Healthcare-Associated Adverse Events in Alternate Level of Care Patients Awaiting Long-Term Care in Hospital
Abstract
:1. Introduction
2. Methods
2.1. Inclusion/Exclusion Criteria
2.2. Sample Selection
2.3. Data Collection
2.4. Statistical Modelling
3. Results
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Health Quality Ontario. System Performance: Long Term Care Home Wait Times. Available online: https://www.hqontario.ca/System-Performance/Long-Term-Care-Home-Performance/Wait-Times (accessed on 10 August 2021).
- Covinsky, K.E.; Palmer, R.M.; Fortinsky, R.H.; Counsell, S.R.; Stewart, A.L.; Kresevic, D.; Burant, C.J.; Landefeld, C.S. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: Increased vulnerability with age. J. Am. Geriatr. Soc. 2003, 51, 451–458. [Google Scholar] [CrossRef]
- Costa, A.P.; Hirdes, J.P. Clinical characteristics and service needs of alternate-level-of-care patients waiting for long-term care in Ontario hospitals. Healthc. Policy 2010, 6, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.D.; Morris, K.; Frood, J. CIHI survey: Alternative level of care in Canada: A summary. Healthc. Q. 2009, 12, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Cancer Care Ontario. Alternate Level of Care (ALC), Reference Manual Version 2; Cancer Care Ontario: Toronto, ON, Canada, 2017. [Google Scholar]
- Costa, A.P.; Poss, J.W.; Peirce, T.; Hirdes, J.P. Acute care inpatients with long-term delayed-discharge: Evidence from a Canadian health region. BMC Health Serv. Res. 2012, 12, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, A.D.; Dai, C.; Srivastava, S.; Smith, C.A.; Gill, S.S. Risk factors, costs and complications of delayed hospital discharge from internal medicine wards at a Canadian academic medical centre: Retrospective cohort study. BMC Health Serv. Res. 2019, 19, 935. [Google Scholar] [CrossRef]
- Kuluski, K.; Im, J.; McGeown, M. “It’sa waiting game” a qualitative study of the experience of carers of patients who require an alternate level of care. BMC Health Serv. Res. 2017, 17, 318. [Google Scholar] [CrossRef] [Green Version]
- McCloskey, R.; Jarrett, P.; Stewart, C. The untold story of being designated an alternate level of care patient. Healthc. Policy 2015, 11, 76. [Google Scholar] [CrossRef] [Green Version]
- Stone, N.D.; Ashraf, M.S.; Calder, J.; Crnich, C.J.; Crossley, K.; Drinka, P.J.; Gould, C.V.; Juthani-Mehta, M.; Lautenbach, E.; Loeb, M.; et al. Surveillance definitions of infections in long-term care facilities: Revisiting the McGeer criteria. Infect. Control Hosp. Epidemiol. 2012, 33, 965–977. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 10 August 2021).
- Harrell, F.E., Jr. Rms: Regression Modeling Strategies. R Package Version 5.1-4. 2019. Available online: https://CRAN.R-project.org/package=rms (accessed on 10 August 2021).
- Dudgeon, S. Rising Tide: The Impact of Dementia on Canadian Society: A Study; Alzheimer Society of Canada: Toronto, ON, Canada, 2010. [Google Scholar]
- McCloskey, R.; Jarrett, P.; Stewart, C.; Nicholson, P. Alternate level of care patients in hospitals: What does dementia have to do with this? Can. Geriatr. J. 2014, 17, 88. [Google Scholar] [CrossRef] [Green Version]
- Mukadam, N.; Sampson, E.L. A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int. Psychogeriatr. 2011, 23, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.N.; Monson, J.R. Hospital-acquired infections. Surgery 2012, 30, 640–644. [Google Scholar] [CrossRef]
- Glance, L.G.; Stone, P.W.; Mukamel, D.B.; Dick, A.W. Increases in mortality, length of stay, and cost associated with hospital-acquired infections in trauma patients. Arch. Surg. 2011, 146, 794–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plowman, R.; Graves, N.; Griffin, M.A.; Roberts, J.A.; Swan, A.V.; Cookson, B.; Taylor, L. The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. J. Hosp. Infect. 2001, 47, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Tuckman, H.P.; Patrick, R.H.; Kountz, D.S.; Kohn, J.L. Cost of hospital-acquired infection. Hosp. Top. 2010, 88, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.; Gravel, D.; Matlow, A.; Embree, J.; LeSaux, N.; Johnston, L.; Suh, K.N.; John, M.; Embil, J.; Henderson, E.; et al. Assessing the magnitude and trends in hospital acquired infections in Canadian hospitals through sequential point prevalence surveys. Antimicrob. Resist. Infect. Control 2016, 5, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avci, M.; Ozgenc, O.; Coskuner, S.A.; Olut, A.I. Hospital acquired infections (HAI) in the elderly: Comparison with the younger patients. Arch. Gerontol. Geriatr. 2012, 54, 247–250. [Google Scholar] [CrossRef]
- Reed, D.; Kemmerly, S.A. Infection control and prevention: A review of hospital-acquired infections and the economic implications. Ochsner J. 2009, 9, 27–31. [Google Scholar]
- Woodford, H.J.; George, J. Diagnosis and management of urinary tract infection in hospitalized older people. J. Am. Geriatr. Soc. 2009, 57, 107–114. [Google Scholar] [CrossRef]
- Beveridge, L.A.; Davey, P.G.; Phillips, G.; McMurdo, M.E. Optimal management of urinary tract infections in older people. Clin. Interv. Aging 2011, 6, 173. [Google Scholar] [CrossRef] [Green Version]
- Burton, L.A.; Price, R.; Barr, K.E.; McAuley, S.M.; Allen, J.B.; Clinton, A.M.; Phillips, G.; Marwick, C.A.; McMurdo, M.E.; Witham, M.D. Hospital-acquired pneumonia incidence and diagnosis in older patients. Age Ageing 2016, 45, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Bisht, R.; Katiyar, A.; Singh, R.; Mittal, P. Antibiotic resistance-A global issue of concern. Asian J. Pharm. Clin. Res. 2009, 2, 34–39. [Google Scholar]
- Carlet, J.; Jarlier, V.; Harbarth, S.; Voss, A.; Goossens, H.; Pittet, D. Ready for a world without antibiotics? The pensières antibiotic resistance call to action. Antimicrob. Resist. Infect. Control 2012, 1, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sillner, A.Y.; Holle, C.L.; Rudolph, J.L. The overlap between falls and delirium in hospitalized older adults: A systematic review. Clin. Geriatr. Med. 2019, 35, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.G.; Davis, D.; Growdon, M.E.; Albuquerque, A.; Inouye, S.K. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015, 14, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Carroll, J.G. Crossing the quality chasm: A new health system for the 21st century. Qual. Manag. Healthc. 2002, 10, 60–61. [Google Scholar] [CrossRef]
- Swinkels, A.; Mitchell, T. Delayed transfer from hospital to community settings: The older person’s perspective. Health Soc. Care Community 2009, 17, 45–53. [Google Scholar] [CrossRef]
- Sutherland, J.M.; Crump, R.T. Alternative level of care: Canada’s hospital beds, the evidence and options. Healthc. Policy 2013, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Manville, M.; Klein, M.C.; Bainbridge, L. Improved outcomes for elderly patients who received care on a transitional care unit. Can. Fam. Physician 2014, 60, e263–e271. [Google Scholar]
- Béland, D.; Marier, P. COVID-19 and long-term care policy for older people in Canada. J. Aging Soc. Policy 2020, 32, 358–364. [Google Scholar] [CrossRef]
- Fisman, D.N.; Bogoch, I.; Lapointe-Shaw, L.; McCready, J.; Tuite, A.R. Risk factors associated with mortality among residents with coronavirus disease 2019 (COVID-19) in long-term care facilities in Ontario, Canada. JAMA Netw. Open 2020, 3, e2015957. [Google Scholar] [CrossRef]
| All(%) | Females | Males | Dementia | No Dementia | |
|---|---|---|---|---|---|
| Age | |||||
| 65–74 | 26 (16.7%) | 12 (16.0%) | 14 (17.5%) | 6 (8.2%) | 20 (24.1%) |
| 75–84 | 46 (29.5%) | 20 (26.7%) | 26 (32.5%) | 28 (38.4%) | 18 (21.7%) |
| 85–94 | 72 (46.2%) | 37 (49.3%) | 34 (42.5%) | 34 (46.6%) | 37 (44.6%) |
| 95 or older | 12 (7.7%) | 6 (8.0%) | 6 (7.5%) | 5 (6.8%) | 8 (9.6%) |
| Sex | |||||
| Male | 81 (51.9%) | -- | 81 (100%) | 30 (41.1%) | 45 (54.2%) |
| Female | 75 (48.1%) | 75 (100%) | -- | 43 (58.9%) | 38 (44.6%) |
| Living situation | |||||
| Retirement home | 31 (19.9%) | 14 (18.7%) | 17 (21.0%) | 15 (20.5%) | 16 (19.3%) |
| Home alone | 48 (30.7%) | 29 (38.7%) | 19 (23.5%) | 15 (20.5%) | 33 (39.8%) |
| Home with partner or family | 72 (46.2%) | 30 (40.0%) | 42 (51.8%) | 41 (56.2%) | 31 (37.3%) |
| Other | 5 (3.2%) | 2 (2.7%) | 3 (3.7%) | 2 (2.7%) | 3 (3.6%) |
| Comorbidities | |||||
| Dementia | 73 (46.8%) | 30 (40.0%) | 43 (53.1%) | 73 (100%) | -- |
| with BPSD | 16 (10.3%) | 6 (8.0%) | 10 (13.3%) | 16 (21.9%) | -- |
| Falls | 49 (31.4%) | 26 (34.7%) | 23 (28.4%) | ||
| Polypharmacy (>10 medications) | 46 (29.5%) | 21 (28%) | 25 (30.9%) | 26 (35.6%) | 20 (24.1%) |
| Osteoarthritis | 46 (29.5%) | 23 (30.7%) | 23 (28.4%) | 18 (24.66%) | 28 (38.4%) |
| Atrial fibrillation | 38 (24.4%) | 22 (29.3%) | 16 (19.7%) | 16 (21.9%) | 22 (26.5%) |
| Diabetes | 37 (23.7%) | 13 (17.3%) | 24 (29.6%) | ||
| Coronary artery disease | 26 (16.7%) | 8 (10.7%) | 18 (22.2%) | 13 (17.8%) | 13 (15.7%) |
| Depression | 23 (14.7%) | 14 (18.7%) | 9 (11.1%) | 10 (13.7%( | 13 (15.7%) |
| Congestive heart failure | 23 (14.7%) | 9 (12.0%) | 14 (27.4%) | 6 (8.2%) | 17 (20.5%) |
| Chronic kidney disease | 20 (12.8 %) | 8 (10.7%) | 12 (14.8%) | 12 (16.4%) | 8 (11.0%) |
| Chronic pain | 16 (10.3%) | 10 (13.3%) | 6 (7.4%) | 5 (6.8%) | 11 (13.2%) |
| Urinary incontinence | 11 (7.1%) | 6 (8%) | 5 (6.2%) | 4 (5.4%) | 7 (8.4%) |
| Parkinson’s disease | 9 (5.8%) | 4 (5.3%) | 5 (6.2%) | 4 (5.4%) | 5 (6.0%) |
| Bowel incontinence | 8 (5.1%) | 5(6.7%) | 3 (3.7%) | 4 (5.4%) | 4 (4.8%) |
| COPD | 8 (5.1%) | 5 (6.7%) | 3 (3.7%) | 3 (4.1%) | 5 (6.0%) |
| Delirium | 5 (3.2%) | 4 (5.3%) | 1 (1.2%) | 3 (4.1%) | 2 (2.4%) |
| All (%) | Females | Males | Dementia | No Dementia | |
|---|---|---|---|---|---|
| <15 days | 34 (21.7) | 16 (21.3%) | 18 (22.5%) | 15 (20.5%) | 19 (22.9%) |
| 15–30 days | 41 (26.2) | 22 (29.3%) | 16 (20.0%) | 9 (12.3%) | 29 (34.9%) |
| 31–60 days | 37 (23.7) | 21 (28.0%) | 18 (22.5%) | 22 (30.1%) | 18 (21.7%) |
| 61–100 days | 18 (11.5) | 9 (12.0%) | 9 (11.3%) | 7 (9.6%) | 11 (13.3%) |
| 101–200 days | 20 (12.8) | 7 (9.3%) | 13 (16.3%) | 15 (20.5%) | 5 (6.0%) |
| 201–300 days | 4 (2.5) | 0 (0%) | 4 (5.0%) | 3 (4.1%) | 1 (1.2%) |
| >300 days | 2 (1.2) | 0 (0%) | 2 (2.5%) | 2 (2.7%) | 0 (0%) |
| All | Females | Males | Dementia | No Dementia | |
|---|---|---|---|---|---|
| Total adverse events | 362 | 156 | 206 | 206 | 156 |
| Infections | 94 | 37 | 57 | 58 | 36 |
| Urinary tract infections | 50 | 22 | 28 | 31 | 19 |
| Respiratory infections | 26 | 6 | 20 | 18 | 8 |
| Skin/soft tissue infections | 14 | 7 | 7 | 8 | 6 |
| Gastrointestinal infections | 3 | 1 | 2 | 0 | 3 |
| Bacteremia | 1 | 1 | 0 | 1 | 0 |
| Non-infectious adverse events | 268 | 119 | 149 | 148 | 120 |
| Delirium | 76 | 35 | 41 | 44 | 32 |
| Falls | 39 | 18 | 21 | 20 | 19 |
| Venothrombotic events | 2 | 2 | 0 | 1 | 1 |
| Pressure ulcers | 22 | 10 | 12 | 8 | 14 |
| Other | 129 | 54 | 75 | 75 | 54 |
| Antimicrobial days | 620 | 247 | 373 | 387 | 233 |
| For urinary infections | 299 | 133 | 166 | 200 | 99 |
| For respiratory infections | 147 | 39 | 108 | 79 | 68 |
| For skin/soft tissue infections | 136 | 51 | 85 | 94 | 42 |
| For gastrointestinal infections | 24 | 10 | 14 | 0 | 24 |
| For bacteremia | 14 | 14 | 0 | 14 | 0 |
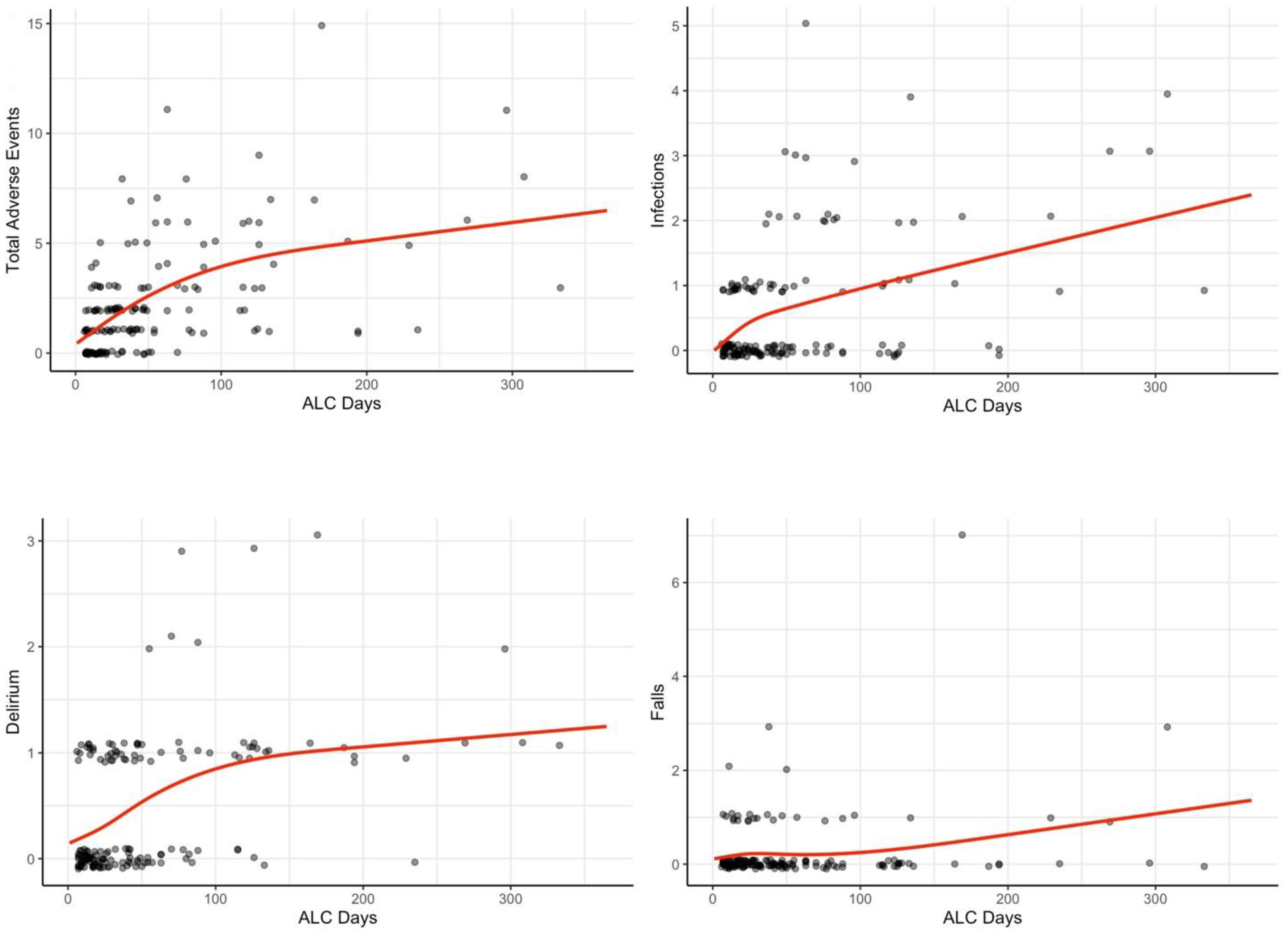
| ALC Days | Adverse Events | Infections | Delirium | Falls |
|---|---|---|---|---|
| 14 | 1.08 | 0.25 | 0.23 | 0.18 |
| 30 | 1.81 | 0.49 | 0.35 | 0.23 |
| 60 | 2.93 | 0.71 | 0.62 | 0.20 |
| 100 | 3.93 | 0.99 | 0.85 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim Fat, G.J.; Gopaul, A.; Pananos, A.D.; Taabazuing, M.-M. Healthcare-Associated Adverse Events in Alternate Level of Care Patients Awaiting Long-Term Care in Hospital. Geriatrics 2022, 7, 81. https://doi.org/10.3390/geriatrics7040081
Lim Fat GJ, Gopaul A, Pananos AD, Taabazuing M-M. Healthcare-Associated Adverse Events in Alternate Level of Care Patients Awaiting Long-Term Care in Hospital. Geriatrics. 2022; 7(4):81. https://doi.org/10.3390/geriatrics7040081
Chicago/Turabian StyleLim Fat, Guillaume J., Aquila Gopaul, A. Demetri Pananos, and Mary-Margaret Taabazuing. 2022. "Healthcare-Associated Adverse Events in Alternate Level of Care Patients Awaiting Long-Term Care in Hospital" Geriatrics 7, no. 4: 81. https://doi.org/10.3390/geriatrics7040081
APA StyleLim Fat, G. J., Gopaul, A., Pananos, A. D., & Taabazuing, M.-M. (2022). Healthcare-Associated Adverse Events in Alternate Level of Care Patients Awaiting Long-Term Care in Hospital. Geriatrics, 7(4), 81. https://doi.org/10.3390/geriatrics7040081






