A Quality Improvement Initiative to Reduce Postoperative Delirium among Cardiac Surgery Patients
Abstract
:1. Introduction
2. Methods
2.1. Context for the QI Project
2.2. Use of an Established QI Model
2.3. Applying the QI Model to Reduce Postoperative Delirium among the Cardiac Surgery Patients
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| POD | Postoperative Delirium |
| rPOD | Reduction of Postoperative Delirium |
| CPAC | Cardiac Pre-Admission Clinic |
| CSIU | Cardiac Surgery Inpatient Unit |
| ICCS | Intensive Care Cardiac Surgery |
References
- Salluh, J.I.; Soares, M.; Teles, J.M.; Ceraso, D.; Raimondi, N.; Nava, V.S.; Blasquez, P.; Ugarte, S.; Ibanez-Guzman, C.; Centeno, J.V.; et al. Delirium epidemiology in critical care (DECCA): An international study. Crit. Care 2010, 14, R210. [Google Scholar] [CrossRef] [Green Version]
- Whitlock, E.L.; Vannucci, A.; Avidan, M.S. Postoperative delirium. Minerva Anestesiol. 2011, 77, 448–456. [Google Scholar] [PubMed]
- Rudolph, J.L.; Inouye, S.K.; Jones, R.; Yang, F.M.; Fong, T.G.; Levkoff, S.E.; Marcantonio, E.R. Marcantonio ER. Delirium: An Independent Predictor of Functional Decline After Cardiac Surgery. J. Am. Geriatr. Soc. 2010, 58, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.H. Delirium in the Cardiac Surgical Intensive Care Unit. Curr. Opin. Anaesthesiol. 2014, 27, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, E.R.; Goldman, L.; Mangione, C.M.; Ludwig, L.E.; Muraca, B.; Haslauer, C.M.; Donaldson, M.C.; Whittemore, A.D.; Sugarbaker, D.J.; Poss, R.; et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA 1994, 271, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.L.; Jones, R.; Rasmussen, L.S.; Silverstein, J.H.; Inouye, S.K.; Marcantonio, E.R. Independent vascular and cognitive risk factors for postoperative delirium. Am. J. Med. 2007, 120, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, E.R.; Flacker, J.M.; Michaels, M.; Resnick, N.M. Delirium is independently associated with poor functional recovery after hip fracture. J. Am. Geriatr. Soc. 2000, 48, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Franco, K.; Litaker, D.; Locala, J.; Bronson, D. The cost of delirium in the surgical patient. Psychosomatics 2001, 42, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.C.; Djaiani, G.; Rudolph, J.L. Detection, Prevention, and Management of Delirium in the Critically Ill Cardiac Patient and Patients Who Undergo Cardiac Procedures. Can. J. Cardiol. 2017, 33, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Boustani, M.; Baker, M.S.; Campbell, N.; Munger, S.; Hui, S.L.; Castelluccio, P.; Farber, M.; Guzman, O.; Ademuyiwa, A.; Miller, D.; et al. Impact and recognition of cognitive impairment among hospitalized elders. J. Hosp. Med. Off. Publ. Soc. Hosp. Med. 2010, 5, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pronovost, P.J.; Berenholtz, S.M.; Needham, D.M. Translating Evidence into Practice: A Model for Large Scale Knowledge Translation. BMJ 2012, 337, 963–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ely, E.W.; Margolin, R.; Francis, J.; May, L.; Truman, B.; Dittus, R.; Speroff, T.; Gautam, S.; Bernard, G.R.; Inouye, S.K. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit. Care Med. 2001, 29, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Gélinas, C.; Fillion, L.; Puntillo, K.A.; Viens, C.; Fortier, M. Validation of the critical-care pain observation tool in adult patients. Am. J. Crit. Care 2006, 15, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.W.; Truman, B.; Shintani, A.; Thomason, J.W.W.; Wheeler, A.P.; Gordon, S.; Francis, J.; Speroff, T.; Gautam, S.; Margolin, R.; et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 2003, 289, 2983–2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ely, E.W.; Inouye, S.K.; Bernard, G.R.; Gordon, S.; Francis, J.; May, L.; Truman, B.; Speroff, T.; Gautam, S.; Margolin, R.; et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001, 286, 2703–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
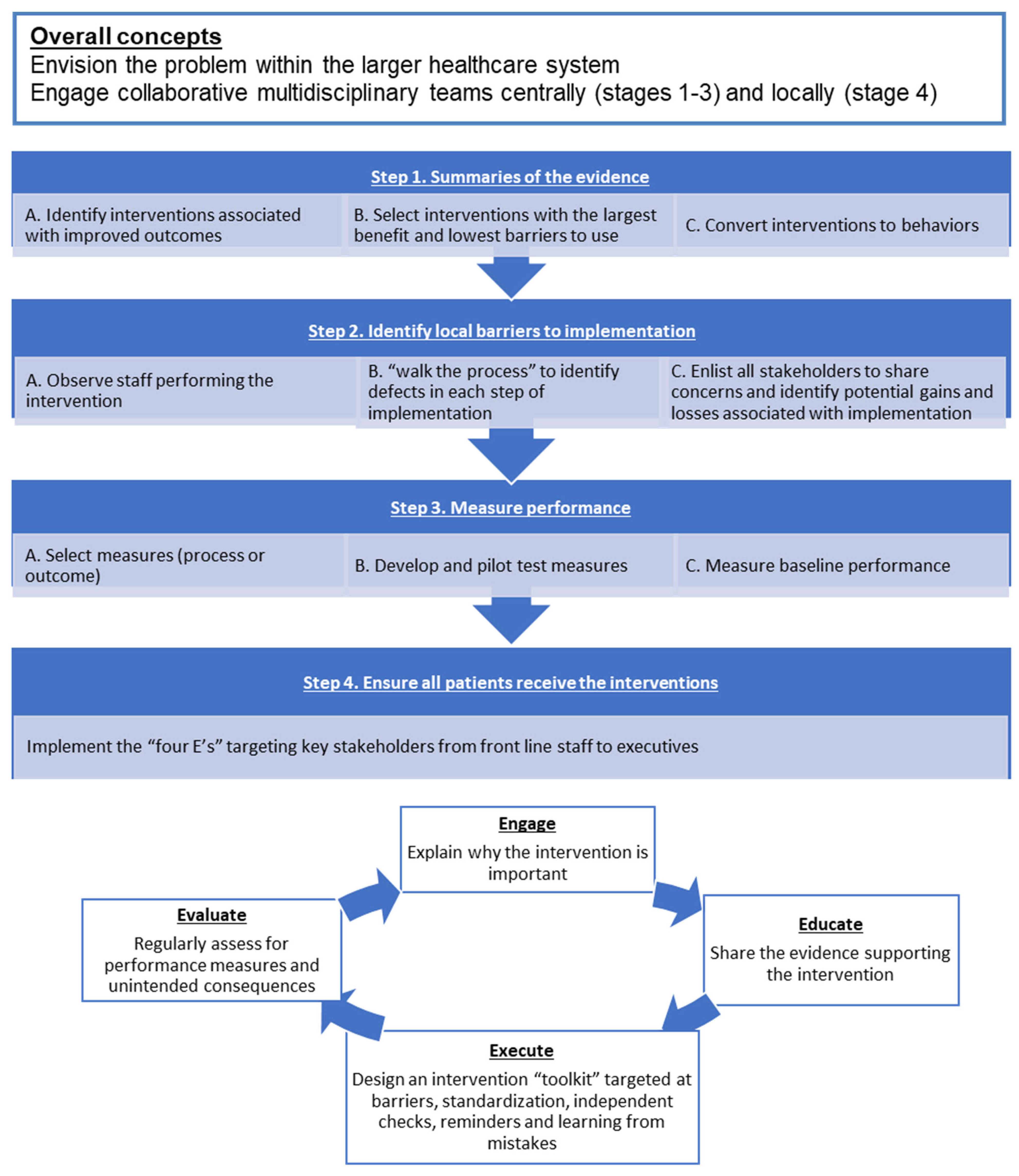
| Step 1: Summarizing Evidence to Identify Potentially Beneficial Interventions | Step 2: Identifying Local Barriers to Implementation | Step 3: Selecting and Developing Performance Measures | Step 4: Ensuring All Patients Receive the Interventions |
|---|---|---|---|
|
|
| E-Engage E-Educate E-Execute E-Evaluate |
| Preoperative Intervention (CPAC, CSIU, 5A)—Preoperative Assessment Package | Operative Intervention | Postoperative Intervention—ICCS |
|---|---|---|
|
|
|
| Nurse education package and Yearly self-learning module | ||
| A Positive CAM Indicates Delirium, a Medical Emergency and Should Be Translated to Following Action | |
|---|---|
| Investigations | Interventions |
|
|
| Barriers | Strategy to Overcome Barriers | |
|---|---|---|
| 1 | Lack of leadership |
|
| 2 | Lack of delirium-related knowledge and training among nursing staff |
|
| 3 | Lack of preoperative baseline risk assessment |
|
| 4 | Over sedation |
|
| 5 | Delirium screening |
|
| 6 | Perceived pain and discomfort screening |
|
| 7 | Early mobilization |
|
| 8 | Interventions aimed at preventing delirium |
|
| 9 | Lack of patient and caregiver engagement |
|
| 10 | Lack of communication with the community physician (family physician) |
|
| Measure (S) | Mode of Assessment |
|---|---|
| Process Measures (Intervention adherence) | |
| Intervention adherence | Rate of completion of baseline risk assessment |
| Rate of completion of delirium assessment | |
| Outcome Measures | |
| Primary outcome | Rates of delirium screening |
| Quality indicators | Number of positive CAM screens in clinical database for patients screened with CAM |
| Number of patients restrained in ICU, Wards | |
| Hospital LOS, ICU LOS | |
| All-cause in hospital mortality | |
| Major adverse cardiac events | |
| Rate of sternal wound infection | |
| Patient Characteristic | Pre-rPOD Intervention (2009–2011) | During-rPOD Intervention (2012–2015) | Post-rPOD Intervention (2016–2018) | p-Value |
|---|---|---|---|---|
| Age | 66 (58–74) | 67 (58–74) | 68 (59–75) | <0.001 |
| Sex (Female) | 28.2% | 27.9% | 27.8% | 0.942 |
| Type of Cardiac Surgery | ||||
| CABG | 60.9% | 46.9% | 45.6% | <0.001 |
| Valve | 14.8% | 20.2% | 23.0% | <0.001 |
| CABG + Valve | 11.2% | 11.3% | 11.1% | 0.969 |
| Other | 13.1% | 21.6% | 20.3% | <0.001 |
| MoCA Score | - | 26 (23–28) | 26 (23–28) | 0.479 |
| CFS (Nursing Assessment) | - | 3 (2–4) | 4 (3–4) | <0.001 |
| Patient Health Questionnaire (Version 9) | - | 1 (0–3) | 2 (0–6) | <0.001 |
| 7.1. Process Measures | During-rPOD Implementation (2012–2016) | Post-rPOD Implementation (2016–2018) | p-Value |
|---|---|---|---|
| 7.1.1 Baseline risk assessment | |||
| MoCA completion rate | 30.0% | 46.6% | <0.001 |
| CFS completion rate | 30.7% | 49.2% | <0.001 |
| 7.1.2 Delirium assessment | |||
| Any CAM Assessment Recorded | 97.3% | 98.5% | 0.002 |
| Any RASS Assessment Recorded | 98.4% | 99.2% | 0.006 |
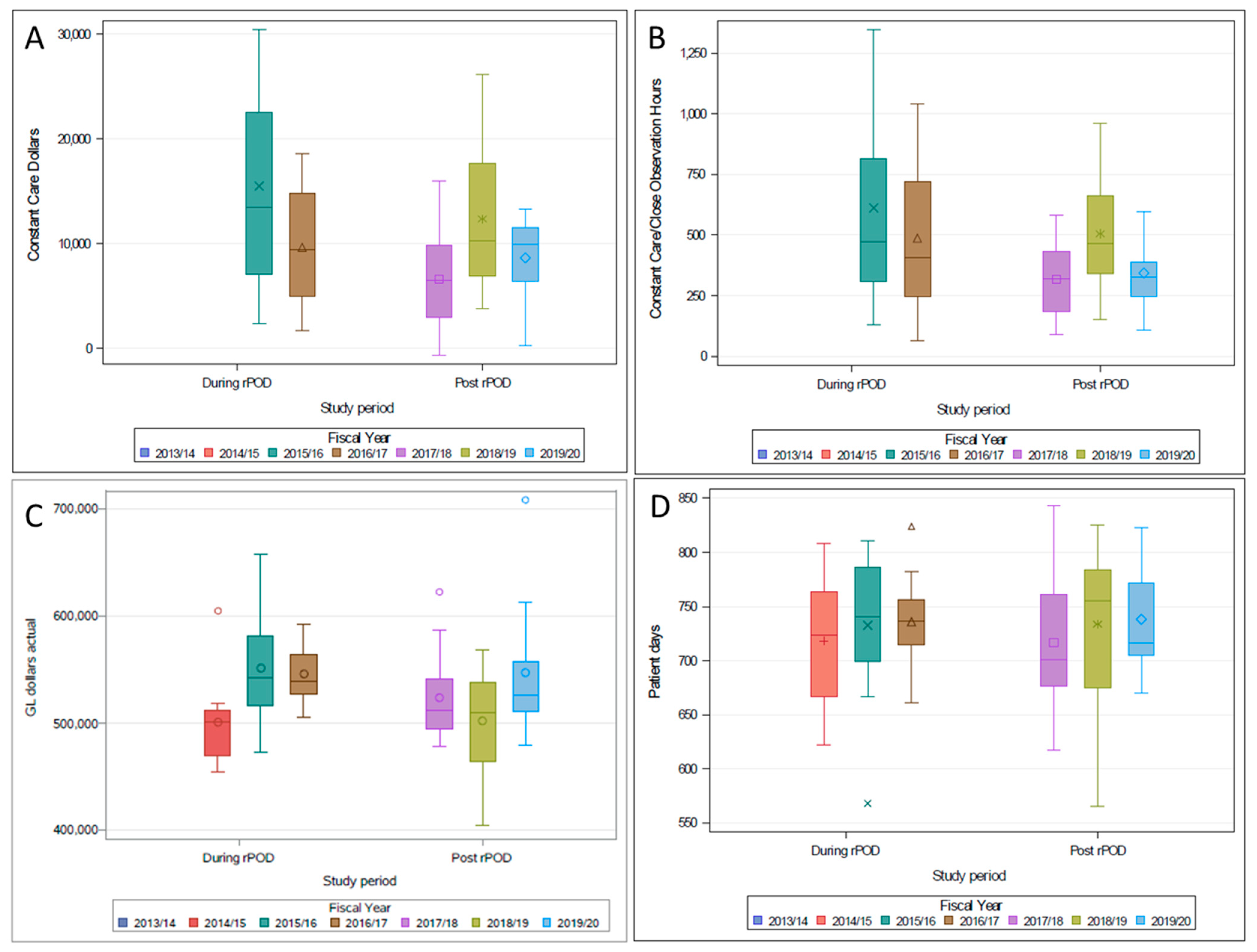
| 7.2. Quality Improvement Measures | Pre-rPOD Intervention (2009–2011) | During-rPOD Intervention (201–2016) | Post-rPOD Intervention (2016–2018) | p-Value |
|---|---|---|---|---|
| 7.2.1 Primary outcome | ||||
| Postoperative delirium screening rates | 9.0% | 23.3% | 19.1% | <0.001 |
| 7.2.2 Quality indicators | ||||
| Number of Positive CAM screens in clinical database for patients screened with CAM | 2 (1–4) | 2 (1–5) | 3 (1–6) | <0.001 |
| Number of patients restrained—ICU | - | 3.0% | 1.2% | <0.001 |
| Number of patients restrained—Ward | - | 0.4% | 0.4% | 0.955 |
| Length of ICU stay for patients screened with delirium (Hours) | 79 (43–161) | 90 (42–165) | 74 (41–147) | 0.329 |
| Length of Hospital Stay (Surgery to Discharge) for patients screened with delirium (Days) | 12 (7–22) | 13 (8–23) | 12 (8–23) | 0.282 |
| Major Adverse Cardiac Events (MI, Stroke, Dialysis, In-Hospital Mortality) | 5.4% | 8.1% | 6.3% | <0.001 |
| Sternal Infection (Superficial or Deep) | 0.2% | 1.3% | 1.3% | <0.001 |
| In-Hospital Mortality | 2.5% | 3.2% | 2.1% | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanjanwala, R.M.; Hiebert, B.; Kent, D.; Warren, S.; Grocott, H.; Arora, R.C. A Quality Improvement Initiative to Reduce Postoperative Delirium among Cardiac Surgery Patients. Geriatrics 2021, 6, 111. https://doi.org/10.3390/geriatrics6040111
Sanjanwala RM, Hiebert B, Kent D, Warren S, Grocott H, Arora RC. A Quality Improvement Initiative to Reduce Postoperative Delirium among Cardiac Surgery Patients. Geriatrics. 2021; 6(4):111. https://doi.org/10.3390/geriatrics6040111
Chicago/Turabian StyleSanjanwala, Rohan M., Brett Hiebert, David Kent, Sandy Warren, Hilary Grocott, and Rakesh C. Arora. 2021. "A Quality Improvement Initiative to Reduce Postoperative Delirium among Cardiac Surgery Patients" Geriatrics 6, no. 4: 111. https://doi.org/10.3390/geriatrics6040111
APA StyleSanjanwala, R. M., Hiebert, B., Kent, D., Warren, S., Grocott, H., & Arora, R. C. (2021). A Quality Improvement Initiative to Reduce Postoperative Delirium among Cardiac Surgery Patients. Geriatrics, 6(4), 111. https://doi.org/10.3390/geriatrics6040111






