Evidence-Informed Approach to De-Prescribing of Atypical Antipsychotics (AAP) in the Management of Behavioral Expressions (BE) in Advanced Neurocognitive Disorders (NCD): Results of a Retrospective Study
Abstract
1. Introduction
Objective
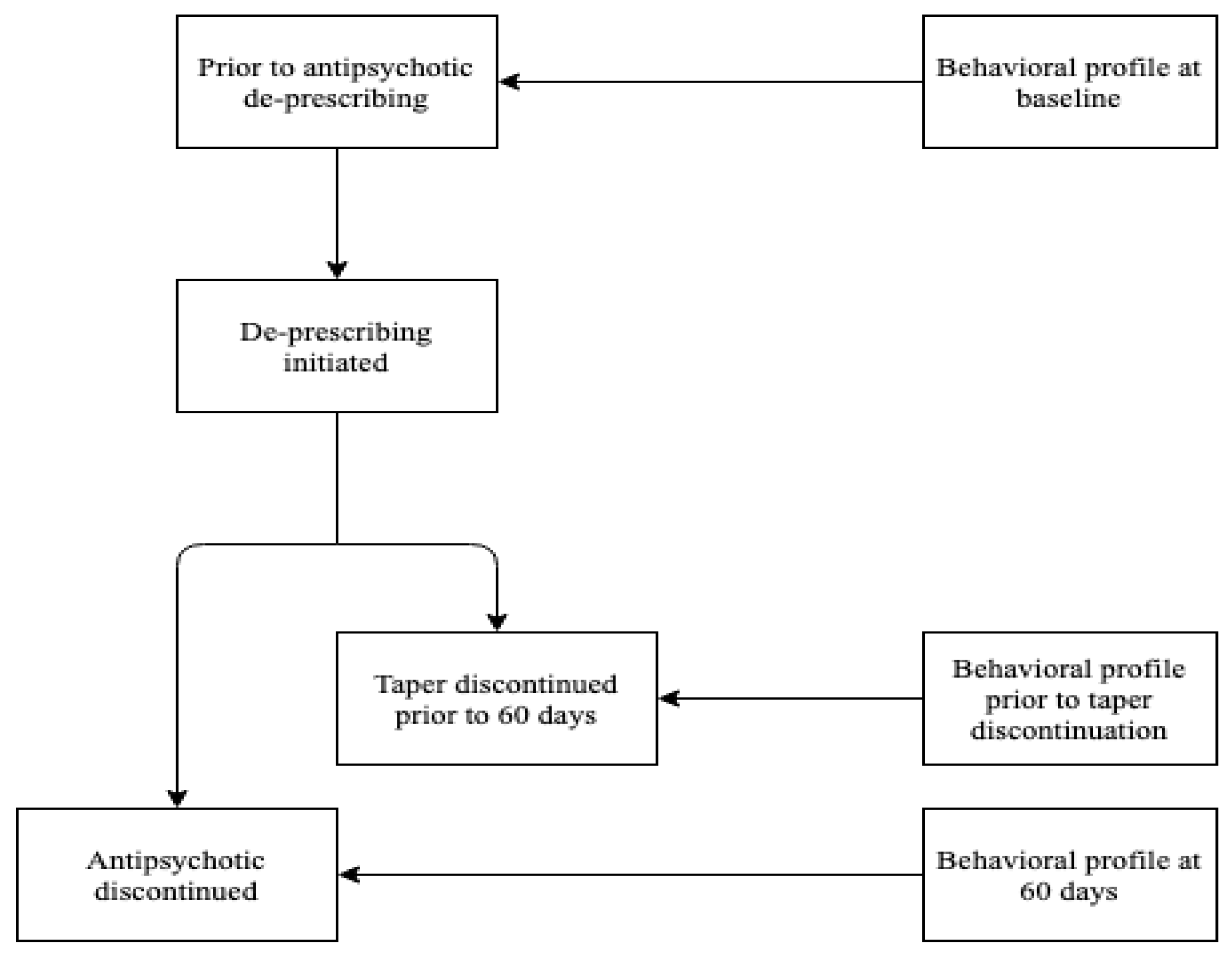
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
4.1. Use of Atypical Antipsychotics for Mis-Identification Expressions
4.2. Use of Atypical Antipsychotics for Goal-Directed Expressions
4.3. Equivocal Response of Vocal Expressions to AAP
4.4. Absence of Response of Dis-Organized Expression to AAP
4.5. Absence of Response of Emotional and Fretful Expressions to AAP
4.6. Absence of Response of Apathy Expressions to AAP
4.7. Absence of Response of Oppositional Expressions to AAP
4.8. Absence of Response of Physical Expressions to AAP
4.9. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013; ISBN 9780890425558. [Google Scholar]
- Cohen-Mansfield, J. Consent and Refusal in Dementia/NCD research: Conceptual and practical consideration. Alzheimer Dis. Assoc. Disord. 2003, 17, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.I.; Sadock, B.J.; Sadock, V.A.; Ruiz, P. Comprehensive Textbook of Psychiatry, 6th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 1995; pp. 250–650. [Google Scholar]
- Cohen-Mansfield, J. Theoretical frameworks for behavioral problems in dementia. Alzheimer’s Care Q 2000, 1, 8–21. [Google Scholar]
- De Medeiros, K.; Robert, P.; Gauthier, S.; Stella, F.; Politis, A.; Leoutsakos, J.; Taragano, F.; Kremer, B.A.; Porsteinsson, A.P.; Geda, Y.E.; et al. The Neuropsychiatric inventory—Clinician-rating scale (NPI-C): Reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int. Psychogeriatr. 2010, 22, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.; Gray, A.; Ayre, G. Psychotic symptoms, aggressions and restlessness in dementia. Rev. Neurol. 1999, 155, 44–52. [Google Scholar]
- Kales, H.C.; Gitlin, L.N.; Lyketsos, C.G. Assessment and management of behavioral and psychological symptoms of dementia. BMJ 2015, 2, 350–369. [Google Scholar] [CrossRef]
- Carrarini, C.; Russo, M.; Dono, F.; Barbone, F.; Rispoli, M.G.; Ferri, L.; Pietro, M.D.; Digiovanni, A.; Ajdinaj, P.; Speranza, R.; et al. Agitation and dementia: Prevention and treatment strategies in acute and chronic conditions. Front. Neurol. 2021, 12, 644317. [Google Scholar] [CrossRef]
- Cloak, N.; Khalili, Y.A. Behavioral and Psychological Symptoms in Dementia; Stat Pearls Publishing: Treasure Island, FL, USA, 2020; PMID 31855379. [Google Scholar]
- Marcinkowska, M.; Sniecikowska, J.; Fajkis, N.; Franczyk, W.; Kolaczkowski, M. Management of dementia-related psychosis, agitation and aggression: A review of the pharmacology and clinical effects of potential drug candidates. CNS Drugs 2020, 34, 243–268. [Google Scholar] [CrossRef]
- Imel, M.; Campbell, J.R. Mapping from a Clinical Terminology to a Classification. 2003. Available online: https://library.ahima.org/doc?oid=61537#.YfC92PgRU2w (accessed on 11 December 2021).
- World Health Organization. World Alzheimer Report 2012. A Public Health Priority. Available online: https://www.who.int/publications/item/dementia-a-public-health-priority (accessed on 26 October 2021).
- Gottesman, R.T.; Stem, Y. Behavioral and psychiatric symptoms of dementia and rate of decline in Alzheimer’s disease. Front. Pharmacol. 2019, 10, 1062. [Google Scholar] [CrossRef]
- Seitz, D.; Sherman, C.; Kirkham, J. Prevalence of psychotropic medication use among older adults with dementia: Meta-Analysis. Am. J. Geriatr. Psychiatry 2015, 23, 154–155. [Google Scholar] [CrossRef]
- Patel, V.; Hope, R.A. A rating scale for aggressive behavior in the elderly—the RACE. Psychol. Med. 1992, 22, 211–221. [Google Scholar] [CrossRef]
- Pulsford, D.; Duxbury, J. Aggressive behavior by people with dementia in residential care settings: A review. J. Psychiatr. Ment. Health Nurs. 2006, 13, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Devanand, D.P.; Mintzer, J.; Schultz, S.K.; Andrews, H.F.; Sultzer, D.L.; Pena, D.; Gupta, S.; Colon, S.; Schimming, C.; Pelton, G.H.; et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. NEJM 2012, 367, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Buhr, G.T.; White, H.K. Difficult behaviors in long-term care patients with dementia. J. Am. Med. Dir. Assoc. 2006, 8, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Samara, M.; Heres, S.; Davis, J.M. Dose equivalents for antipsychotic drugs: The DDD method. Schizophr. Bull. 2016, 42, 90–94. [Google Scholar] [CrossRef]
- Smith, M.; Buckwalter, K. Behaviors associated with dementia. Am. J. Nurs. 2005, 105, 40–52. [Google Scholar] [CrossRef]
- Macfarlane, S.; Cunningham, C. Limiting antipsychotic drugs in dementia. Aust. Prescr. 2021, 44, 8–11. [Google Scholar] [CrossRef]
- Ma, H.; Huang, Y.; Cong, Z.; Wang, Y.; Jiang, W.; Gao, S.; Zhu, G. The efficacy and safety of atypical antipsychotics for the treatment of dementia: A meta-analysis of randomized placebo-controlled trials. J. Alzheimer’s Dis. 2014, 42, 915–937. [Google Scholar] [CrossRef]
- Leeuwen, E.V.; Petrovik, M.; Mieke, L.v.D.; Sutter, A.I.D.; Stichele, R.V.; Declercq, T.; Christiaens, C. Withdrawal versus continuation of long-term antipsychotic drug use for behavioral and psychological symptoms in older people with dementia. Cochrane Database Syst. Rev. 2018, 30, CD007726. [Google Scholar] [CrossRef]
- Kleijer, B.C.; Marum, R.J.; Egberts, A.C.G.; Jansen, P.A.F.; Frijters, D.; Heerdink, E.R.; Ribbe, M. The course of behavioural problems in elderly nursing home patients with dementia when treated with antipsychotics. Int. Psychogeriatr. 2009, 21, 931–940. [Google Scholar] [CrossRef]
- Tampi, R.R.; Tampi, D.J.; Balachandran, S.; Srinivasan, S. Antipsychotic use in dementia: A systematic review of benefits and risks from meta-analysis. Ther. Adv. Chronic Dis. 2016, 7, 229–245. [Google Scholar] [CrossRef]
- Bjerre, L.M.; Farrell, B.; Hogel, M.; Lemay, G.; McCarthy, L.; Raman-Wilms, L.; Rojas-Fernandez, C.; Sinha, S.; Thompson, W.; Welch, V.; et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia Evidence-based clinical practice guideline. Can. Fam. Physician 2018, 64, 17–27. [Google Scholar] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000; ISBN 0890420254. [Google Scholar]
- Algase, D.L.; Beck, C.; Kolanowski, A.; Whall, A.; Berent, S.; Richards, C.; Beattie, E. Need-driven dementia-compromised behavior: An alternative view of disruptive behavior. Am. J. Alzheimer’s Dis. Other Dement. 1996, 11, 10–19. [Google Scholar] [CrossRef]
- Kovach, C.R.; Noonan, P.E.; Schlidt, A.M.; Wells, T. A model of consequences of need-driven, dementia-compromised behavior. J. Nurs. Scholarsh. 2005, 37, 134–140. [Google Scholar] [CrossRef] [PubMed]
- PIECES.TM Learning and Development Model. Supporting Relationships for Changing Health and Health Care. Available online: https://pieceslearning.com/model/ (accessed on 15 September 2021).
- Advanced Gerontological Education. Gentle Persuasive Approaches. (GPA). GPA Education is for Everyone Who Works with Older Adults. Available online: https://ageinc.ca/about-gpa-2/ (accessed on 23 March 2021).
- Speziale, J.; Black, E.; Coatworth-Puspoky, R.; Ross, T.; O’Regan, T. Moving forward: Evaluating a curriculum for managing responsive behaviors in a geriatric psychiatry inpatient population. Gerontologist 2009, 49, 570–576. [Google Scholar] [CrossRef]
- Lyketsos, G.; Oscar, L.; Beverly, J.; Fitzpatrick, A.L.; Breitner, J.; Dekosky, S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA 2002, 288, 1475–1483. [Google Scholar] [CrossRef]
- Luthra, A.S. The Meaning of Behaviors in Dementia/Neurocognitive Disorders: New Terminology, Classification, and Behavioral Management; Common Ground Publishing LLC: Vancouver, BC, Canada, 2014; ISBN 1612295320. [Google Scholar]
- Luthra, A.S. Reliability and Validity of a New Behavioral Scale to Measure Behavioral and Psychological Symptoms in Dementias (BPSD): Luthra’s Behavioral Assessment and Intervention Response (LuBAIR) Scale. Am. J. Geriatr. Psychiatry 2015, 23, 154. [Google Scholar] [CrossRef]
- The Classification of Behaviors in Persons with Dementia: The Meaning of Behavioral Expressions in Persons with Dementia. Available online: https://www.youtube.com/channel/UCSFOpEaa0x2l8X-0GZodHlA/featured (accessed on 25 July 2021).
- Luthra, A.S. The Meaning of Behavioral Expressions in Persons with Dementia. Available online: http://dementiabehaviors.com/ (accessed on 15 June 2021).
- Benjamin, D.J.; Berger, J.O.; Johannesson, M.; Nosek, B.A.; Wagenmakers, E.J.; Berk, R.; Bollen, K.A.; Brembs, B.; Brown, L.; Camerer, C.; et al. Redefine statistical significance. Nat. Hum. Behav. 2018, 2, 6–10. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Association: New York, NY, USA, 1988; ISBN 0-8058-0283-5. [Google Scholar]
- Ralph, S.J.; Espinet, A.J. Increased All-Cause Mortality by Antipsychotic Drugs: Updated Review and Meta-Analysis in Dementia and General Mental Health Care. J. Alzheimer’s Dis. Rep. 2018, 2, 1–26. [Google Scholar] [CrossRef]
- Atkinson, R.C.; Shiffrin, R.M. Human Memory: A proposed system and its Control Processes. Psychol. Learn. Motiv. 1968, 2, 89–195. [Google Scholar] [CrossRef]
- Ruths, S.; Straand, J.; Nygaard, H.A.; Aarsland, D. Stopping antipsychotic drug therapy in demented nursing home patients: A randomized, placebo-controlled study—The Bergen District Nursing Home Study (BEDNURS). Int. J. Geriatr. Psychiatry 2008, 23, 889–895. [Google Scholar] [CrossRef]
- Siegel, D.J. Memory: An overview, with emphasis on developmental interpersonal and neurobiological aspects. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Nevid, J. Psychology: Concepts and Applications, 3rd ed.; Wadsworth Cengage Learning: Belmont, NY, USA, 2009; ISBN 0547148143. [Google Scholar]
- Anderson, R.C. The Notion of Schemata and the Educational Enterprise: General Discussion of the Conference; Lawrence Erlbaum: New Jersey, NY, USA, 1984; pp. 415–431. ISBN 9781315271644. [Google Scholar]
- Kietzman, M. Information Processing. In Perspectives in Psychological Experimentation: Towards the Year 2000; Sarris, V., Praducci, A., Eds.; Hillsdale Erlbaum: New Jersey, NY, USA, 1984; pp. 291–309. ISBN 0898592887. [Google Scholar]
- Kietzman, M. Perception, cognition, and information processing. In Comprehensive Textbook of Psychiatry, 4th ed.; Kaplin, H.I., Sadock, B., Eds.; Williams and Wilkins: Baltimore, UK, 1985; pp. 157–158. ISBN 0683045105. [Google Scholar]
- Bridges-Parlet, S.; Knopman, D.; Steffes, S. Withdrawal of neuroleptic medications from institutionalized dementia patients: Results of a double blind, baseline-treatment controlled pilot study. J. Geriatr. Psychiatry Neurol. 1997, 10, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.J. Theory of Affordances. In Perceiving Acting, and Knowing: Toward an Ecological Psychology; Shaw, R., Bransford, J., Eds.; Lawrence Erlbaum: Hillsdale, NJ, USA, 1979; pp. 67–82. ISBN 0898599598. [Google Scholar]
- Maslow, A.H. A theory of human motivation. Psychol. Rev. 1943, 50, 370–396. [Google Scholar] [CrossRef]
- Bergh, S.; Selbæk, G.; Engedal, K. Dementia antipsychotics and antidepressants discontinuation study (DESEP) [Discontinuation of antipsychotics and antidepressants among patients with dementia and BPSD living in nursing homes—A 24 weeks double blind RCT]. U/S Natl. Libr. Med. 2011, 344, 1566. [Google Scholar]
- Perry, W.; McIlwain, M.; Kloezeman, K.; Henry, B.L.; Minassian, A. Diagnosis and characterization of mania: Quantifying increased energy and activity in the human behavioral pattern monitor. Psychiatry Res. 2016, 30, 278–283. [Google Scholar] [CrossRef][Green Version]
- Hanwella, R.; de Silva, V.A. Signs and symptoms of acute mania: A factor analysis. BMC Psychiatry 2011, 11, 137. [Google Scholar] [CrossRef]
- Johnson, S.L.; Sandrow, D.; Meyer, B.; Winters, R.; Miller, I.; Solomon, D.; Keitner, G. Increases in Manic Symptoms After Life Events Involving Goal Attainment. J. Abnorm. Psychol. 2000, 109, 721–727. [Google Scholar] [CrossRef]
- Bailey, M.R.; Williamson, C.; Mezias, C.; Winiger, V.; Silver, R.; Balsam, P.D.; Simpson, E.H. The effects of pharmacological modulation of the serotonin 2C receptor on goal-directed behavior in mice. Psychopharmacology 2015, 233, 615–624. [Google Scholar] [CrossRef]
- Conn, K.A.; Burne, T.H.; Kesby, J.P. Subcortical Dopamine and Cognition in Schizophrenia: Looking Beyond Psychosis in Preclinical Models. Front. Neurosci. 2020, 14, 542. [Google Scholar] [CrossRef]
- Sabic, D.; Sabic, A.; Bacic-Becirovic, A. Major depressive disorder and difference between genders. Mater. Socio-Med. 2021, 33, 105–108. [Google Scholar] [CrossRef]
- Sharma, A.; McClellan, J. Emotional and Behavioral Dysregulation in Severe Mental Illness. Child Adolesc. Psychiatr. Clin. N. Am. 2021, 30, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, W.; Walczak-Kozłowska, T.; Walczak, E.; Bikun, B.D. Nursing care in bipolar disorder—Case study. Pomeranian J. Life Sci. 2021, 67, 54–61. [Google Scholar] [CrossRef]
- Myrbakk, E.; von Tetzchner, S. Psychiatric disorders and behavior problems in people with intellectual disability. Res. Dev. Disabil. 2008, 29, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.B.; Brotchie, H.; Graham, R.K. Vitamin D and depression. J. Affect. Disord. 2017, 208, 56–61. [Google Scholar] [CrossRef]
- Hoffart, A.; Johnson, S.U.; Ebrahimi, O.V. The network of stress-related states and depression and anxiety symptoms during the COVID-19 lockdown. J. Affect. Disord. 2021, 294, 671–678. [Google Scholar] [CrossRef]
- Everitt, N.; Broadbent, J.; Richardson, B.; Smyth, J.M.; Heron, K.; Teague, S.; Fuller-Tyszkiewicz, M. nExploring the features of an app-based just-in-time intervention for depression. J. Affect. Disord. 2021, 291, 279–287. [Google Scholar] [CrossRef]
- Szymkowicz, S.M.; Jones, J.D.; Timblin, H.; Ryczek, C.; Taylor, W.D.; May, P.E. Apathy as a Within-Person Mediator of Depressive Symptoms and Cognition in Parkinson’s disease: Longitudinal Mediation Analyses. Am. J. Geriatr. Psychiatry 2021, 21, 1064–7481. [Google Scholar] [CrossRef]
- Seetharaman, A.; Chauhan, V.S.; Adhvaryu, A.; Prakash, J. Lorazepam challenge test: A unique clinical response in catatonia. Ind. Psychiatry J. 2021, 30, 235–236. [Google Scholar] [CrossRef]
- van Rooij, B.; Sokol, D.D. The Cambridge Handbook Compliance; Cambridge University Press: Cambridge, UK, 2021; ISBN 9781108759458. [Google Scholar]
- Ye, X.; Nair, R.; Durrett, C. Connecting Attributions and QA Model Behavior on Realistic Counterfactuals; Association for Computational Linguistics: Punta Cana, Dominican Republic, 2021; pp. 5496–5512. [Google Scholar]
- Greenwood, P.M. Functional plasticity in cognitive aging: Review and hypothesis. Neuropsychology 2007, 21, 657–673. [Google Scholar] [CrossRef]
- Gao, R.L.; Lim, K.S.; Luthra, A.S. Discontinuation of antipsychotics treatment for elderly patients within a specialized behavioural unit: A retrospective review. Int. J. Clin. Pharm. 2021, 43, 212–219. [Google Scholar] [CrossRef]
| Impairment in Regulation of Sensorium (Based in Information Processing Theories) | Impairment in Emotional Regulatory Circuits (Based in Theories on Regulation of Emotions) |
|---|---|
| Disorganized Expressions (DOE) | Vocal Expressions (VE) |
| Appearing “vacant” or “lost” in facial expressions Disorganized thinking, unintelligible or gabled speech Rapid shifts in, or incongruence of, emotional states Inappropriate mixing of food or dressing, layering of clothes, smearing fecal matter, playing in the toilet bowl, etc. Playing with “things” in the air, responding to auditory hallucination & picking “things” from the body or furniture. Mental or physical lethargy , or general functional decline | Verbally responsive (brief explosive burst, argumentative, quarrelsome) Talking loud and fast, manic-like behavior Yelling and screaming to get things done Rattling bed rails/table tops, persistently calling out for staff/family member Uttering noises or making repetitive sounds |
| Emotional Expressions (EE) | |
| Appearing sad, tearful or irritable Expressing themes of despair, morbidity, somatic complaints or self-deprecating comments Mimicking/mocking or being dismissive Sarcastic or teasing, being derogatory, critical and negative of others Expressing feelings or rejection or increased sensitivity to others comments | |
| Impairment in Information Processing Pathways (Based in Information Processing Theories) | Fretful-Trepidated Expressions (FE) |
| Expressing worry, fear, forbidding, or catastrophe | |
| Mis-Identification Expressions (MiE) | Fearful or scared facial expressions |
| Misidentification of persons, places, objects Misidentification of sounds, smells, tastes, or touch Misidentification of events, or occurrences Misperception or misinterpretation of comments or behaviors of others | Anxious or distressed facial expressions Clingy or “latching on”, wringing of hands, rubbing face/body Hoarding or collecting |
| Impairment in Motivational circuits (Based on Motivational Theories) | Impairment in Self-Monitoring and Regulatory Circuits. (Based in Theories on Regulation of Social Behaviors) |
| Goal-Directed Expressions (GDE) | Oppositional Expressions (OE) |
| Goal-directed thinking: I am going home to my kids/to the bank; I am getting married to day; where can I pay my bills Goal-directed activities: rummaging, hoarding, empting drawers etc. stripping of clothes, rearranging furniture or fixing items in melleu bed/chair exiting or exit seeking; intrusiveness or purposeful wandering | Negotiating around care and other needs Working against the care provider (pulling pants up during incontinent care, buttoning up shirt when needing to undress) Evasive to directions from care provider (ignoring etc.) Resistive to care, medications, or meals (pursing lips at meals, clamping/crossing legs, closed, tightening arms, resisting rolling/turning etc.) Barricading and territorialism of space or belongings |
| Physically Responsive Expressions (PE) | |
| Self-abusive Pulling, pushing, grabbing Kicking, biting, scratching, punching, twisting, head-butting, spitting at someone Throwing things, breaking objects, tipping furniture | |
| Apathy Expressions (AE) | |
| Indifference and/or lack of concern re-self and environment Lack of self-initiation, low social engagement (interpersonal interactions and milieu structure, poor resistance Emotional indifference and/or lack of emotional remorse | |
| Importuning Expressions (IE) | |
| Persistently seeking reassurance or asking for assistance Behaving in ways for demands to be met immediately (repeatedly asking to be toileted or for medications, etc.) Shadowing staff (following closely, crowding staff member’s personal space) Attention seeking or “manipulative” behaviors (repeatedly empting soiled line cart, throwing food on the floor, etc.) | Sexual Expressions (SE) |
| Verbally sexual (comments, gestures, innuendos) Physically sexual (grabbing breasts, buttocks) Self-stimulation | |
| Motor expressions (ME) | |
| Roaming, pacing, wandering Fidgety, pocking in chair, restless, agitated Seemingly driven, “on the go”, wheelchair propelling, chair/bed exiting |
| Patient Number | Age | Date | Behavioural Categories | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disorganized Expressions | Mis-Identification Expressions | Goal-Directed Express-ions | Vocal Expressions | Emotional Expressions | Fretful-Trepidated Expressions | Importuning Expressions | Apathy Expressions | Oppositional Expressions | Physical Expressions | Sexual Expressions | Motor Expressions | |||
| 001S | Age: 76M | 31/12/2018 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 8/1/2019 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 002S | Age: 96M | 27/06/2019 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| 15/09/2019 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 003S | Age: 87F | 20/06/2018 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| 12/8/2018 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 004S | Age: 70M | 5/7/2019 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| 11/10/2019 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 005S | Age: 81 M | 23/03/2019 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| 1/6/2019 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 006S | Age: 76M | 19/10/2018 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| 22/05/2019 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 007S | Age: 81F | 8/8/2018 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 18/11/2019 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | ||
| 008S | Age: 81M | 4/5/2018 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 29/09/2020 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 009S | Age: 83M | 11/10/2018 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 9/8/2019 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | ||
| 010S | Age: 73F | 27/04/2018 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 3/8/2018 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 011S | Age: 87M | 13/02/2019 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 5/6/2019 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 012S | Age: 81M | 16/11/2018 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 6/5/2019 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 013S | Age: 84M | 21/11/2018 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 30/03/2019 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 014S | Age: 75M | 5/6/2019 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
| 3/2/2020 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 015S | Age: 82M | 7/12/2019 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| 2/2/2020 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 016S | Age: 88M | 23/08/2017 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 9/12/2017 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| 017S | Age: 91F | 21/03/2021 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 9/5/2021 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ||
| Behavioural Categories | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Number | Age | Date | Disorganized Expressions | Mis-Identification Expressions | Goal-Directed Expressions | Vocal Expressions | Emotional Expressions | Fretful-Trepida-ted Expressions | Importuning Expressions | Apathy Expressions | Oppositional Expressions | Physical Expressions | Sexual Expressions | Motor Expressions |
| 001F | Age: 62 F | 23/07/2017 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| 14/05/2016 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 002F | Age: 80 M | 21/01/2019 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| 28/02/2019 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 003F | Age: 75M | 2/12/2018 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 |
| 25/09/2018 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 004F | Age: 84M | 5/4/2019 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| 17/04/2019 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 005F | Age: 81 F | 23/01/2019 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 |
| 5/3/2019 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | ||
| 006F | Age: 77M | 27/02/2019 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 3/7/2019 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ||
| 007F | Age: 98F | 29/03/2019 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| 4/5/2019 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | ||
| 008F | Age: 88 M | 19/11/2018 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| 30/07/2020 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | ||
| 009F | Age: 60 F | 25/11/2015 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| 14/02/2016 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 010F | Age: 74M | 5/2/2016 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| 5/5/2016 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 011F | Age: 72M | 11/12/2018 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| 9/4/2019 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 012F | Age: 79F | 28/05/2016 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 31/08/2016 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 013F | Age: 87M | 18/12/2017 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 23/01/2018 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 014F | Age: 87F | 14/11/2018 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| 21/11/2018 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 015F | Age: 88 M | 17/01/2017 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
| 21/07/2017 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 016F | Age: 77M | 20/12/2018 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
| 14/03/2019 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 017F | Age: 92F | 25/04/2018 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 |
| 25/07/2018 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 018F | Age: 88F | 21/11/2017 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| 18/02/2018 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | ||
| 019F | Age: 76F | 5/1/2019 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 3/10/2019 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 020F | Age: 81M | 25/11/2019 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| 23/01/2020 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | ||
| 021F | Age: 83F | 23/01/2020 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| 9/2/2020 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 022F | Age: 92M | 8/3/2020 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| 27/10/2020 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| 023F | Age: 76M | 26/05/2020 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| 30/07/2020 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | ||
| Variables | Assessment #1 | Assessment #2 | ||
|---|---|---|---|---|
| Behavioural Category | Disorganized Expressions | Present | 0 | 0 |
| Not Present | 40 | 40 | ||
| Mis-Identification Expressions | Present | 1 | 21 | |
| Not Present | 39 | 19 | ||
| Goal-Directed Expressions | Present | 1 | 22 | |
| Not Present | 39 | 18 | ||
| Vocal Expressions | Present | 24 | 35 | |
| Not Present | 16 | 5 | ||
| Emotional Expressions | Present | 40 | 38 | |
| Not Present | 0 | 2 | ||
| Fretful-Trepidated Expressions | Present | 16 | 14 | |
| Not Present | 24 | 26 | ||
| Importuning Expressions | Present | 14 | 18 | |
| Not Present | 26 | 22 | ||
| Apathy Expressions | Present | 2 | 1 | |
| Not Present | 38 | 39 | ||
| Oppositional Expressions | Present | 39 | 40 | |
| Not Present | 4 | 0 | ||
| Physical Expressions | Present | 36 | 37 | |
| Not Present | 4 | 3 | ||
| Sexual Expressions | Present | 4 | 2 | |
| Not Present | 36 | 38 | ||
| Motor Expression | Present | 33 | 37 | |
| Not Present | 7 | 3 |
| Independent Variables | Dependent Variables | F Value | p |
|---|---|---|---|
| Disorganized Expression | Time | - | - |
| De-Prescription | |||
| Mis Identification Expression | Time | 136.56 | <0.0001 ** |
| De-Prescription | 100.94 | <0.0001 ** | |
| Goal-Directed Expression | Time | 219.00 | <0.0001 ** |
| De-Prescription | 161.87 | <0.0001 ** | |
| Vocal Expression | Time | 8.48 | 0.0047 * |
| De-Prescription | 2.05 | 0.1560 | |
| Emotional Expression | Time | - | - |
| De-Prescription | |||
| Fretful Trepidated Expression | Time | - | - |
| De-Prescription | |||
| Importuning Expression | Time | - | - |
| De-Prescription | |||
| Apathy Expression | Time | - | - |
| De-Prescription | |||
| Oppositional Expression | Time | - | - |
| De-Prescription | |||
| Physical Expression | Time | - | - |
| De-Prescription | |||
| Sexual Expression | Time | - | - |
| De-Prescription | |||
| Motor Expression | Time | 1.94 | 0.1680 |
| De-Prescription | 6.78 | 0.01107 * |
| Variables | Successful% (N) | Failed% (N) | x2 (p) | ||
|---|---|---|---|---|---|
| Behavioral Category | Disorganized Expressions | Present | 0.0 (0) | 0.0 (0) | - |
| Not Present | 100 (17) | 100 (23) | |||
| Mis-Identification Expressions | Present | 0.0 (0) | 91.30 (21) | 29.119 (p < 0.0001) | |
| Not Present | 100 (17) | 8.70(2) | |||
| Goal-Directed Expressions | Present | 0.0 (0) | 95.62 (22) | 32.374 (p < 0.0001) | |
| Not Present | 100 (17) | 4.348 (1) | |||
| Vocal Expressions | Present | 76.47 (13) | 95.62 (22) | 1.7684 (p = 0.184) | |
| Not Present | 23.53 (4) | 4.348 (1) | |||
| Emotional Expressions | Present | 100 (0)) | 91.30 (21) | 0.2638 (p = 0.6075) | |
| Not Present | 0 (17) | 8.70 (2) | |||
| Fretful-Trepidated Expressions | Present | 35.39 (6) | 34.78 (8) | 0.000 (p = 1) | |
| Not Present | 64.71 (11) | 65.22 (15) | |||
| Importuning Expressions | Present | 41.18 (7) | 47.88 (11) | 0.0093 (p = 0.0237) | |
| Not Present | 58.82 (10) | 52.17 (12) | |||
| Apathy Expressions | Present | 0.0 (0) | 4.348 (1) | 0.000 (p = 1) | |
| Not Present | 100 (17) | 95.62 (22) | |||
| Oppositional Expressions | Present | 100 (17) | 100(23) | - | |
| Not Present | 0 (0) | 0.0 (0) | |||
| Physical Expressions | Present | 88.24 (15) | 95.62 (22) | 0.0746 (p = 0.7847) | |
| Not Present | 11.76 (2) | 4.348 (1) | |||
| Sexual Expressions | Present | 5.88 (1) | 4.348 (1) | 0.000 (p = 1) | |
| Not Present | 94.12 (16) | 95.62 (22) | |||
| Motor Expression | Present | 82.35 (14) | 100 (23) | 2.2129 (p = 0.1369) | |
| Not Present | 17.65 (3) | 0.0 (0) | |||
| n Behavior Present | Mean | Mean of the Differences | t | df | p | Cohen’s d | ||
|---|---|---|---|---|---|---|---|---|
| Disorganized Expressions | First Assessment | 0 | 0 | - | 39 | - | - | |
| Last Assessment | 0 | |||||||
| Mis-Identification Expressions | First Assessment | 1 | 0.025 | −0.5 | −6.245 | 39 | <0.0001 | −0.9874 |
| Last Assessment | 21 | 0.525 | ||||||
| Goal-Directed Expressions | First Assessment | 1 | 0.025 | −0.525 | −6.566 | 39 | <0.0001 | −1.0381 |
| Last Assessment | 22 | 0.55 | ||||||
| Vocal Expressions | First Assessment | 24 | 0.6 | −0.275 | −3.846 | 39 | 0.0004 | −0.6081 |
| Last Assessment | 35 | 0.875 | ||||||
| Emotional Expressions | First Assessment | 40 | 1 | 0.05 | 1.4327 | 39 | 0.1599 | 0.2265 |
| Last Assessment | 38 | 0.95 | ||||||
| Fretful-Trepidated Expressions | First Assessment | 16 | 0.4 | 0.05 | 0.7026 | 39 | 0.4865 | 0.1111 |
| Last Assessment | 14 | 0.35 | ||||||
| Importuning Expressions | First Assessment | 14 | 0.4 | −0.05 | −0.703 | 39 | 0.4865 | −0.1111 |
| Last Assessment | 18 | 0.45 | ||||||
| Apathy Expressions | First Assessment | 2 | 0.05 | 0.025 | 1 | 39 | 0.3235 | 0.1581 |
| Last Assessment | 1 | 0.025 | ||||||
| Oppositional Expressions | First Assessment | 39 | 0.975 | −0.025 | −1 | 39 | 0.3235 | −0.1581 |
| Last Assessment | 40 | 1 | ||||||
| Physical Expressions | First Assessment | 36 | 0.9 | −0.025 | −0.573 | 39 | 0.5703 | −0.0905 |
| Last Assessment | 37 | 0.925 | ||||||
| Sexual Expressions | First Assessment | 4 | 0.1 | 0.05 | 1.4327 | 39 | 0.1599 | 0.2265 |
| Last Assessment | 2 | 0.05 | ||||||
| Motor Expression | First Assessment | 33 | 0.825 | −0.1 | −1.275 | 39 | 0.2099 | −0.2016 |
| Last Assessment | 37 | 0.925 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luthra, A.S.; Gao, R.L.; Remers, S.; Carducci, P.; Sue, J. Evidence-Informed Approach to De-Prescribing of Atypical Antipsychotics (AAP) in the Management of Behavioral Expressions (BE) in Advanced Neurocognitive Disorders (NCD): Results of a Retrospective Study. Geriatrics 2022, 7, 14. https://doi.org/10.3390/geriatrics7010014
Luthra AS, Gao RL, Remers S, Carducci P, Sue J. Evidence-Informed Approach to De-Prescribing of Atypical Antipsychotics (AAP) in the Management of Behavioral Expressions (BE) in Advanced Neurocognitive Disorders (NCD): Results of a Retrospective Study. Geriatrics. 2022; 7(1):14. https://doi.org/10.3390/geriatrics7010014
Chicago/Turabian StyleLuthra, Atul Sunny, Raymond LinBin Gao, Shannon Remers, Peter Carducci, and Joanna Sue. 2022. "Evidence-Informed Approach to De-Prescribing of Atypical Antipsychotics (AAP) in the Management of Behavioral Expressions (BE) in Advanced Neurocognitive Disorders (NCD): Results of a Retrospective Study" Geriatrics 7, no. 1: 14. https://doi.org/10.3390/geriatrics7010014
APA StyleLuthra, A. S., Gao, R. L., Remers, S., Carducci, P., & Sue, J. (2022). Evidence-Informed Approach to De-Prescribing of Atypical Antipsychotics (AAP) in the Management of Behavioral Expressions (BE) in Advanced Neurocognitive Disorders (NCD): Results of a Retrospective Study. Geriatrics, 7(1), 14. https://doi.org/10.3390/geriatrics7010014






