | 5 (71.4) | 2 (28.6) | 7 (0.7) |
| Clinical Frailty Scale (CFS) | |||
| 1, Very Fit | 6 (30.0) | 14 (70.0) | 20 (2.0) |
| 2, Fit | 18 (29.5) | 43 (70.5) | 61 (6.11) |
| 3, Managing well | 39 (27.1) | 105 (72.9) | 144 (14.4) |
| 4, Very Mildly frail | 50 (35.0) | 93 (65.0) | 143 (14.3) |
| 5, Mildly frail | 56 (34.8) | 105 (65.2) | 161 (16.3) |
| 6, Moderately Frail | 89 (40.8) | 129 (59.2) | 218 (21.8) |
| 7, Severely frail | 87 (44.4) | 109 (55.6) | 196 (19.6) |
| 8, Very severely frail | 32 (58.2) | 23 (41.8) | 55 (5.5) |
| Crude Odds Ratio (OR) | Base Model Adjusted OR (aOR) & | Base + Age aOR | Base + CFS (Binary) aOR | Base + mFI (Binary) aOR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p | aOR (95%CI) | p | aOR (95%CI) | p | aOR (95%CI) | p | aOR (95%CI) | p | |
| Constant | 0.52 (0.36–0.75) | <0.001 | 0.25 (0.16–0.40) | <0.001 | 0.126 (0.07–0.22) | <0.001 | 0.145 (0.09–0.24) | <0.001 | 0.199 (0.12–0.32) | <0.001 |
| Sex (Female) | Ref | - | ||||||||
| Male | 1.32 (1.01–1.72) | 0.044 | 1.26 (0.96–1.65) | 0.10 | 1.33 (1.01–1.76) | 0.043 | 1.41 (1.07–1.87) | 0.016 | 1.30 (0.99–1.72) | 0.06 |
| Smoking (Never/Ex) | Ref | - | ||||||||
| Current smoker | 0.78 (0.42–1.45) | 0.44 | 0.77 (0.41–1.44) | 0.41 | 0.81 (0.43–1.54) | 0.52 | 0.69 (0.36–1.29) | 0.25 | 0.76 (0.41–1.43) | 0.40 |
| Elevated CRP (≥40) | 2.37 (1.75–3.21) | <0.001 | 2.33 (1.72–3.16) | <0.001 | 2.56 (1.88–3.49) | <0.001 | 2.47 (1.81–3.36) | <0.001 | 2.34 (1.73–3.18) | <0.001 |
| Age Group (65–74) | ||||||||||
| 75–84 yrs | 1.87 (1.34–2.62) | <0.001 | 2.01 (1.43–2.84) | <0.001 | ||||||
| 85–94 yrs | 2.18 (1.50–3.17) | <0.001 | 2.58 (1.75–3.81) | <0.001 | ||||||
| 95 or older | 4.64 (1.78–12.13) | 0.002 | 5.49 (2.09–14.39) | <0.001 | ||||||
| CFS (Not Frail) | Ref | - | ||||||||
| Frail | 1.83 (1.36–2.45) | <0.001 | 2.12 (1.56–2.872.87) | <0.001 | ||||||
| mFI (Not-Frail) | Ref | - | ||||||||
| Frail | 1.49 (1.13–1.96) | 0.004 | 1.53 (1.16–2.03) | 0.003 | ||||||
| CFS (1–4) | Ref- | Ref- | ||||||||
| CFS 5 | 1.27 (0.84–1.90) | 0.26 | ||||||||
| CFS 6 | 1.88 (1.30–2.73) | 0.001 | ||||||||
| CFS 7–8 | 2.33 (1.62–3.45) | <0.001 | ||||||||
| Crude Odds Ratio (OR) | Base Model Adjusted OR (aOR) & | Base + Age aOR | Base + CFS (Binary) aOR | Base + mFI (Binary) aOR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p | aOR (95%CI) | p | aOR (95%CI) | p | aOR (95%CI) | p | aOR (95%CI) | p | |
| Constant | 2.64 (1.77–3.92) | <0.001 | 2.62 (1.62–4.23) | <0.001 | 1.74 (1.02–2.96) | 0.04 | 1.47 (0.91–2.36) | 0.12 | 2.22 (1.35–3.65) | 0.002 |
| Sex (Female) | Ref- | |||||||||
| Male | 1.06 (0.79–1.41) | 0.71 | 1.06 (0.79–1.42) | 0.70 | 1.13 (0.84–1.73) | 0.43 | 1.21 (0.90–1.64) | 0.21 | 1.08 (0.81–1.45) | 0.59 |
| Smoking (Never/Ex) | Ref- | |||||||||
| Current smoker | 0.69 (0.37–1.29) | 0.25 | 0.69 (0.37–1.29) | 0.25 | 0.75 (0.40–1.42) | 0.38 | 0.60 (0.32–1.14) | 0.12 | 0.69 (0.37–1.29) | 0.25 |
| Elevated CRP (≥40) | 0.99 (0.73–1.35) | 0.98 | 0.99 (0.73–1.35) | 0.95 | 1.06 (0.78–1.46) | 0.70 | 1.04 (0.76–1.43) | 0.81 | 0.99 (0.72–1.35) | 0.92 |
| Age Group (65–74) | ||||||||||
| 75–84 yrs | 1.23 (0.88–1.71) | 0.23 | 1.24 (0.88–1.73) | 0.22 | ||||||
| 85–94 yrs | 2.49 (1.64–3.79) | <0.001 | 2.52 (1.65–3.86) | <0.001 | ||||||
| 95 or older | 2.19 (0.70–6.84) | 0.18 | 2.24 (0.71–7.04) | 0.17 | ||||||
| CFS (Not Frail) | Ref- | |||||||||
| Frail | 2.32 (1.71–3.14) | <0.001 | 2.44 (1.79–3.30) | <0.001 | ||||||
| mFI (Not-Frail) | Ref- | |||||||||
| Frail | 1.39 (1.03–1.86) | 0.03 | 1.39 (1.04–1.88) | 0.028 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, B.; Keevil, V.L.; Anand, A.; Osuafor, C.N.; Goudie, R.J.B.; Preller, J.; Lowry, M.; Clunie, S.; Shenkin, S.D.; McCarthy, K.; et al. The Prognostic and Discriminatory Utility of the Clinical Frailty Scale and Modified Frailty Index Compared to Age. Geriatrics 2022, 7, 87. https://doi.org/10.3390/geriatrics7050087
Carter B, Keevil VL, Anand A, Osuafor CN, Goudie RJB, Preller J, Lowry M, Clunie S, Shenkin SD, McCarthy K, et al. The Prognostic and Discriminatory Utility of the Clinical Frailty Scale and Modified Frailty Index Compared to Age. Geriatrics. 2022; 7(5):87. https://doi.org/10.3390/geriatrics7050087
Chicago/Turabian StyleCarter, Ben, Victoria L. Keevil, Atul Anand, Christopher N. Osuafor, Robert J. B. Goudie, Jacobus Preller, Matthew Lowry, Sarah Clunie, Susan D. Shenkin, Kathryn McCarthy, and et al. 2022. "The Prognostic and Discriminatory Utility of the Clinical Frailty Scale and Modified Frailty Index Compared to Age" Geriatrics 7, no. 5: 87. https://doi.org/10.3390/geriatrics7050087
APA StyleCarter, B., Keevil, V. L., Anand, A., Osuafor, C. N., Goudie, R. J. B., Preller, J., Lowry, M., Clunie, S., Shenkin, S. D., McCarthy, K., Hewitt, J., & Quinn, T. J. (2022). The Prognostic and Discriminatory Utility of the Clinical Frailty Scale and Modified Frailty Index Compared to Age. Geriatrics, 7(5), 87. https://doi.org/10.3390/geriatrics7050087








