Abstract
As the population ages and the prevalence of heart failure increases, cardiologists and geriatricians can expect to see more elderly patients with heart failure in their everyday practice. With the advancement of medical care and technology, the options for heart failure management have expanded, though current guidelines are based on studies of younger populations, and the evidence in older populations is not as robust. Pharmacologic therapy remains the cornerstone of heart failure management and has improved long-term mortality. Prevention of sudden cardiac death with implantable devices is being more readily utilized in older patients. Advanced therapies have provided more options for end-stage heart failure, though its use is still limited in older patients. In this review, we discuss the current guidelines for medical management of heart failure in older adults, as well as the expanding literature on advanced therapies, such as heart transplantation in older patients with end-stage heart failure. We also discuss the importance of a multidisciplinary care approach including consideration of non-medical co-morbidities such as frailty and cognitive decline.
1. Introduction
With the advancement of medicine, technology, and preventative care, the average life expectancy at birth has increased and is expected to continue rising. Worldwide, there has been an increase in the population 65 years and older [1,2,3,4] as well as the very elderly population greater than 85 years old [5]. By 2050, it is estimated that 17 percent of the world population and 21 percent of the United States (US) population will be above the age of 65 years [4,6]. Within the US, average total life expectancy is expected to reach 85.6 years by 2060 [1]. With the aging population, cardiologists and geriatricians can expect to see an increase in the number of older patients with multimorbidity and cardiovascular disease.
The prevalence of heart failure increases significantly with age and is estimated to affect more than 6 million adults in the US [3]. Prevalence almost doubles from approximately 6 percent of the population between 60 and 79 years to 11 percent of those greater than 80 years old [3]. The higher prevalence in the older population is likely attributed to age-related heart failure risk factors such as hypertension, coronary artery disease, diabetes, and structural and functional changes. Age plays a role in clinical characteristics and prognostic factors and outcomes in older patients. Older patients hospitalized with heart failure are more often female and have a higher prevalence of preserved ejection fraction and more significant valvular disease, though lower rates of traditional risk factors such as ischemic heart disease and diabetes [7]. Additionally, heart failure in older adults is associated with cognitive decline, frailty, and malnutrition and can significantly decrease quality of life [8]. The advancement of medicine and technology has resulted in a wider-range of treatment options for patients with heart failure. However, even though half of all patients with heart failure are over 75 years old, most clinical trials assess much younger cohorts. Applying conventional guidelines formulated using trial data derived from younger populations may call for special considerations for older patients, but often they do not provide concrete evidence-based recommendations. It is important to consider how age, comorbidities, and geriatric syndromes impact the management options, including medical and advanced therapies, for our older patients. This review was developed through an extensive PubMed search and review of pivotal trials of guideline-directed medical and device therapy for heart failure, with a specific focus on those addressing the geriatric population, polypharmacy, cognitive decline, and frailty.
2. Medical Management
2.1. Pharmacologic Therapy
Long-term heart failure mortality has improved with guideline-directed pharmacologic therapy and treatment of risk factors, though absolute mortality remains approximately 50% within five years of diagnosis [3]. Current heart failure guidelines [9,10] provide a number of Class I recommendations for medications in the management of heart failure with reduced ejection fraction, including renin–angiotensin receptor blockers or angiotensin receptor neprilysin inhibitors, beta blockers, aldosterone receptor antagonists, and loop diuretics for patients with evidence of fluid retention (Central Figure). Additionally, the sodium–glucose cotransporter-2 inhibitors dapagliflozin and empagliflozin were recently FDA approved for the management of heart failure with reduced ejection fraction [11,12]. These medications appear to also be beneficial in heart failure with preserved EF [13,14], which is particularly important given its increased prevalence in the elderly population.

Patients greater than 75 years old are under-represented in pivotal trials supporting the use of guideline-directed medical therapies. These trials also excluded those who did not meet criteria for creatinine clearance, heart rate, and systolic blood pressure. For example, the US Carvedilol Heart Failure study excluded patients with a systolic blood pressure greater than 160 mmHg and heart rate less than 68 beats per minute, and the average age of participants was 58 years old [15]. The SOLVD study evaluating the benefit of enalapril excluded patients greater than 80 years old and those who had an elevated creatinine or “any other disease that might substantially shorten survival or impede participation in a long-term trial [16]”. The average age of participants was 61 years old. While these trials have demonstrated mortality benefit with use of the studied medications, it serves as a reminder that trial populations often do not reflect real world patient populations. Pharmacologic therapy needs to be carefully selected in older adults for many reasons. With aging, there are changes in drug metabolism and patient body composition that can impact volume of distribution and plasma concentrations of medications. Older patients are also more likely to have concomitant renal or liver disease that raise the risk of medication-related adverse events. The presence of sick sinus syndrome and orthostatic hypotension may also impact the appropriateness of pharmacologic therapy in older patients. In the absence of clear guidance on when to discontinue or how to prioritize pharmacologic therapy in older patients, many clinicians continue to prescribe these medications despite a risk–benefit ratio that is less than clear. Target doses for older populations are not well established, and one meta-analysis suggested no benefit of angiotensin converting enzyme inhibitors after age 75 [17]. The potential of adverse effects should be routinely assessed when balancing the risks and benefits of pharmacologic therapies, particularly when life expectancy is shorter, decreasing the time horizon for potential benefit from pharmacologic therapy. This should include ongoing assessment of an older patient’s quality of life and priorities for medical care—living longer, maintaining current health, or comfort.
2.2. Defibrillators and Cardiac Resynchronization Therapy
In addition to pharmacologic therapy, primary prevention of sudden cardiac death is a routine part of guideline-directed therapy in select patients with cardiomyopathy and heart failure. Implantable cardioverter-defibrillator (ICD) therapy is recommended for patients with a left ventricular ejection fraction of 35% or less and New York Heart Association (NYHA) class II or III symptoms. Cardiac resynchronization therapy (CRT) is recommended for patients with a left ventricular ejection fraction of 35% or less and left bundle-branch block with a QRS duration of greater than 150 milliseconds, in addition to NYHA class II–IV symptoms [10]. Notably, the guidelines currently do not provide an age cutoff for those who should receive ICD and CRT implantations.
Although the mortality benefits of these devices have been shown in landmark trials [18,19,20,21], older adults were not well represented in the treatment groups, with the average age being between 58 and 64 years across these studies. Nonetheless, more than 40% of new ICDs and CRTs are implanted in patients >70 years old and more than 10% in patients >80 years old [22,23]. ICD therapy can reduce mortality, but it does not have any effect on symptoms or quality of life. In contrast, older patients have been shown to benefit symptomatically and echocardiographically from cardiac resynchronization similar to their younger counterparts [24,25]. From a mortality standpoint, the etiology of cardiomyopathy likely drives the benefit older patients receive from the addition of a defibrillator to resynchronization therapy. A study by Wang et al. found no difference in survival benefit in patients ≥75 years old with non-ischemic cardiomyopathy [26]. Gras et al. evaluated patients with ischemic and non-ischemic cardiomyopathy and found improved all-cause death in patients >75 years old with ischemic cardiomyopathy, whereas no benefit was seen in those with non-ischemic cardiomyopathy [27].
It is important to note that recommendations for device therapy are for patients with an expected meaningful survival of greater than one year, though decision making can be challenging given concurrent comorbidities, geriatric conditions, and how “meaningful survival” is defined. Additionally, procedural complications and routine maintenance and monitoring of implanted devices must be considered. As such, it is important to assess patients on an individualized basis when deciding who would be an appropriate candidate to refer for defibrillator or cardiac resynchronization therapies. Decision aids for ICDs are available and can be helpful in facilitating these conversations [28]. It would be reasonable to pursue placement of an ICD in an older patient with a life expectancy greater than one year and meaningful quality of life. For older patients with an ICD already in place, it can be important to discuss whether to deactivate shock therapy as their medical condition declines to avoid painful shocks at the end of life. Conversation around the risks and benefits of generator change is also important when batteries reach end of life.
3. Advanced Therapies
Despite evidence-based medical and device-based therapies, about 10 percent of heart failure patients progress to “advanced” or end-stage heart failure. Advanced heart failure is characterized by progressive symptoms including exercise intolerance, weight loss, refractory volume overload, and hypotension [29]. When heart failure reaches the advanced stage, numerous treatment options remain, including intravenous inotropes, left ventricular assist devices, and cardiac transplantation. However, these therapies all carry trade-offs in the form of serious adverse events or loss of independence that can impact quality of life. Thorough assessment of patient comorbidities and discussions about patient and caregiver preferences are critical for assessing the appropriateness of these therapies in the elderly.
3.1. Inotropes
Positive inotropic drugs such as dobutamine and milrinone improve hemodynamics in decompensated heart failure with low cardiac output and evidence of poor end-organ perfusion. They are commonly used short-term in patients with cardiogenic shock to maintain systemic perfusion while the acute precipitating event is treated or to bridge patients to a more durable advanced therapy. Due to concerns regarding increased mortality associated with their use, long-term inotropes are not routinely recommended. However, for select patients who cannot be weaned from inotropes or who receive significant symptomatic benefit from their use, long-term inotropes can be considered. Accordingly, current heart failure guidelines provide a Class IIb recommendation for long-term ambulatory use of inotropes as a palliative option for patients with end-stage heart failure who are not candidates for advanced therapies [10]. While continuous inotropes have be shown to be associated with higher mortality and adverse events [30,31,32], their use for improving symptoms and reducing hospitalizations is appropriate in the palliative setting [33,34,35,36]. Although there are limited data focused on the effects of inotropes in the elderly, given the limited options older patients with end-stage heart failure face, inotropes can be a therapeutic option for carefully selected patients. When utilizing inotropes, palliative care assistance should be considered to help manage symptoms and develop an end of life care plan.
3.2. Ventricular Assist Devices
Improvement in mechanical circulatory support technology has led to an increase in left ventricular assist device (LVAD) implantations, decreased in-hospital mortality, and improved survival [37,38]. The average patient age at LVAD implant remains approximately 57 years old [38], but as the population of patients with end-stage heart failure ages, the number of older patients undergoing LVAD implants for destination therapy is increasing. Analysis of data from the National Inpatient Sample found that the number of LVAD implants in patients greater than 65 years old has increased from 20% in 2007 to 33% in 2014 [39] and that adults greater than 75 years old receiving implants increased from 3.5% in 2003 to 10.5% in 2014 [40]. Data from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) reveal that the proportion of patients greater than 75 years receiving an LVAD has remained approximately 5% in recent years [41].
Existing data regarding outcomes in older adults with LVADs remain limited and contradictory. Analyses of INTERMACS database and National Inpatient Sample of older adults concluded that age remains a predictor of poor outcomes after LVAD implantation [41,42]. Patients greater than 75 years have lower survival rates and are less likely to be discharged home compared with patients younger than 55 years [41]. In contrast, an analysis of the Mechanical Circulatory Support Research Network registry revealed that age greater than 70 years was not a strong predictor of mortality, with a one-year survival similar to all destination therapy patients [43]. Another study of patients greater than 70 years old who received the HeartMate II LVAD showed good functional recovery, survival, and quality of life at two years, concluding that age alone should not be a contraindication for LVAD therapy [44].
Potential complications such as bleeding, which older patients are more likely to suffer from [41,43], infection, stroke, and pump thrombosis are additional factors to consider when deciding whether your older patient would be a candidate for LVAD. However, significant recent advances in LVAD technology have improved outcomes and hemocompatibility-related adverse events [45]. While survival with newer-generation LVADs remains lower in the elderly when compared with younger populations, elderly patients have shown similar improvements in quality of life and functional capacity, while having a lower rate of late major complications [46]. Despite these encouraging findings, use of LVADs in the elderly has not increased in the most recent era [46]. Proactive discussions, with the assistance of palliative care, about the risks and benefits of LVAD therapy in the elderly are critical to allow for timely implantation before elderly patients become too frail or have other end-organ dysfunction that will limit successful outcomes or eliminate potential LVAD candidacy.
3.3. Cardiac Transplantation
Cardiac transplantation remains the best therapeutic option for patients with end-stage heart failure. In the early era of heart transplantation, older patients were not considered transplant candidates, and further screening was recommended for patients over the age of 50 years old [47]. Due to improved outcomes in older patients and advances in post-transplant care, the 2006 International Society for Heart Lung Transplantation listing criteria for heart transplantation provided a Class IIb recommendation for carefully selected patients greater than 70 years old to be considered for transplantation [48], a recommendation that has carried onto the most recent 2016 guidelines [49]. The change in recommendations was based on data demonstrating comparable survival in older patients compared with younger patients. Weiss et al. reviewed the United Network for Organ Sharing database and found the five-year survival of patients greater than 60 years old to be nearly 70%—an acceptable rate compared with 75% in younger patients [50]. Goldstein et al. found that although patients in their 70s had lower survival compared with patients in their 60s, they still benefited significantly from cardiac transplant, with a median survival of 8.5 years compared with 9.8 years in the younger patients [51]. Other studies have found that the outcomes of transplant recipients in their 70s were similar to recipients in their 60s without significant difference in morbidity or mortality [52,53]. Additionally, older patients were less likely to experience rejection than their younger cohort [51,52]. These studies have suggested that age itself should not be an exclusion criterion to be considered for transplantation and that the proportion of heart transplants in older patients has been steadily increasing (Figure 1). However, careful consideration of a patient’s other end-organ function, frailty, and ability to recover from major surgery are a critical part of the evaluation for heart transplantation in the older population.
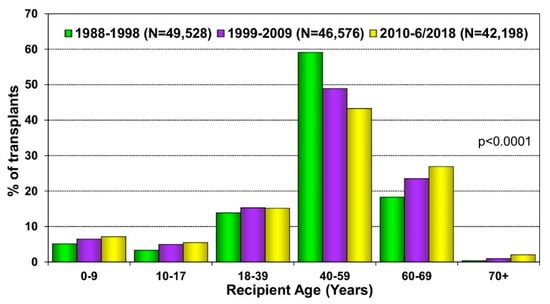
Figure 1.
Recipient age distribution (adult and pediatric) by era. Reprinted with permission from Khush KK, Wida CS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report—2019; focus theme: Donor and recipient size match. J Heart Lung Transplant; 2019; 38: 1056–1066.
4. Additional Considerations
When caring for older patients with heart failure, a multifactorial approach should be taken that includes assessment and management of associated geriatric syndromes such as cognitive impairment, frailty, and malnutrition, which can play a role in hospitalization, quality of life, morbidity, and mortality.
4.1. Polypharmacy
Polypharmacy, defined as five or more medications, is nearly universal in heart failure patients due to multimorbidity and guideline-based medication recommendations. Heart failure patients take an average of 10 medications, all of which carry risk for drug interactions and adverse reactions [54]. There is an increasing prevalence of polypharmacy in older adults, estimated to have grown from 24% in 2000 to 39% in 2012 amongst adults 65 years and older [55]. Though many trials have excluded older, multimorbid adults, guidelines do not provide concrete recommendations for discontinuation in older adults, and thus, medications are often continued indefinitely. Increased medication burden can decrease functional capacity and quality of life, as well as increasing the risks of side effects and adverse events. This then can lead to a prescribing cascade [56], which is the prescription of additional medications used to treat the side effects, further increasing the medication burden in older patients.
Recognizing the need to re-examine the benefits and risks of medication use, especially in older adults prone to adverse events, the process known as deprescribing is gaining momentum worldwide. Deprescribing focuses on removing or reducing unnecessary or potentially harmful medication use with the goal of improving outcomes, taking into account an individual’s overall physiologic status, stage of life, and goals of care [56]. Ongoing deprescribing trials focus on populations with higher co-morbidity burden and patients with possible symptoms related to medication use and can examine the safety of deprescribing. For example, the TRED-HF trial was a small, randomized trial of withdrawal of heart failure medications in patients with dilated cardiomyopathy with improved ejection fraction. Patients deemed recovered from dilated cardiomyopathy relapsed following treatment withdrawal, implying that indefinite treatment for cardiomyopathy may be necessary [57]. Although the trial examined a younger population, the implications of the trial could aid in the decision of which medications may or may not be appropriate to discontinue given the high incidence of heart failure in the older population.
For providers and patients interested in pursuing deprescribing, Krishnaswami et al. provide a five-step approach to deprescribing that includes: (1) reviewing and reconciling medications, (2) assessing the risk of adverse drug events, (3) assessing candidacy of individual medications, (4) prioritizing drug discontinuation, and (5) discontinuing medications and implementing monitoring protocols [56]. The decision and process to deprescribe should include patients, their families, and health care personnel such as pharmacists who can help to analyze medication lists and identify potential drug interactions and specific medications to review.
4.2. Cognitive Decline
The prevalence of cognitive impairment is approximately 16 to 20 percent of the general population and 40 percent amongst patients with heart failure [58], with an even higher prevalence in patients with more advanced symptoms [59]. Older adults are at risk for not only age-related cognitive changes such as dementia but also cardiovascular etiologies as well, such as cardio-cerebral syndrome in heart failure. The pathophysiology of cognitive impairment in patients with heart failure is multifactorial, and proposed mechanisms include decreased cerebral perfusion and neurohormonal changes [60]. Clinical manifestations include abnormalities in learning, memory, psychomotor speed, executive function, and complex attention [8,61]. Cognitive decline has been associated with worse quality of life, spousal/caregiver distress, readmission risk, and increased mortality risk [62,63]. Reviews of the literature have suggested that while heart failure patients are at increased risk for cognitive decline, this may be modified by treatment such as cardiac transplantation [64,65]. Further investigation to identify the mechanisms of cognitive decline in heart failure and therapeutic interventions is needed. Identification of cognitive impairment through routine screening may help clinicians investigate potential reversible causes, as well as aiding in providing multidisciplinary care.
4.3. Frailty
Frailty is a syndrome that is characterized by the exaggerated decline in function and increased physiological vulnerability to stressors [66], commonly seen in older patients with heart failure. The prevalence of frailty ranges from 10% to 60% [66] and is estimated to be approximately 50% in community-dwelling patients with heart failure and 75% amongst hospitalized patients [67]. The biological mechanism of frailty includes up-regulation of inflammatory markers that leads to hormonal dysregulation and a downstream catabolic state and muscle wasting [8]. Frailty has been associated with increased disability, mortality, and hospitalization [68,69]. In a recent prospective study following hospitalized patients greater than 65 years old, advancing age was strongly associated with increased frailty domains, including physical and social frailty, and was associated with higher mortality, heart failure readmissions, and all-cause death [70]. In patients with advanced heart failure, frailty prior to transplantation was found to be associated with increased mortality and hospitalization post-transplant [71].
Frailty assessment is instrumental in helping to guide treatment plans that will maximize patients’ likelihood of a positive outcome and should be implemented as part of routine heart failure management in older adults. The Fried phenotype method (Table 1) is a widely used assessment model, and frailty based on the Fried phenotype is consistently associated with worse clinical outcomes, greater functional impairment, and poor quality of life in older, community-dwelling individuals [72]. An additional importance of recognizing frailty is not just prognostic value. Physical activity is associated with better outcomes, and in those who are frail, increased physical activity can lower the risk of all-cause mortality [73]. The recent REHAB-HF trial found that early, tailored rehabilitation intervention resulted in greater improvement in physical function in older patients hospitalized with decompensated heart failure [74]. Frail patients in particular may benefit from the aforementioned deprescribing strategy described above. This should especially be considered if medical therapy contributes to orthostatic symptoms or fatigue that limits physical rehabilitation or if pill burden limits oral intake that contributes to malnutrition. Unfortunately, no evidence currently exists to guide which medications to prioritize for deprescribing. Individual patient priorities and comorbidities should therefore influence medication changes.

Table 1.
Fried Frailty Criteria [72].
5. Conclusions
As the population ages and medical therapy improves, the number of older heart failure patients is expected to increase as well. Although medical therapy has improved outcomes in heart failure, mortality and hospitalization remain high. Older patients were historically underrepresented or excluded from the landmark trials that led to the current heart failure guidelines, though we continue to apply the current recommendations to our older patients.
Heart transplantation remains the best therapeutic option for end-stage heart failure, though age remains a barrier to transplant at many institutions. Alternatively, technological advances have allowed more patients to receive LVAD in end-stage heart failure, but older patients are more prone to complications. Newer LVAD technology may mitigate those complications, but the number of older LVAD patients has not increased in recent years. In our older patients with heart failure, it is also important to take a multifactorial approach to care, while considering geriatric syndromes, polypharmacy, and expected survival when prescribing and offering advanced therapies.
Author Contributions
E.L. and B.C.L. contributed equally to conceptualization, writing, and revising of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Medina, L.; Sabo, S.; Vespa, J. Living Longer: Historical and Projected Life Expectancy in the United States, 1960 to 2060; US Department of Commerce, US Census Bureau: Suitland, MD, USA, 2020; Volume P25-1145.
- Roberts, A.; Ogunwole, S.; Blackslee, L.; Rabe, M. The Population 65 Years and Older in the United States: 2016. American Community Survey Reports. 2018; Volume ACS-38. Available online: https://www.census.gov/content/dam/Census/library/publications/2018/acs/ACS-38.pdf (accessed on 28 September 2021).
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Goodkind, D.; Kowal, P. An Aging World: 2015; U.S. Census Bureau: Suitland, MD, USA, 2016; Volume P95/16-1.
- Ortman, J.; Velkoff, V.; Hogan, H. An Aging Nation: The Older Population in the United States; Population Estimates and Projections; US Census Bureau: Washington, DC, USA, 2014; Volume P25-1140.
- West, L.; Cole, S.; Goodkind, D.; He, W. 65+ in the United States: 2010; US Census Bureau: Washington, DC, USA, 2014; Volume P23-212.
- Stein, G.Y.; Kremer, A.; Shochat, T.; Bental, T.; Korenfeld, R.; Abramson, E.; Ben-Gal, T.; Sagie, A.; Fuchs, S. The Diversity of Heart Failure in a Hospitalized Population: The Role of Age. J. Card. Fail. 2012, 18, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Gorodeski, E.Z.; Goyal, P.; Hummel, S.L.; Krishnaswami, A.; Goodlin, S.J.; Hart, L.L.; Forman, D.E.; Wenger, N.K.; Kirkpatrick, J.N.; Alexander, K.P. Domain Management Approach to Heart Failure in the Geriatric Patient: Present and Future. J. Am. Coll. Cardiol. 2018, 71, 1921–1936. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Card. Fail. 2017, 23, 628–651. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Borlaug, B.A.; Kitzman, D.W.; Shah, S.J.; Tang, F.; Khariton, Y.; Malik, A.O.; Khumri, T.; Umpierrez, G.; et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat. Med. 2021, 27, 1954–1960. [Google Scholar] [CrossRef]
- Packer, M.; Bristow, M.R.; Cohn, J.N.; Colucci, W.; Fowler, M.B.; Gilbert, E.M.; Shusterman, N.H.; U.S. Carvedilol Heart Failure Study Group. The Effect of Carvedilol on Morbidity and Mortality in Patients with Chronic Heart Failure. N. Engl. J. Med. 1996, 334, 1349–1355. [Google Scholar] [CrossRef]
- The SOLVD Investigators; Yusuf, S.; Pitt, B.; Davis, C.E.; Hood, W.B.; Cohn, J.N. Effect of Enalapril on Survival in Patients with Reduced Left Ventricular Ejection Fractions and Congestive Heart Failure. N. Engl. J. Med. 1991, 325, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Flather, M.D.; Yusuf, S.; Køber, L.; Pfeffer, M.; Hall, A.; Murray, G.; Torp-Pedersen, C.; Ball, S.; Pogue, J.; Moyé, L.; et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: A systematic overview of data from individual patients. Lancet 2000, 355, 1575–1581. [Google Scholar] [CrossRef]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Klein, H.; Levine, J.H.; Saksena, S.; Waldo, A.L.; Wilber, D.; et al. Improved Survival with an Implanted Defibrillator in Patients with Coronary Disease at High Risk for Ventricular Arrhythmia. N. Engl. J. Med. 1996, 335, 1933–1940. [Google Scholar] [CrossRef]
- Kadish, A.; Dyer, A.; Daubert, J.P.; Quigg, R.; Estes, N.M.; Anderson, K.P.; Calkins, H.; Hoch, D.; Goldberger, J.; Shalaby, A.; et al. Prophylactic Defibrillator Implantation in Patients with Nonischemic Dilated Cardiomyopathy. N. Engl. J. Med. 2004, 350, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Bardy, G.H.; Lee, K.L.; Mark, D.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an Implantable Cardioverter–Defibrillator for Congestive Heart Failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac Resynchronization in Chronic Heart Failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.E.; Kay, G.N.; Plumb, V.J.; McElderry, H.T.; Doppalapudi, H.; Yamada, T.; Shafiroff, J.; Syed, Z.A.; Shkurovich, S. Implantable cardioverter-defibrillator prescription in the elderly. Heart Rhythm 2009, 6, 1136–1143. [Google Scholar] [CrossRef]
- Hammill, S.C.; Kremers, M.S.; Kadish, A.H.; Stevenson, L.W.; Heidenreich, P.A.; Lindsay, B.D.; Mirro, M.J.; Radford, M.J.; McKay, C.; Wang, Y.; et al. Review of the ICD Registry’s Third Year, Expansion to include Lead Data and Pediatric ICD Procedures, and Role for Measuring Performance. Heart Rhythm 2009, 6, 1397–1401. [Google Scholar] [CrossRef]
- Martens, P.; Verbrugge, F.H.; Nijst, P.; Dupont, M.; Mullens, W. Mode of Death in Octogenarians Treated With Cardiac Resynchronization Therapy. J. Card. Fail. 2016, 22, 970–977. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Dupont, M.; De Vusser, P.; Rivero-Ayerza, M.; Van Herendael, H.; Vercammen, J.; Jacobs, L.; Verhaert, D.; Vandervoort, P.; Tang, W.W.; et al. Response to cardiac resynchronization therapy in elderly patients (≥70 years) and octogenarians. Eur. J. Heart Fail. 2013, 15, 203–210. [Google Scholar] [CrossRef]
- Wang, Y.; Sharbaugh, M.S.; Althouse, A.D.; Mulukutla, S.; Saba, S. Cardiac resynchronization therapy pacemakers versus defibrillators in older non-ischemic cardiomyopathy patients. Indian Pacing Electrophysiol. J. 2019, 19, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Gras, M.; Bisson, A.; Bodin, A.; Herbert, J.; Babuty, D.; Pierre, B.; Clementy, N.; Fauchier, L. Mortality and cardiac resynchronization therapy with or without defibrillation in primary prevention. Europace 2020, 22, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- A Decision Aid for Implantable Cardioverter-Defibrillators (ICD) for Patients with Heart Failure Considering an ICD Who Are at Risk for Sudden Cardiac Death (Primary Prevention). 2017. Available online: https://patientdecisionaid.org/wp-content/uploads/2020/03/ICD-Pamphlet-4.13.21.pdf (accessed on 28 September 2021).
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure: Epidemiology, Diagnosis, and Therapeutic Approaches. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Gattis, W.A.; Uretsky, B.F.; Adams, K.F.; McNulty, S.E.; Grossman, S.H.; McKenna, W.J.; Zannad, F.; Swedberg, K.; Gheorghiade, M.; et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: Insights from the Flolan International Randomized Survival Trial (FIRST). Am. Heart J. 1999, 138, 78–86. [Google Scholar] [CrossRef]
- Packer, M.; Carver, J.R.; Rodeheffer, R.J.; Ivanhoe, R.J.; Dibianco, R.; Zeldis, S.M.; Hendrix, G.H.; Bommer, W.J.; Elkayam, U.; Kukin, M.L.; et al. Effect of Oral Milrinone on Mortality in Severe Chronic Heart Failure. N. Engl. J. Med. 1991, 325, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Cuffe, M.S.; Califf, R.M.; Adams, J.K.F.; Benza, R.L.; Bourge, R.C.; Colucci, W.; Massie, B.M.; O’Connor, C.M.; Pina, I.L.; Quigg, R.; et al. Short-term Intravenous Milrinone for Acute Exacerbation of Chronic Heart FailureA Randomized Controlled Trial. JAMA 2002, 287, 1541–1547. [Google Scholar] [CrossRef]
- Martens, P.; Vercammen, J.; Ceyssens, W.; Jacobs, L.; Luwel, E.; Van Aerde, H.; Potargent, P.; Renaers, M.; Dupont, M.; Mullens, W. Effects of intravenous home dobutamine in palliative end-stage heart failure on quality of life, heart failure hospitalization, and cost expenditure. ESC Heart Fail. 2018, 5, 562–569. [Google Scholar] [CrossRef]
- Oliva, F.; Latini, R.; Politi, A.; Staszewsky, L.; Maggioni, A.P.; Nicolis, E.; Mauri, F. Intermittent 6-month low-dose dobutamine infusion in severe heart failure: DICE Multicenter Trial. Am. Heart J. 1999, 138, 247–253. [Google Scholar] [CrossRef]
- Cesario, D.; Clark, J.; Maisel, A. Beneficial effects of intermittent home administration of the inotrope/vasodilator milrinone in patients with end-stage congestive heart failure: A preliminary study. Am. Heart. J. 1998, 135, 121–129. [Google Scholar] [CrossRef]
- Chernomordik, F.; Freimark, D.; Arad, M.; Shechter, M.; Matetzky, S.; Savir, Y.; Shlomo, N.; Peled, A.; Goldenberg, I.; Peled, Y. Quality of life and long-term mortality in patients with advanced chronic heart failure treated with intermittent low-dose intravenous inotropes in an outpatient setting. ESC Heart Fail. 2017, 4, 122–129. [Google Scholar] [CrossRef]
- Briasoulis, A.; Inampudi, C.; Akintoye, E.; Adegbala, O.; Alvarez, P.; Bhama, J. Trends in Utilization, Mortality, Major Complications, and Cost After Left Ventricular Assist Device Implantation in the United States (2009 to 2014). Am. J. Cardiol. 2018, 121, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.J.; Shah, P.; Kiernan, M.S.; Cornwell, W.K.; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann. Thorac. Surg. 2021, 111, 778–792. [Google Scholar] [CrossRef]
- Rali, A.S.; Ranka, S.; Acharya, P.; Buechler, T.; Weidling, R.; Mastoris, I.; Taduru, S.; Abicht, T.; Haglund, N.; Sauer, A.J.; et al. Comparison of Trends, Mortality, and Readmissions After Insertion of Left Ventricular Assist Devices in Patients <65 Years vs. ≥65 Years. Am. J. Cardiol. 2020, 128, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Rali ASV, V.; Masoomi, R.; Shah, Z.; Gupta, B.; Haglund, N.; Abicht, T.; Sauer, A. Trends in LVADs in the Geriatric Population: Demographics for 2003–2014. J. Card. Fail. 2017, 23, S17. [Google Scholar] [CrossRef][Green Version]
- Caraballo, C.; DeFilippis, E.M.; Nakagawa, S.; Ravindra, N.G.; Miller, P.E.; Mezzacappa, C.; McCullough, M.; Gruen, J.; Levin, A.; Reinhardt, S.; et al. Clinical Outcomes After Left Ventricular Assist Device Implantation in Older Adults: An INTERMACS Analysis. JACC: Heart Fail. 2019, 7, 1069–1078. [Google Scholar] [CrossRef]
- Gazda, A.J.; Kwak, M.J.; Akkanti, B.; Nathan, S.; Kumar, S.; de Armas, I.S.; Baer, P.; Patel, B.; Kar, B.; Gregoric, I.D. Complications of LVAD utilization in older adults. Heart Lung 2021, 50, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Singh, R.; Pagani, F.D.; Desai, S.S.; Haglund, N.A.; Dunlay, S.M.; Maltais, S.; Aaronson, K.D.; Stulak, J.M.; Davis, M.E.; et al. Ventricular Assist Device Therapy in Older Patients With Heart Failure: Characteristics and Outcomes. J. Card. Fail. 2016, 22, 981–987. [Google Scholar] [CrossRef]
- Adamson, R.M.; Stahovich, M.; Chillcott, S.; Baradarian, S.; Chammas, J.; Jaski, B.; Hoagland, P.; Dembitsky, W. Clinical Strategies and Outcomes in Advanced Heart Failure Patients Older Than 70 Years of Age Receiving the HeartMate II Left Ventricular Assist Device: A Community Hospital Experience. J. Am. Coll. Cardiol. 2011, 57, 2487–2495. [Google Scholar] [CrossRef]
- Mehra, M.R.; Goldstein, D.J.; Uriel, N.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Ewald, G.A.; et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N. Engl. J. Med. 2018, 378, 1386–1395. [Google Scholar] [CrossRef]
- Emerson, D.; Chikwe, J.; Catarino, P.; Hassanein, M.; Deng, L.; Cantor, R.S.; Roach, A.; Cole, R.; Esmailian, F.; Kobashigawa, J.; et al. Contemporary Left Ventricular Assist Device Outcomes in an Aging Population: An STS INTERMACS Analysis. J. Am. Coll. Cardiol. 2021, 78, 883–894. [Google Scholar] [CrossRef]
- Miller, L.W. Listing criteria for cardiac transplantation: Results of an American Society of Transplant Physicians-National Institutes of Health conference. Transplantation 1998, 66, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Kobashigawa, J.; Starling, R.; Russell, S.; Uber, P.A.; Parameshwar, J.; Mohacsi, P.; Augustine, S.; Aaronson, K.; Barr, M. Listing Criteria for Heart Transplantation: International Society for Heart and Lung Transplantation Guidelines for the Care of Cardiac Transplant Candidates—2006. J. Heart Lung Transplant. 2006, 25, 1024–1042. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Weiss, E.S.; Nwakanma, L.U.; Patel, N.D.; Yuh, D.D. Outcomes in Patients Older Than 60 Years of Age Undergoing Orthotopic Heart Transplantation: An Analysis of the UNOS Database. J. Heart Lung Transplant. 2008, 27, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.J.; Bello, R.; Shin, J.J.; Stevens, G.; Zolty, R.; Maybaum, S.; D’Alessandro, D. Outcomes of cardiac transplantation in septuagenarians. J. Heart Lung Transplant. 2012, 31, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.B.; Lu, D.; Mentz, R.J.; Rogers, J.G.; Milano, C.A.; Felker, G.; Hernandez, A.F.; Patel, C.B. Cardiac transplantation for older patients: Characteristics and outcomes in the septuagenarian population. J. Heart Lung Transplant. 2016, 35, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Blanche, C.; Blanche, D.A.; Kearney, B.; Sandhu, M.; Czer, L.S.; Kamlot, A.; Hickey, A.; Trento, A. Heart transplantation in patients seventy years of age and older: A comparative analysis of outcome. J. Thorac. Cardiovasc. Surg. 2001, 121, 532–541. [Google Scholar] [CrossRef]
- Gastelurrutia, P.; Benrimoj, S.C.; Espejo, J.; Tuneu, L.; Mangues, M.A.; Bayes-Genis, A. Negative Clinical Outcomes Associated With Drug-Related Problems in Heart Failure (HF) Outpatients: Impact of a Pharmacist in a Multidisciplinary HF Clinic. J. Card. Fail. 2011, 17, 217–223. [Google Scholar] [CrossRef]
- Kantor, E.D.; Rehm, C.D.; Haas, J.S.; Chan, A.T.; Giovannucci, E.L. Trends in Prescription Drug Use Among Adults in the United States From 1999–2012. JAMA 2015, 314, 1818–1831. [Google Scholar] [CrossRef]
- Krishnaswami, A.; Steinman, M.A.; Goyal, P.; Zullo, A.R.; Anderson, T.S.; Birtcher, K.K.; Goodlin, S.J.; Maurer, M.S.; Alexander, K.P.; Rich, M.W.; et al. Deprescribing in Older Adults With Cardiovascular Disease. J. Am. Coll. Cardiol. 2019, 73, 2584–2595. [Google Scholar] [CrossRef]
- Halliday, B.P.; Wassall, R.; Lota, A.S.; Khalique, Z.; Gregson, J.; Newsome, S.; Jackson, R.; Rahneva, T.; Wage, R.; Smith, G.; et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): An open-label, pilot, randomised trial. Lancet 2019, 393, 61–73. [Google Scholar] [CrossRef]
- Cannon, J.A.; Moffitt, P.; Perez-Moreno, A.C.; Walters, M.R.; Broomfield, N.; Mcmurray, J.; Quinn, T.J. Cognitive Impairment and Heart Failure: Systematic Review and Meta-Analysis. J. Card. Fail. 2017, 23, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Harkness, K.; Demers, C.; Heckman, G.; McKelvie, R.S. Screening for Cognitive Deficits Using the Montreal Cognitive Assessment Tool in Outpatients ≥65 Years of Age With Heart Failure. Am. J. Cardiol. 2011, 107, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Havakuk, O.; King, K.; Grazette, L.; Yoon, A.J.; Fong, M.; Bregman, N.; Elkayam, U.; Kloner, R.A. Heart Failure-Induced Brain Injury. J. Am. Coll. Cardiol. 2017, 69, 1609–1616. [Google Scholar] [CrossRef]
- Leto, L.; Feola, M. Cognitive impairment in heart failure patients. J. Geriatr. Cardiol. 2014, 11, 316–328. [Google Scholar] [CrossRef]
- Yzeiraj, E.; Tam, D.M.; Gorodeski, E.Z. Management of Cognitive Impairment in Heart Failure. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 4. [Google Scholar] [CrossRef]
- Agarwal, K.S.; Kazim, R.; Xu, J.; Borson, S.; Taffet, G.E. Unrecognized Cognitive Impairment and Its Effect on Heart Failure Readmissions of Elderly Adults. J. Am. Geriatr. Soc. 2016, 64, 2296–2301. [Google Scholar] [CrossRef]
- Vogels, R.L.C.; Scheltens, P.; Schroeder-Tanka, J.M.; Weinstein, H.C. Cognitive impairment in heart failure: A systematic review of the literature. Eur. J. Heart Fail. 2007, 9, 440–449. [Google Scholar] [CrossRef]
- Hajduk, A.M.; Kiefe, C.I.; Person, S.D.; Gore, J.G.; Saczynski, J.S. Cognitive Change in Heart Failure: A systematic review. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 451–460. [Google Scholar] [CrossRef]
- Afilalo, J.; Alexander, K.P.; Mack, M.J.; Maurer, M.S.; Green, P.; Allen, L.A.; Popma, J.J.; Ferrucci, L.; Forman, D.E. Frailty Assessment in the Cardiovascular Care of Older Adults. J. Am. Coll. Cardiol. 2014, 63, 747–762. [Google Scholar] [CrossRef]
- Pandey, A.; Kitzman, D.; Reeves, G. Frailty Is Intertwined with Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Heart Fail. 2019, 7, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lupón, J.; Vidán, M.T.; Ferguson, C.; Gastelurrutia, P.; Newton, P.J.; Macdonald, P.S.; Bueno, H.; Bayés-Genís, A.; Woo, J.; et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008251. [Google Scholar] [CrossRef] [PubMed]
- Vidán, M.T.; Blaya-Novakova, V.; Sánchez, E.; Ortiz, J.; Serra-Rexach, J.A.; Bueno, H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur. J. Heart Fail. 2016, 18, 869–875. [Google Scholar] [CrossRef]
- Matsue, Y.; Kamiya, K.; Saito, H.; Saito, K.; Ogasahara, Y.; Maekawa, E.; Konishi, M.; Kitai, T.; Iwata, K.; Jujo, K.; et al. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: The FRAGILE-HF cohort study. Eur. J. Heart Fail. 2020, 22, 2112–2119. [Google Scholar] [CrossRef]
- Macdonald, P.S.; Gorrie, N.; Brennan, X.; Aili, S.R.; De Silva, R.; Jha, S.R.; Fritis-Lamora, R.; Montgomery, E.; Wilhelm, K.; Pierce, R.; et al. The impact of frailty on mortality after heart transplantation. J. Heart Lung Transplant. 2021, 40, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Higueras-Fresnillo, S.; Cabanas-Sánchez, V.; Lopez-Garcia, E.; Esteban-Cornejo, I.; Banegas, J.R.; Sadarangani, K.P.; Rodríguez-Artalejo, F.; Martinez-Gomez, D. Physical Activity and Association Between Frailty and All-Cause and Cardiovascular Mortality in Older Adults: Population-Based Prospective Cohort Study. J. Am. Geriatr. Soc. 2018, 66, 2097–2103. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Whellan, D.J.; Duncan, P.; Pastva, A.M.; Mentz, R.J.; Reeves, G.R.; Nelson, M.B.; Chen, H.; Upadhya, B.; Reed, S.D.; et al. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. N. Engl. J. Med. 2021, 385, 203–216. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).