The Association between Poor Diet Quality, Physical Fatigability and Physical Function in the Oldest-Old from the Geisinger Rural Aging Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Demographic and Health-Related Characteristics
2.3. Questionnaire Assessments
2.4. Twenty-Four-Hour Dietary Recalls
2.5. Statistical Analysis
3. Results
3.1. Demographic and Health-Related Characteristics in Individuals Reporting More vs. Less Perceived Physical Fatigability
3.2. Healthy Eating Index Data by Fatigability Status
3.3. Macro- and Micronutrient Intake Data by Fatigability Status
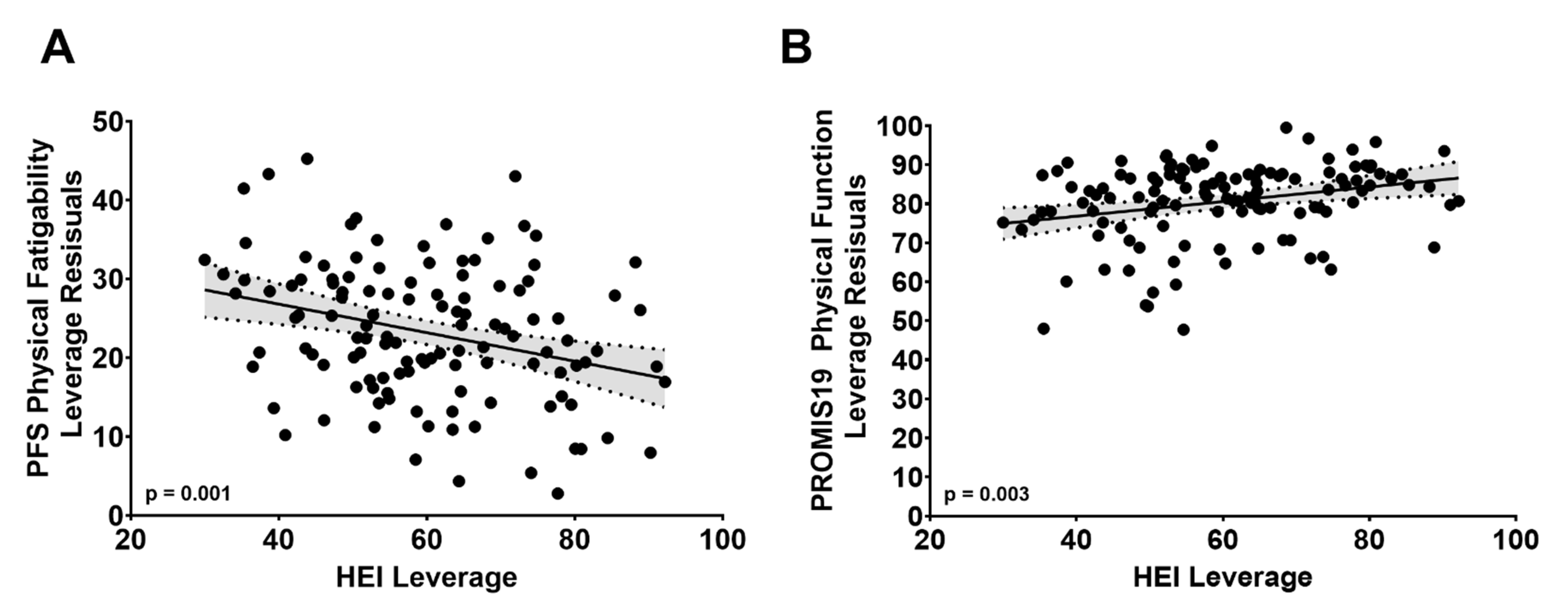
3.4. PFS Physical Score by HEI Total Score
3.5. PROMIS19 Physical Function Score by HEI Total Score
3.6. PFS Physical Score by PROMIS19 Physical Function Score
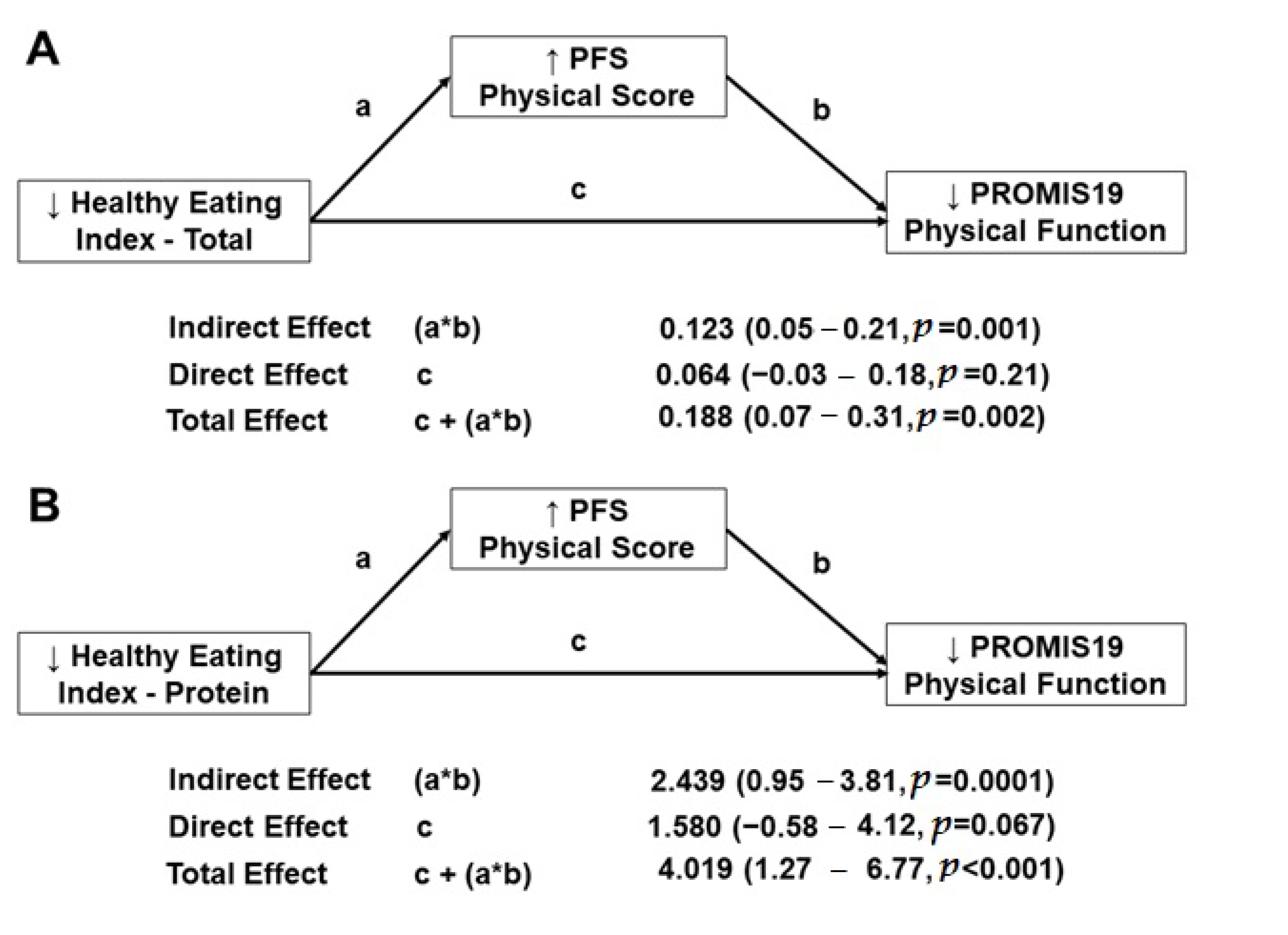
3.7. Mediation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Manini, T.M.; Clark, B.C. Dynapenia and aging: An update. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 28–40. [Google Scholar] [CrossRef] [PubMed]
- U.S. Census Bureau, Population Division. Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States: April 1, 2010 to July 1, 2019 (NC-EST2019-AGESEX). Released June 2020. Available online: https://www.census.gov/data/tables/time-series/demo/popest/2010s-national-detail.html#par_textimage_1537638156 (accessed on 13 April 2021).
- Negm, A.M.; Kennedy, C.C.; Pritchard, J.M.; Ioannidis, G.; Vastis, V.; Marr, S.; Patterson, C.; Misiaszek, B.; Woo, T.K.W.; Thabane, L.; et al. The short performance physical battery is associated with one-year emergency department visits and hospitalization. Can. J. Aging Rev. Can. Du Vieil. 2019, 38, 507–511. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Onder, G.; Zamboni, V.; Manini, T.; Shorr, R.I.; Russo, A.; Bernabei, R.; Pahor, M.; Landi, F. Physical function and self-rated health status as predictors of mortality: Results from longitudinal analysis in the Ilsirente Study. BMC Geriatr. 2008, 8, 34. [Google Scholar] [CrossRef]
- Mani, D.; Almuklass, A.M.; Hamilton, L.D.; Vieira, T.M.; Botter, A.; Enoka, R.M. Motor unit activity, force steadiness, and perceived fatigability are correlated with mobility in older adults. J. Neurophysiol. 2018, 120, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Simonsick, E.M.; Schrack, J.A.; Santanasto, A.J.; Studenski, S.A.; Ferrucci, L.; Glynn, N.W. Pittsburgh Fatigability Scale: One-page predictor of mobility decline in mobility-intact older adults. J. Am. Geriatr. Soc. 2018, 66, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.S.; Gmelin, T.; Renner, S.W.; Boudreau, R.M.; Martin, S.; Wojczynski, M.K.; Christensen, K.; Andersen, S.L.; Cosentino, S.; Santanasto, A.J.; et al. Evaluation of the bidirectional relations of perceived physical fatigability and physical activity on slower gait speed. J. Gerontol. A Biol. Sci. Med. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, M.; Vandewoude, M.; Perkisas, S.; Van Gossum, A. Undernutrition in community dwelling elderly. E-SPEN J. 2013, 8, e213–e215. [Google Scholar] [CrossRef]
- Vandewoude, M.F.J.; van Wijngaarden, J.P.; De Maesschalck, L.; Luiking, Y.C.; Van Gossum, A. The prevalence and health burden of malnutrition in belgian older people in the community or residing in nursing homes: Results of the Nutriaction ii Study. Aging Clin. Exp. Res. 2019, 31, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Brownie, S. Why are elderly individuals at risk of nutritional deficiency? Int. J. Nurs. Pr. 2006, 12, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Irving, B.A.; Robinson, M.M.; Nair, K.S. Age effect on myocellular remodeling: Response to exercise and nutrition in humans. Ageing Res. Rev. 2012, 11, 374–389. [Google Scholar] [CrossRef]
- Van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: A systematic review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11.e13. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, S.; Aspray, T.J.; Bauer, J.M.; Cederholm, T.; Hemsworth, J.; Hill, T.R.; McPhee, J.S.; Piasecki, M.; Seal, C.; Sieber, C.C.; et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.T.; Mossakowski, A.A.; Baar, K.; Alcazar, J.; Martin-Rincon, M.; Alegre, L.M.; Ara, I.; Calbet, J.A.L.; Hinkley, J.M.; Coen, P.M.; et al. Commentaries on viewpoint: Rejuvenation of the term sarcopenia. J. Appl. Physiol. 2019, 126, 257–262. [Google Scholar]
- Alley, D.E.; Koster, A.; Mackey, D.; Cawthon, P.; Ferrucci, L.; Simonsick, E.M.; Yu, B.; Hardy, S.; Goodpaster, B.; Sarkisian, C.; et al. Hospitalization and change in body composition and strength in a population-based cohort of older persons. J. Am. Geriatr. Soc. 2010, 58, 2085–2091. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The Health, Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, B.; Patel, K.V.; Fried, L.F.; Robinson-Cohen, C.; de Boer, I.H.; Harris, T.; Murphy, R.A.; Satterfield, S.; Goodpaster, B.H.; Shlipak, M.; et al. Association of muscle endurance, fatigability, and strength with functional limitation and mortality in the Health Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Martone, A.M.; Marzetti, E.; Calvani, R.; Picca, A.; Tosato, M.; Santoro, L.; Di Giorgio, A.; Nesci, A.; Sisto, A.; Santoliquido, A.; et al. Exercise and protein intake: A synergistic approach against sarcopenia. Biomed. Res. Int. 2017, 2017, 2672435. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.L.; Mirtallo, J.; Compher, C.; Dhaliwal, R.; Forbes, A.; Grijalba, R.F.; Hardy, G.; Kondrup, J.; Labadarios, D.; Nyulasi, I.; et al. Adult starvation and disease-related malnutrition: A proposal for etiology-based diagnosis in the clinical practice setting from the international consensus guideline committee. Clin. Nutr. 2010, 29, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Franz, K.; Otten, L.; Muller-Werdan, U.; Doehner, W.; Norman, K. Severe weight loss and its association with fatigue in old patients at discharge from a geriatric hospital. Nutrients 2019, 11, 2415. [Google Scholar] [CrossRef]
- Bollwein, J.; Diekmann, R.; Kaiser, M.J.; Bauer, J.M.; Uter, W.; Sieber, C.C.; Volkert, D. Dietary quality is related to frailty in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.L.; Kita, K.; Fish, J.; Heydt, D.; Frey, C. Nutrition risk screening characteristics of rural older persons: Relation to functional limitations and health care charges. Am. J. Clin. Nutr. 1997, 66, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Glynn, N.W.; Santanasto, A.J.; Simonsick, E.M.; Boudreau, R.M.; Beach, S.R.; Schulz, R.; Newman, A.B. The Pittsburgh Fatigability Scale for older adults: Development and validation. J. Am. Geriatr. Soc. 2015, 63, 130–135. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Liu, Y.H.; Gao, X.; Mitchell, D.C.; Wood, G.C.; Bailey, R.K.; Still, C.D.; Jensen, G.L. Validation of a diet quality screening tool for use in the oldest old. J. Nutr. Gerontol. Geriatr. 2019, 38, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Popham, M.; Santanasto, A.J.; Hardy, R.; Glynn, N.W.; Kuh, D. Are BMI and inflammatory markers independently associated with physical fatigability in old age? Int. J. Obes. 2019, 43, 832–841. [Google Scholar] [CrossRef]
- LaSorda, K.R.; Gmelin, T.; Kuipers, A.L.; Boudreau, R.M.; Santanasto, A.J.; Christensen, K.; Renner, S.W.; Wojczynski, M.K.; Andersen, S.L.; Cosentino, S.; et al. Epidemiology of perceived physical fatigability in older adults: The Long Life Family Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 75, e81–e88. [Google Scholar] [CrossRef]
- National Cancer Institute. Developing the Healthy Eating Index. Available online: https://epi.grants.cancer.gov/hei/developing.html#2015 (accessed on 15 June 2020).
- Hagan, K.A.; Grodstein, F. The Alternative Healthy Eating Index and physical function impairment in men. J. Nutr. Health Aging 2019, 23, 459–465. [Google Scholar] [CrossRef]
- Hagan, K.A.; Chiuve, S.E.; Stampfer, M.J.; Katz, J.N.; Grodstein, F. Greater adherence to the Alternative Healthy Eating Index is associated with lower incidence of physical function impairment in the Nurses’ Health Study. J. Nutr. 2016, 146, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Stefler, D.; Hu, Y.; Malyutina, S.; Pajak, A.; Kubinova, R.; Peasey, A.; Pikhart, H.; Rodriguez-Artalejo, F.; Bobak, M. Mediterranean diet and physical functioning trajectories in eastern Europe: Findings from the HAPIEE Study. PLoS ONE 2018, 13, e0200460. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Houston, D.K.; Locher, J.L.; Ellison, K.J.; Gropper, S.; Buys, D.R.; Zizza, C.A. Higher Healthy Eating Index-2005 scores are associated with better physical performance. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 93–99. [Google Scholar] [CrossRef]
- Morris, S.; Cater, J.D.; Green, M.A.; Johnstone, A.M.; Brunstrom, J.M.; Stevenson, E.J.; Williams, E.A.; Corfe, B.M. Inadequacy of protein intake in older uk adults. Geriatrics 2020, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.M.; Wertheim, B.C.; LaCroix, A.Z.; Prentice, R.L.; Neuhouser, M.L.; Tinker, L.F.; Kritchevsky, S.; Shikany, J.M.; Eaton, C.; Chen, Z.; et al. Biomarker-calibrated protein intake and physical function in the Women’s Health Initiative. J. Am. Geriatr. Soc. 2013, 61, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Tooze, J.A.; Garcia, K.; Visser, M.; Rubin, S.; Harris, T.B.; Newman, A.B.; Kritchevsky, S.B.; Health, A.B. Protein intake and mobility limitation in community-dwelling older adults: The Health ABC Study. J. Am. Geriatr. Soc. 2017, 65, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, K.F.; Brussaard, J.H.; Kruizinga, A.G.; Telman, J.; Lowik, M.R. Socio-economic status, dietary intake and 10 y trends: The Dutch National Food Consumption Survey. Eur. J. Clin. Nutr. 2003, 57, 128–137. [Google Scholar] [CrossRef]
- Mendonca, N.; Granic, A.; Mathers, J.C.; Hill, T.R.; Siervo, M.; Adamson, A.J.; Jagger, C. Prevalence and determinants of low protein intake in very old adults: Insights from the Newcastle 85+ Study. Eur. J. Nutr. 2018, 57, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznaric, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.L.; Verdijk, L.B.; van Loon, L.J.C. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc. Natl. Acad. Sci. USA 2006, 103, 17589–17594. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M.; Lauretani, F.; Bandinelli, S.; Bos, A.; Corsi, A.M.; Simonsick, E.M.; Ferrucci, L. Magnesium and muscle performance in older persons: The InCHIANTI Study. Am. J. Clin. Nutr. 2006, 84, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Caruso, M.G.; Maggi, S. Dietary magnesium and incident frailty in older people at risk for knee osteoarthritis: An eight-year longitudinal study. Osteoporos. Int. 2018, 29, 1253. [Google Scholar] [CrossRef] [PubMed]
- Lo Piano, F.; Corsonello, A.; Corica, F. Magnesium and elderly patient: The explored paths and the ones to be explored: A review. Magnes. Res. 2019, 32, 1–15. [Google Scholar]
- Halfdanarson, T.R.; Kumar, N.; Li, C.Y.; Phyliky, R.L.; Hogan, W.J. Hematological manifestations of copper deficiency: A retrospective review. Eur. J. Haematol. 2008, 80, 523–531. [Google Scholar] [CrossRef]
- Balducci, L. Anemia, fatigue and aging. Transfus. Clin. Biol. 2010, 17, 375–381. [Google Scholar] [CrossRef]
- Pesta, D.H.; Tsirigotis, D.N.; Befroy, D.E.; Caballero, D.; Jurczak, M.J.; Rahimi, Y.; Cline, G.W.; Dufour, S.; Birkenfeld, A.L.; Rothman, D.L.; et al. Hypophosphatemia promotes lower rates of muscle ATP synthesis. FASEB J. 2016, 30, 3378–3387. [Google Scholar] [CrossRef]
- Balboa-Castillo, T.; Struijk, E.A.; Lopez-Garcia, E.; Banegas, J.R.; Rodriguez-Artalejo, F.; Guallar-Castillon, P. Low vitamin intake is associated with risk of frailty in older adults. Age Ageing 2018, 47, 872–879. [Google Scholar] [CrossRef]
- Struijk, E.A.; Lana, A.; Guallar-Castillon, P.; Rodriguez-Artalejo, F.; Lopez-Garcia, E. Intake of b vitamins and impairment in physical function in older adults. Clin. Nutr. 2018, 37, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Roth-Maier, D.A.; Kettler, S.I.; Kirchgessner, M. Availability of vitamin b6 from different food sources. Int. J. Food Sci. Nutr. 2002, 53, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Nygard, L.K.; Dahl, L.; Mundal, I.; Saltyte Benth, J.; Rokstad, A.M.M. Protein intake, protein mealtime distribution and seafood consumption in elderly norwegians: Associations with physical function and strength. Geriatrics 2020, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Loeser, R.F.; Hsu, F.C.; Booth, S.L.; Nevitt, M.; Simonsick, E.M.; Strotmeyer, E.S.; Vermeer, C.; Kritchevsky, S.B.; Health, A.B.C.S. Vitamin k status and lower extremity function in older adults: The Health Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1348–1355. [Google Scholar] [CrossRef]
| Characteristics | Physical Fatigability Status | ||
|---|---|---|---|
| More, ≥15 | Less, <15 | p-Value | |
| n | 98 | 24 | |
| Age | 0.097 | ||
| 80–84 years, % | 38.8 | 54.2 | |
| 85–89 years, % | 41.8 | 41.7 | |
| 90+ years, % | 19.4 | 4.2 | |
| Female, % | 58.2 | 37.5 | 0.069 |
| History of diabetes, % | 27.6 | 16.7 | 0.256 |
| History of coronary artery disease, % | 46.9 | 33.3 | 0.224 |
| History of hypertension, % | 84.7 | 83.3 | 0.870 |
| History of obstructive sleep apnea, % | 15.3 | 12.5 | 0.724 |
| History of depression, % | 3.1 | 4.2 | 0.791 |
| History of osteoarthritis, % | 56.1 | 41.7 | 0.203 |
| History of liver disease, % | 1.0 | 0.0 | 0.507 |
| Body Mass Index (BMI, kg/m2), mean ± SD | 28.6 ± 4.9 | 27.9 ± 4.8 | 0.490 |
| PROMIS Physical Function, mean ± SD | 78.5 ± 11.4 | 89.3 ± 5.1 | <0.0001 |
| Characteristics | Physical Fatigability Status | ||
|---|---|---|---|
| More, ≥15 | Less, <15 | p-Value | |
| n | 98 | 24 | |
| HEI | 59.0 ± 1.6 | 66.8 ± 3.3 | 0.028 |
| HEI-Total Vegetables | 3.5 ± 0.1 | 4.1 ± 0.3 | 0.066 |
| HEI-Greens and Beans | 1.8 ± 0.2 | 3.0 ± 0.4 | 0.019 |
| HEI-Total Fruits | 3.1 ± 0.2 | 3.5 ± 0.4 | 0.357 |
| HEI-Whole Fruits | 3.6 ± 0.2 | 3.9 ± 0.4 | 0.459 |
| HEI-Whole Grains | 5.6 ± 0.4 | 6.1 ± 0.7 | 0.467 |
| HEI-Total Dairy | 6.5 ± 0.3 | 6.6 ± 0.6 | 0.850 |
| HEI-Total Protein | 4.3 ± 0.1 | 4.8 ± 0.2 | 0.027 |
| HEI-Seafood and Plant Protein | 2.7 ± 0.2 | 3.7 ± 0.4 | 0.035 |
| HEI-Fatty Acids | 3.5 ± 0.3 | 4.0 ± 0.7 | 0.522 |
| HEI-Sodium | 5.6 ± 0.3 | 5.7 ± 0.7 | 0.927 |
| HEI-Refined Grains | 6.4 ± 0.4 | 7.5 ± 0.8 | 0.204 |
| HEI-Added Sugars | 7.1 ± 0.3 | 7.9 ± 0.6 | 0.230 |
| HEI-Saturated Fats | 5.2 ± 0.4 | 6.1 ± 0.7 | 0.298 |
| Characteristics | Physical Fatigability Status | ||
|---|---|---|---|
| More, ≥15 | Less, <15 | p-Value | |
| n | 98 | 24 | |
| Fat, g | 55.6 ± 1.1 | 53.5 ± 2.2 | 0.376 |
| Carbohydrates, g | 193.0 ± 3.1 | 194.0 ± 6.3 | 0.885 |
| Protein, g | 57.1 ± 1.3 | 64.7 ± 2.7 | 0.010 |
| Fiber, g | 16.4 ± 0.6 | 19.9 ± 1.2 | 0.007 |
| Vit A, μg RAE | 677.7 ± 30.3 | 814.7 ± 62.4 | 0.044 |
| Vit D, μg | 4.8 ± 0.3 | 5.4 ± 0.7 | 0.379 |
| Vit E, mg AT | 8.2 ± 0.6 | 8.8 ± 1.2 | 0.671 |
| Vit K, μg | 75.3 ± 11.7 | 130.0 ± 24.0 | 0.037 |
| Vit B6, mg | 1.5 ± 0.1 | 1.9 ± 0.1 | 0.001 |
| Vit C, mg | 77.0 ± 5.4 | 94.1 ± 11.1 | 0.156 |
| Folate, μg | 430.6 ± 18.1 | 485.8 ± 37.1 | 0.169 |
| Ca++, mg | 727.2 ± 25.3 | 779.2 ± 52.0 | 0.355 |
| Mg++, mg | 223.1 ± 6.3 | 265.5 ± 12.9 | 0.003 |
| Zn++, mg | 8.3 ± 0.3 | 9.5 ± 0.5 | 0.037 |
| Cu++, mg | 0.89 ± 0.02 | 1.02 ± 0.05 | 0.020 |
| Mn++, mg | 3.1 ± 0.1 | 3.8 ± 0.3 | 0.014 |
| Phosphorous, mg | 961.3 ± 21.0 | 1083.9 ± 43.2 | 0.009 |
| Choline, mg | 267.4 ± 8.4 | 295.0 ± 17.3 | 0.141 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, B.; Liu, Y.-H.; Stampley, J.; Wood, G.C.; Mitchell, D.C.; Jensen, G.L.; Gao, X.; Glynn, N.W.; Still, C.D.; Irving, B.A. The Association between Poor Diet Quality, Physical Fatigability and Physical Function in the Oldest-Old from the Geisinger Rural Aging Study. Geriatrics 2021, 6, 41. https://doi.org/10.3390/geriatrics6020041
Davis B, Liu Y-H, Stampley J, Wood GC, Mitchell DC, Jensen GL, Gao X, Glynn NW, Still CD, Irving BA. The Association between Poor Diet Quality, Physical Fatigability and Physical Function in the Oldest-Old from the Geisinger Rural Aging Study. Geriatrics. 2021; 6(2):41. https://doi.org/10.3390/geriatrics6020041
Chicago/Turabian StyleDavis, Brett, Yi-Hsuan Liu, James Stampley, G. Craig Wood, Diane C. Mitchell, Gordon L. Jensen, Xiang Gao, Nancy W. Glynn, Christopher D. Still, and Brian A. Irving. 2021. "The Association between Poor Diet Quality, Physical Fatigability and Physical Function in the Oldest-Old from the Geisinger Rural Aging Study" Geriatrics 6, no. 2: 41. https://doi.org/10.3390/geriatrics6020041
APA StyleDavis, B., Liu, Y.-H., Stampley, J., Wood, G. C., Mitchell, D. C., Jensen, G. L., Gao, X., Glynn, N. W., Still, C. D., & Irving, B. A. (2021). The Association between Poor Diet Quality, Physical Fatigability and Physical Function in the Oldest-Old from the Geisinger Rural Aging Study. Geriatrics, 6(2), 41. https://doi.org/10.3390/geriatrics6020041







