Deterioration, Compensation and Motor Control Processes in Healthy Aging, Mild Cognitive Impairment and Alzheimer’s Disease
Abstract
1. Introduction
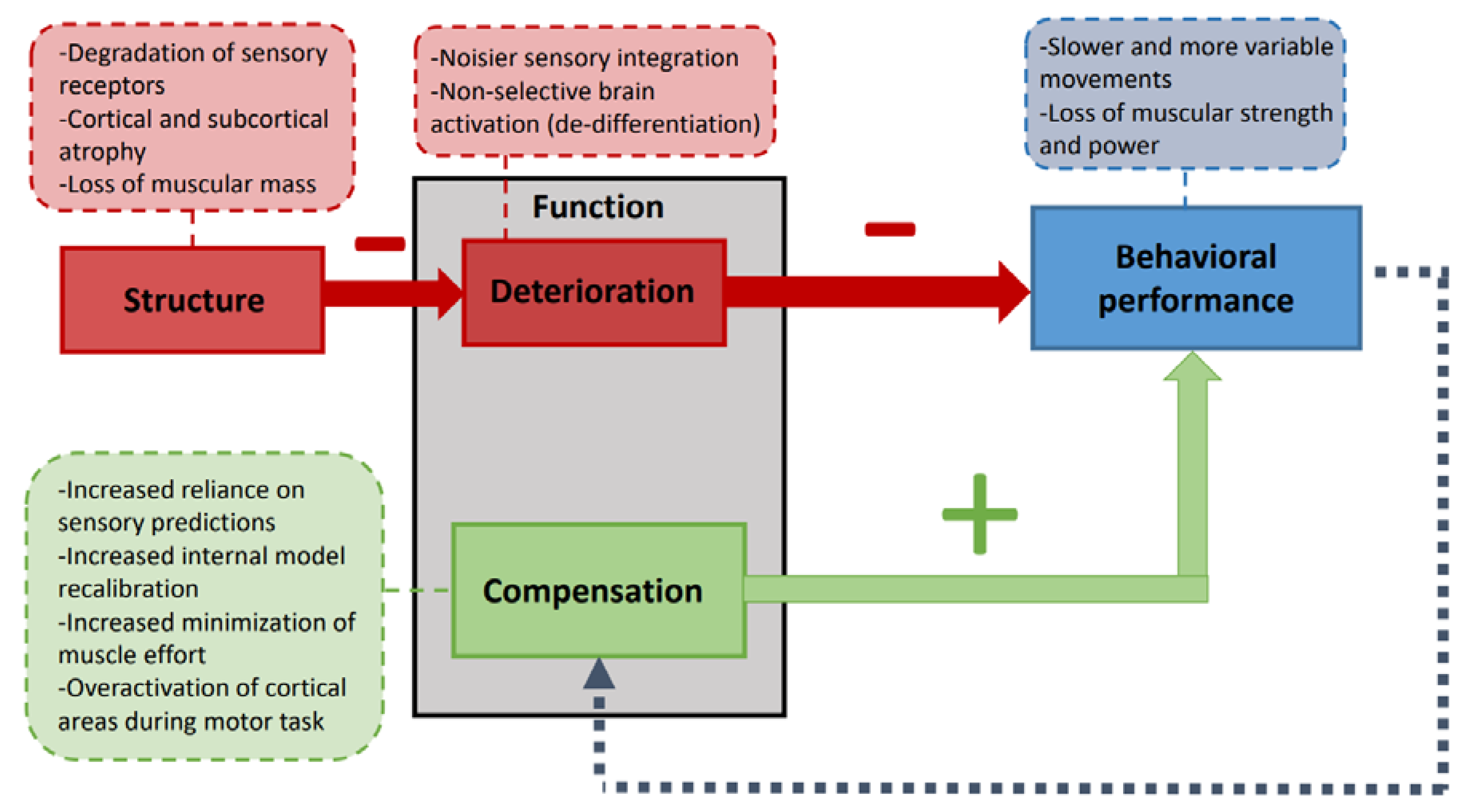
2. Deterioration and Compensation during Healthy Aging
3. Mechanistic Studies of Motor Function in Healthy Aging
4. Motor Function Studies in Pathological Aging: Alzheimer’s Disease (AD) and Mild Cognitive Impairment (MCI)
5. Final Remarks and Futures Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Population Prospects—Population Division—United Nations. Available online: https://population.un.org/wpp/Download/Standard/Population/ (accessed on 24 February 2021).
- Wimo, A.; Jönsson, L.; Bond, J.; Prince, M.; Winblad, B. Alzheimer Disease International The worldwide economic impact of dementia 2010. Alzheimer’s Dement. 2013, 9, 1–11.e3. [Google Scholar] [CrossRef]
- Takizawa, C.; Thompson, P.L.; Van Walsem, A.; Faure, C.; Maier, W.C. Epidemiological and Economic Burden of Alzheimer’s Disease: A Systematic Literature Review of Data across Europe and the United States of America. J. Alzheimer’s Dis. 2014, 43, 1271–1284. [Google Scholar] [CrossRef]
- Jia, J.; Wei, C.; Chen, S.; Li, F.; Tang, Y.; Qin, W.; Zhao, L.; Jin, H.; Xu, H.; Wang, F.; et al. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimer’s Dement. 2018, 14, 483–491. [Google Scholar] [CrossRef]
- Hardy, S.E.; Kang, Y.; Studenski, S.A.; Degenholtz, H.B. Ability to Walk 1/4 Mile Predicts Subsequent Disability, Mortality, and Health Care Costs. J. Gen. Intern. Med. 2011, 26, 130. [Google Scholar] [CrossRef]
- Cummings, S.R.; Studenski, S.; Ferrucci, L. A Diagnosis of Dismobility—Giving Mobility Clinical Visibility: A Mobility Working Group Recommendation. JAMA J. Am. Med Assoc 2014, 311, 2061–2062. [Google Scholar] [CrossRef]
- Musich, S.; Wang, S.S.; Ruiz, J.; Hawkins, K.; Wicker, E. The impact of mobility limitations on health outcomes among older adults. Geriatr. Nurs. 2018, 39, 162–169. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Ciol, M.A.; Yorkston, K.M.; Hoffman, J.M.; Chan, L. Mobility Limitations in the Medicare Population: Prevalence and Sociodemographic and Clinical Correlates. J. Am. Geriatr. Soc. 2005, 53, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Cooper, R.; Shardell, M.; Simonsick, E.M.; Schrack, J.A.; Kuh, D. Age-Related Change in Mobility: Perspectives From Life Course Epidemiology and Geroscience. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2016, 71, 1184–1194. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait Speed and Survival in Older Adults. JAMA J. Am. Med. Assoc. 2011, 305, 50. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, J.; Neyens, J.C.L.; Van Rossum, E.; Spreeuwenberg, M.D.; De Witte, L.P. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: A systematic review. BMC Geriatr. 2011, 11, 33. [Google Scholar] [CrossRef]
- Newman, A.B.; Simonsick, E.M.; Naydeck, B.L.; Boudreau, R.M.; Kritchevsky, S.B.; Nevitt, M.C.; Pahor, M.; Satterfield, S.; Brach, J.S.; Studenski, S.A.; et al. Association of Long-Distance Corridor Walk Performance With Mortality, Cardiovascular Disease, Mobility Limitation, and Disability. JAMA 2006, 295, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.M.; Barnes, B.; Speechley, M.; Hunter, S.W.M.; Doherty, T.J.; Duque, G.; Gopaul, K.; Sposato, L.A.; Casas-Herrero, A.; Borrie, M.J.; et al. Disentangling Cognitive-Frailty: Results From the Gait and Brain Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2016, 71, 1476–1482. [Google Scholar] [CrossRef]
- Verghese, J.; Wang, C.; Lipton, R.B.; Holtzer, R. Motoric Cognitive Risk Syndrome and the Risk of Dementia. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2013, 68, 412–418. [Google Scholar] [CrossRef]
- Hunter, S.K.; Pereira, H.M.; Keenan, K.G. The aging neuromuscular system and motor performance. J. Appl. Physiol. 2016, 121, 982–995. [Google Scholar] [CrossRef]
- Clark, B.C.; Woods, A.J.; Clark, L.A.; Criss, C.R.; Shadmehr, R.; Grooms, D.R. The Aging Brain & the Dorsal Basal Ganglia: Implications for Age-Related Limitations of Mobility. Adv. Geriatr. Med. Res. 2019, 1. [Google Scholar] [CrossRef]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Sorond, F.A.; Cruz-Almeida, Y.; Clark, D.J.; Viswanathan, A.; Scherzer, C.R.; De Jager, P.; Csiszar, A.; Laurienti, P.J.; Hausdorff, J.M.; Chen, W.G.; et al. Aging, the Central Nervous System, and Mobility in Older Adults: Neural Mechanisms of Mobility Impairment. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2015, 70, 1526–1532. [Google Scholar] [CrossRef]
- Rosso, A.L.; Studenski, S.A.; Chen, W.G.; Aizenstein, H.J.; Alexander, N.B.; Bennett, D.A.; Black, S.E.; Camicioli, R.; Carlson, M.C.; Ferrucci, L.; et al. Aging, the Central Nervous System, and Mobility. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2013, 68, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Papegaaij, S.; Taube, W.; Baudry, S.; Otten, E.; Hortobágyi, T. Aging Causes a Reorganization of Cortical and Spinal Control of Posture. Front. Aging Neurosci. 2014, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Anderson, N.D.; Locantore, J.K.; McIntosh, A.R. Aging Gracefully: Compensatory Brain Activity in High-Performing Older Adults. Neuroimage 2002, 17, 1394. [Google Scholar] [CrossRef]
- Cabeza, R. Hemispheric Asymmetry Reduction in Older Adults: The HAROLD Model. Psychol. Aging 2002, 17, 85. [Google Scholar] [CrossRef]
- Logan, J.M.; Sanders, A.L.; Snyder, A.Z.; Morris, J.C.; Buckner, R.L. Under-Recruitment and Nonselective Recruitment: Dissociable Neural Mechanisms Associated with Aging. Neuronology 2002, 33, 827. [Google Scholar]
- Buckner, R.; Logan, J.M. Frontal Contributions to Episodic Memory Encoding in the Young and Elderly. Cogn. Neurosci. Mem. Encoding Retr. 2002, 59. [Google Scholar]
- Wang, L.; Zhang, Y.; Zhang, J.; Sang, L.; Li, P.; Yan, R.; Qiu, M.; Liu, C. Aging Changes Effective Connectivity of Motor Networks During Motor Execution and Motor Imagery. Front. Aging Neurosci. 2019, 11, 312. [Google Scholar] [CrossRef]
- Mattay, V.; Fera, F.; Tessitore, A.; Hariri, A.; Das, S.; Callicott, J.; Weinberger, D. Neurophysiological correlates of age-related changes in human motor function. Neurology 2002, 58, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.S.; Frackowiak, R.S.J. Age-related changes in the neural correlates of motor performance. Brain 2003, 126, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Heuninckx, S.; Wenderoth, N.; Swinnen, S.P. Systems Neuroplasticity in the Aging Brain: Recruiting Additional Neural Resources for Successful Motor Performance in Elderly Persons. J. Neurosci. 2008, 28, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Heuninckx, S.; Wenderoth, N.; Debaere, F.; Peeters, R.; Swinnen, S.P. Neural Basis of Aging: The Penetration of Cognition into Action Control. J. Neurosci. 2005, 25, 6787–6796. [Google Scholar] [CrossRef] [PubMed]
- Turesky, T.K.; Turkeltaub, P.E.; Eden, G.F. An Activation Likelihood Estimation Meta-Analysis Study of Simple Motor Movements in Older and Young Adults. Front. Aging Neurosci. 2016, 8, 238. [Google Scholar] [CrossRef]
- McGregor, K.M.; Craggs, J.G.; Benjamin, M.L.; Crosson, B.; White, K.D. Age-Related Changes in Motor Control During Unimanual Movements. Brain Imaging Behav. 2009, 3, 317–331. [Google Scholar] [CrossRef]
- Hutchinson, S.; Kobayashi, M.; Horkan, C.M.; Pascual-Leone, A.; Alexander, M.P.; Schlaug, G. Age-Related Differences in Movement Representation. Neuroimage 2002, 17, 1720. [Google Scholar] [CrossRef]
- Ward, N.S.; Swayne, O.B.; Newton, J.M. Age-dependent changes in the neural correlates of force modulation: An fMRI study. Neurobiol. Aging 2008, 29, 1434–1446. [Google Scholar] [CrossRef]
- Calautti, C.; Serrati, C.; Baron, J.C. Effects of Age on Brain Activation during Auditory-Cued Thumb-to-Index Opposition: A Positron Emission Tomography Study. Stroke 2001, 32, 139. [Google Scholar] [CrossRef] [PubMed]
- Langan, J.; Peltier, S.J.; Bo, J.; Fling, B.W.; Welsh, R.C.; Seidler, R.D. Functional implications of age differences in motor system connectivity. Front. Syst. Neurosci. 2010, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Boudrias, M.-H.; Gonçalves, C.S.; Penny, W.D.; Park, C.-H.; Rossiter, H.E.; Talelli, P.; Ward, N.S. Age-related changes in causal interactions between cortical motor regions during hand grip. NeuroImage 2012, 59, 3398–3405. [Google Scholar] [CrossRef] [PubMed]
- Naccarato, M.; Calautti, C.; Jones, P.; Day, D.; Carpenter, T.; Baron, J.-C. Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? An fMRI study. NeuroImage 2006, 32, 1250–1256. [Google Scholar] [CrossRef]
- Riecker, A.; Gröschel, K.; Ackermann, H.; Steinbrink, C.; Witte, O.; Kastrup, A. Functional significance of age-related differences in motor activation patterns. NeuroImage 2006, 32, 1345–1354. [Google Scholar] [CrossRef]
- Bernard, J.A.; Seidler, R.D. Evidence for motor cortex dedifferentiation in older adults. Neurobiol. Aging 2012, 33, 1890–1899. [Google Scholar] [CrossRef]
- Park, D.C.; Polk, T.A.; Park, R.; Minear, M.; Savage, A.; Smith, M.R. From The Cover: Aging reduces neural specialization in ventral visual cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 13091–13095. [Google Scholar] [CrossRef]
- Li, S.-C. Lindenberger, Cross-Level Unification: A Computational Exploration of the Link between Deterioration of Neurotransmitter Systems and Dedifferentiation of Cognitive Abilities in Old Age. In Cognitive Neuroscience of Memory; Hogrefe & Huber: Toronto, ON, Canada, 1999; pp. 103–146. [Google Scholar]
- Harada, T.; Miyai, I.; Suzuki, M.; Kubota, K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp. Brain Res. 2009, 193, 445–454. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Lustig, C. Brain aging: Reorganizing discoveries about the aging mind. Curr. Opin. Neurobiol. 2005, 15, 245–251. [Google Scholar] [CrossRef]
- Wu, T.; Hallett, M. The influence of normal human ageing on automatic movements. J. Physiol. 2005, 562, 605–615. [Google Scholar] [CrossRef]
- Pousson, M.; Lepers, R.; Van Hoecke, J. Changes in isokinetic torque and muscular activity of elbow flexors muscles with age. Exp. Gerontol. 2001, 36, 1687–1698. [Google Scholar] [CrossRef]
- Buckles, V.D. Age-Related Slowing. In Sensorimotor Impairment in the Elderly; Metzler, J.B., Ed.; Springer: Dordrecht, The Netherlands, 1993; pp. 73–87. [Google Scholar]
- Darling, W.; Cooke, J.; Brown, S. Control of simple arm movements in elderly humans. Neurobiol. Aging 1989, 10, 149–157. [Google Scholar] [CrossRef]
- Goble, D.J.; Coxon, J.P.; Wenderoth, N.; Van Impe, A.; Swinnen, S.P. Proprioceptive sensibility in the elderly: Degeneration, functional consequences and plastic-adaptive processes. Neurosci. Biobehav. Rev. 2009, 33, 271–278. [Google Scholar] [CrossRef]
- Zalewski, C.K. Aging of the Human Vestibular System. Semin. Hear. 2015, 36, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Saftari, L.N.; Kwon, O.-S. Ageing vision and falls: A review. J. Physiol. Anthr. 2018, 37, 11. [Google Scholar] [CrossRef] [PubMed]
- Hoffstaedter, F.; Grefkes, C.; Roski, C.; Caspers, S.; Zilles, K.; Eickhoff, S.B. Age-related decrease of functional connectivity additional to gray matter atrophy in a network for movement initiation. Beiträge Ref. Anat. Entwickelungsgeschichte 2015, 220, 999–1012. [Google Scholar] [CrossRef]
- Salat, D.H.; Buckner, R.L.; Snyder, A.Z.; Greve, D.N.; Desikan, R.S.R.; Busa, E.; Morris, J.C.; Dale, A.M.; Fischl, B. Thinning of the Cerebral Cortex in Aging. Cereb. Cortex 2004, 14, 721–730. [Google Scholar] [CrossRef]
- Buss, C.; Rasmussen, J.; Bischof, G.N.; Sele, S.; Jäncke, L.; Liem, F.; Mérillat, S. Decline Variability of Cortical and Subcortical Regions in Aging: A Longitudinal Study. Front. Hum. Neurosci. 2020, 14, 363. [Google Scholar]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Wolpe, N.; Can, C.-; Ingram, J.N.; Tsvetanov, K.A.; Geerligs, L.; Kievit, R.A.; Henson, R.N.; Wolpert, D.M.; Rowe, J.B. Ageing increases reliance on sensorimotor prediction through structural and functional differences in frontostriatal circuits. Nat. Commun. 2016, 7, 13034. [Google Scholar] [CrossRef]
- Helsen, W.F.; Van Halewyck, F.; Levin, O.; Boisgontier, M.P.; Lavrysen, A.; Elliott, D. Manual aiming in healthy aging: Does proprioceptive acuity make the difference? AGE 2016, 38, 1–19. [Google Scholar] [CrossRef]
- Hoellinger, T.; McIntyre, J.; Jami, L.; Hanneton, S.; Cheron, G.; Roby-Brami, A. A strategy of faster movements used by elderly humans to lift objects of increasing weight in ecological context. Neuroscience 2017, 357, 384–399. [Google Scholar] [CrossRef] [PubMed]
- Vandevoorde, K.; Orban de Xivry, J.J. Internal Model Recalibration Does Not Deteriorate with Age While Motor Adaptation Does. Neurobiol. Aging 2019, 80, 138. [Google Scholar] [CrossRef] [PubMed]
- Poirier, G.; Papaxanthis, C.; Mourey, F.; Gaveau, J. Motor Planning of Vertical Arm Movements in Healthy Older Adults: Does Effort Minimization Persist With Aging? Front. Aging Neurosci. 2020, 12, 37. [Google Scholar] [CrossRef]
- Berret, B.; Darlot, C.; Jean, F.; Pozzo, T.; Papaxanthis, C.; Gauthier, J.P. The Inactivation Principle: Mathematical Solutions Minimizing the Absolute Work and Biological Implications for the Planning of Arm Movements. PLoS Comput. Biol. 2008, 4, e1000194. [Google Scholar] [CrossRef]
- Gaveau, J.; Berret, B.; Demougeot, L.; Fadiga, L.; Pozzo, T.; Papaxanthis, C. Energy-related optimal control accounts for gravitational load: Comparing shoulder, elbow, and wrist rotations. J. Neurophysiol. 2014, 111, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Gaveau, J.; Berret, B.; E Angelaki, D.; Papaxanthis, C. Direction-dependent arm kinematics reveal optimal integration of gravity cues. eLife 2016, 5, e16394. [Google Scholar] [CrossRef]
- Gaveau, J.; Grospretre, S.; Angelaki, D.; Papaxanthis, C. A Cross-Species Neural Integration of Gravity for Motor Optimisation. BioRxiv 2019, 728857. [Google Scholar]
- Casteran, M.; Hilt, P.M.; Mourey, F.; Manckoundia, P.; French, R.; Thomas, E. Shifts in Key Time Points and Strategies for a Multisegment Motor Task in Healthy Aging Subjects. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2018, 73, 1609–1617. [Google Scholar] [CrossRef]
- Bock, O. Components of sensorimotor adaptation in young and elderly subjects. Exp. Brain Res. 2004, 160, 259–263. [Google Scholar] [CrossRef]
- Buch, E.R.; Young, S.; Contreras-Vidal, J.L. Visuomotor Adaptation in Normal Aging. Learn. Mem. 2003, 10, 55–63. [Google Scholar] [CrossRef]
- Anguera, J.A.; Reuter-Lorenz, P.A.; Willingham, D.T.; Seidler, R.D. Failure to Engage Spatial Working Memory Contributes to Age-related Declines in Visuomotor Learning. J. Cogn. Neurosci. 2011, 23, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Bock, O.; Girgenrath, M. Relationship between sensorimotor adaptation and cognitive functions in younger and older subjects. Exp. Brain Res. 2005, 169, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Hegele, M.; Heuer, H. Adaptation to a direction-dependent visuomotor gain in the young and elderly. Psychol. Res. 2008, 74, 21–34. [Google Scholar] [CrossRef]
- King, B.R.; Fogel, S.M.; Albouy, G.; Doyon, J. Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Front. Hum. Neurosci. 2013, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Velilla, N.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Sáez De Asteasu, M.L.; Lucia, A.; Galbete, A.; García-Baztán, A.; Alonso-Renedo, J.; González-Glaría, B.; Gonzalo-Lázaro, M.; et al. Effect of Exercise Intervention on Functional Decline in Very Elderly Patients During Acute Hospitalization: A Randomized Clinical Trial. JAMA Intern. Med. 2019, 179, 28. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, J.W.; Ghazanfar, A.A.; Gomez-Marin, A.; MacIver, M.A.; Poeppel, D. Neuroscience Needs Behavior: Correcting a Reductionist Bias. Neuron 2017, 93, 480–490. [Google Scholar] [CrossRef]
- Angelaki, D.E.; Gu, Y.; DeAngelis, G.C. Multisensory integration: Psychophysics, neurophysiology, and computation. Curr. Opin. Neurobiol. 2009, 19, 452–458. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Verghese, J.; Robbins, M.; Holtzer, R.; Zimmerman, M.; Wang, C.; Xue, X.; Lipton, R.B. Gait Dysfunction in Mild Cognitive Impairment Syndromes. J. Am. Geriatr. Soc. 2008, 56, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.; Muir, S.W.; Speechley, M. Dual-Task Complexity Affects Gait in People With Mild Cognitive Impairment: The Interplay Between Gait Variability, Dual Tasking, and Risk of Falls. Arch. Phys. Med. Rehabil. 2012, 93, 293–299. [Google Scholar] [CrossRef]
- Suttanon, P.; Hill, K.D.; Said, C.M.; Dodd, K.J. A Longitudinal Study of Change in Falls Risk and Balance and Mobility in Healthy Older People and People with Alzheimer Disease. Am. J. Phys. Med. Rehabil. 2013, 92, 676–685. [Google Scholar] [CrossRef]
- Suttanon, P.; Hill, K.D.; Said, C.M.; LoGiudice, D.; Lautenschlager, N.T.; Dodd, K.J. Balance and Mobility Dysfunction and Falls Risk in Older People with Mild to Moderate Alzheimer Disease. Am. J. Phys. Med. Rehabil. 2012, 91, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chan, J.S.; Yan, J.H. Mild cognitive impairment affects motor control and skill learning. Rev. Neurosci. 2016, 27, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.; Oteng-Amoako, A.; Speechley, M.; Gopaul, K.; Beauchet, O.; Annweiler, C.; Muir-Hunter, S.W. The Motor Signature of Mild Cognitive Impairment: Results From the Gait and Brain Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 69, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Boripuntakul, S.; Lord, S.R.; Brodie, M.A.D.; Smith, S.T.; Methapatara, P.; Wongpakaran, N.; Sungkarat, S. Spatial variability during gait initiation while dual tasking is increased in individuals with mild cognitive impairment. J. Nutr. Health Aging 2013, 18, 307–312. [Google Scholar] [CrossRef]
- Doi, T.; Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Uemura, K.; Anan, Y.; Suzuki, T. Cognitive function and gait speed under normal and dual-task walking among older adults with mild cognitive impairment. BMC Neurol. 2014, 14, 67. [Google Scholar] [CrossRef]
- Tarnanas, I.; Papagiannopoulos, S.; Kazis, D.; Wiederhold, M.; Widerhold, B.; Tsolaki, M. Reliability of a novel serious game using dual-task gait profiles to early characterize aMCI. Front. Aging Neurosci. 2015, 7, 50. [Google Scholar] [CrossRef]
- Pau, M.; Mulas, I.; Putzu, V.; Asoni, G.; Viale, D.; Mameli, I.; Leban, B.; Allali, G. Smoothness of Gait in Healthy and Cognitively Impaired Individuals: A Study on Italian Elderly Using Wearable Inertial Sensor. Sensors 2020, 20, 3577. [Google Scholar] [CrossRef]
- Gillain, S.; Warzee, E.; Lekeu, F.; Wojtasik, V.; Maquet, D.; Croisier, J.L.; Salmon, E.; Petermans, J. The Value of Instrumental Gait Analysis in Elderly Healthy, MCI or Alzheimer’s Disease Subjects and a Comparison with Other Clinical Tests Used in Single and Dual-Task Conditions. Ann. Phys. Rehabil. Med. 2009, 52, 453. [Google Scholar] [CrossRef] [PubMed]
- Maquet, D.; Lekeu, F.; Warzee, E.; Gillain, S.; Wojtasik, V.; Salmon, E.; Petermans, J.; Croisier, J.L. Gait Analysis in Elderly Adult Patients with Mild Cognitive Impairment and Patients with Mild Alzheimer’s Disease: Simple versus Dual Task: A Preliminary Report. Clin. Physiol. Funct. Imaging 2010, 30, 51. [Google Scholar] [CrossRef]
- Choi, J.S.; Oh, H.S.; Kang, D.W.; Mun, K.R.; Choi, M.H.; Lee, S.J.; Yang, J.W.; Chung, S.C.; Mun, S.W.; Tack, G.R. Comparison of Gait and Cognitive Function among the Elderly with Alzheimer’s Disease, Mild Cognitive Impairment and Healthy. Int. J. Precis. Eng. Manuf. 2011, 12, 169. [Google Scholar] [CrossRef]
- Deschamps, T.; Beauchet, O.; Annweiler, C.; Cornu, C.; Mignardot, J.-B. Postural control and cognitive decline in older adults: Position versus velocity implicit motor strategy. Gait Posture 2014, 39, 628–630. [Google Scholar] [CrossRef]
- Jeon, S.Y.; Han, S.J.; Jeong, J.H.; Fregni, F. Effect of exercise on balance in persons with mild cognitive impairment. Neurorehability 2014, 35, 271–278. [Google Scholar] [CrossRef]
- Tangen, G.G.; Engedal, K.; Bergland, A.; Moger, T.A.; Mengshoel, A.M. Relationships Between Balance and Cognition in Patients With Subjective Cognitive Impairment, Mild Cognitive Impairment, and Alzheimer Disease. Phys. Ther. 2014, 94, 1123–1134. [Google Scholar] [CrossRef]
- Bahureksa, L.; Najafi, B.; Saleh, A.; Sabbagh, M.; Coon, D.; Mohler, M.J.; Schwenk, M. The Impact of Mild Cognitive Impairment on Gait and Balance: A Systematic Review and Meta-Analysis of Studies Using Instrumented Assessment. Gerontology 2017, 63, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Sparto, P.J.; Rosso, A.L.; Divecha, A.A.; Metti, A.L.; Rosano, C. Shared neural substrates of cognitive function and postural control in older adults. Alzheimer’s Dement. 2020, 16, 621–629. [Google Scholar] [CrossRef]
- Gras, L.Z.; Kanaan, S.F.; McDowd, J.M.; Colgrove, Y.M.; Burns, J.; Pohl, P.S. Balance and Gait of Adults With Very Mild Alzheimer Disease. J. Geriatr. Phys. Ther. 2015, 38, 1–7. [Google Scholar] [CrossRef]
- Jor’Dan, A.J.; McCarten, J.R.; Rottunda, S.; Stoffregen, T.A.; Manor, B.; Wade, M.G. Dementia alters standing postural adaptation during a visual search task in older adult men. Neurosci. Lett. 2015, 593, 101–106. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Lee, H.; Chung, I.-S.; Yi, H.-A. Relationship between postural instability and subcortical volume loss in Alzheimer’s disease. Medicine 2017, 96, e7286. [Google Scholar] [CrossRef]
- Yan, J.H.; Rountree, S.; Massman, P.; Doody, R.S.; Li, H. Alzheimer’s disease and mild cognitive impairment deteriorate fine movement control. J. Psychiatr. Res. 2008, 42, 1203–1212. [Google Scholar] [CrossRef]
- Schröter, A.; Mergl, R.; Bürger, K.; Hampel, H.; Möller, H.-J.; Hegerl, U. Kinematic Analysis of Handwriting Movements in Patients with Alzheimer’s Disease, Mild Cognitive Impairment, Depression and Healthy Subjects. Dement. Geriatr. Cogn. Disord. 2003, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Buracchio, T.; Dodge, H.H.; Howieson, D.B.; Wasserman, D.; Kaye, J. The Trajectory of Gait Speed Preceding Mild Cognitive Impairment. Arch. Neurol. 2010, 67, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Bangert, A.S.; Balota, D.A. Keep Up the Pace: Declines in Simple Repetitive Timing Differentiate Healthy Aging from the Earliest Stages of Alzheimer’s Disease. J. Int. Neuropsychol. Soc. 2012, 18, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Colella, D.; Guerra, A.; Paparella, G.; Cioffi, E.; Di Vita, A.; Trebbastoni, A.; Berardelli, A.; Bologna, M. Motor dysfunction in mild cognitive impairment as tested by kinematic analysis and transcranial magnetic stimulation. Clin. Neurophysiol. 2021, 132, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Albert, M.; Belleville, S.; Craik, F.I.M.; Duarte, A.; Grady, C.L.; Lindenberger, U.; Nyberg, L.; Park, D.C.; Reuter-Lorenz, P.A.; et al. Maintenance, Reserve and Compensation: The Cognitive Neuroscience of Healthy Ageing. Nat. Rev. Neurosci. 2018, 19, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Roe, C.M.; Xiong, C.; Miller, J.P.; Morris, J.C. Education and Alzheimer disease without dementia: Support for the cognitive reserve hypothesis. Neurology 2007, 68, 223–228. [Google Scholar] [CrossRef]
- Groot, C.; Van Loenhoud, A.C.; Barkhof, F.; Van Berckel, B.N.; Koene, T.; Teunissen, C.C.; Scheltens, P.; Van Der Flier, W.M.; Ossenkoppele, R. Differential effects of cognitive reserve and brain reserve on cognition in Alzheimer disease. Neurology 2017, 90, e149–e156. [Google Scholar] [CrossRef]
- Marvel, C.L.; Morgan, O.P.; Kronemer, S.I. How the motor system integrates with working memory. Neurosci. Biobehav. Rev. 2019, 102, 184–194. [Google Scholar] [CrossRef]
- Michely, J.; Volz, L.; Hoffstaedter, F.; Tittgemeyer, M.; Eickhoff, S.; Fink, G.; Grefkes, C. Network connectivity of motor control in the ageing brain. NeuroImage Clin. 2018, 18, 443–455. [Google Scholar] [CrossRef]
- Salzman, T.; Aboualmagd, A.; Badawi, H.; Tobón-Vallejo, D.; Kim, H.; Dahroug, L.; Laamarti, F.; El Saddik, A.; Fraser, S. Prefrontal Cortex Involvement during Dual-Task Stair Climbing in Healthy Older Adults: An fNIRS Study. Brain Sci. 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Albers, M.W.; Gilmore, G.C.; Kaye, J.; Murphy, C.; Wingfield, A.; Bennett, D.A.; Boxer, A.L.; Buchman, A.S.; Cruickshanks, K.J.; Devanand, D.P.; et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 70–98. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.T.; Wilson, R.S.; Beck, T.L.; Bienias, J.L.; Bennett, D.A. Motor Dysfunction in Mild Cognitive Impairment and the Risk of Incident Alzheimer Disease. Arch. Neurol. 2006, 63, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Albert, M.; Brandt, J.; Blacker, D.; Hadjigeorgiou, G.; Papadimitriou, A.; Dubois, B.; Sarazin, M.; Wegesin, D.; Marder, K.; et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology 2005, 64, 1696–1703. [Google Scholar] [CrossRef]
- Camicioli, R.; Licis, L. Motor impairment predicts falls in specialized Alzheimer care units. Alzheimer Dis. Assoc. Disord. 2004, 18, 214. [Google Scholar] [PubMed]
- Ansai, J.H.; De Andrade, L.P.; Masse, F.A.A.; Gonçalves, J.; Takahashi, A.C.D.M.; Vale, F.A.C.; Rebelatto, J.R. Risk Factors for Falls in Older Adults With Mild Cognitive Impairment and Mild Alzheimer Disease. J. Geriatr. Phys. Ther. 2019, 42, E116–E121. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, H.; Parvaneh, S.; Mohler, J.; Wendel, C.; Zamrini, E.; O’Connor, K.; Toosizadeh, N. Can motor function uncertainty and local instability within upper-extremity dual-tasking predict amnestic mild cognitive impairment and early-stage Alzheimer’s disease? Comput. Biol. Med. 2020, 120, 103705. [Google Scholar] [CrossRef]
- Dubois, B.; Albert, M.L. Amnestic MCI or prodromal Alzheimer’s disease? Lancet Neurol. 2004, 3, 246–248. [Google Scholar] [CrossRef]
- Chang, K.-V.; Hsu, T.-H.; Wu, W.-T.; Huang, K.-C.; Han, D.-S. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164.e7–1164.e15. [Google Scholar] [CrossRef]
- Morris, R.; Lord, S.; Bunce, J.; Burn, D.; Rochester, L. Gait and cognition: Mapping the global and discrete relationships in ageing and neurodegenerative disease. Neurosci. Biobehav. Rev. 2016, 64, 326–345. [Google Scholar] [CrossRef]
- Marquis, S.; Moore, M.M.; Howieson, D.B.; Sexton, G.; Payami, H.; Kaye, J.A.; Camicioli, R. Independent predictors of cognitive decline in healthy elderly persons. Arch. Neurol. 2002, 59, 601–606. [Google Scholar] [CrossRef]
- Chou, M.-Y.; Nishita, Y.; Nakagawa, T.; Tange, C.; Tomida, M.; Shimokata, H.; Otsuka, R.; Chen, L.-K.; Arai, H. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poirier, G.; Ohayon, A.; Juranville, A.; Mourey, F.; Gaveau, J. Deterioration, Compensation and Motor Control Processes in Healthy Aging, Mild Cognitive Impairment and Alzheimer’s Disease. Geriatrics 2021, 6, 33. https://doi.org/10.3390/geriatrics6010033
Poirier G, Ohayon A, Juranville A, Mourey F, Gaveau J. Deterioration, Compensation and Motor Control Processes in Healthy Aging, Mild Cognitive Impairment and Alzheimer’s Disease. Geriatrics. 2021; 6(1):33. https://doi.org/10.3390/geriatrics6010033
Chicago/Turabian StylePoirier, Gabriel, Alice Ohayon, Adrien Juranville, France Mourey, and Jeremie Gaveau. 2021. "Deterioration, Compensation and Motor Control Processes in Healthy Aging, Mild Cognitive Impairment and Alzheimer’s Disease" Geriatrics 6, no. 1: 33. https://doi.org/10.3390/geriatrics6010033
APA StylePoirier, G., Ohayon, A., Juranville, A., Mourey, F., & Gaveau, J. (2021). Deterioration, Compensation and Motor Control Processes in Healthy Aging, Mild Cognitive Impairment and Alzheimer’s Disease. Geriatrics, 6(1), 33. https://doi.org/10.3390/geriatrics6010033






