Looking for the “Little Things”: A Multi-Disciplinary Approach to Medicines Monitoring for Older People Using the ADRe Resource
Abstract
1. Introduction
2. Prescribing as an Expression of Professional Power
3. ADRs in Older Adults: Systems Failure or Systemic Challenge
4. ADRe and Systematic Medicines Management as a Route to Effective Change
5. How Is ADRe Different?
6. What Does ADRe Entail?
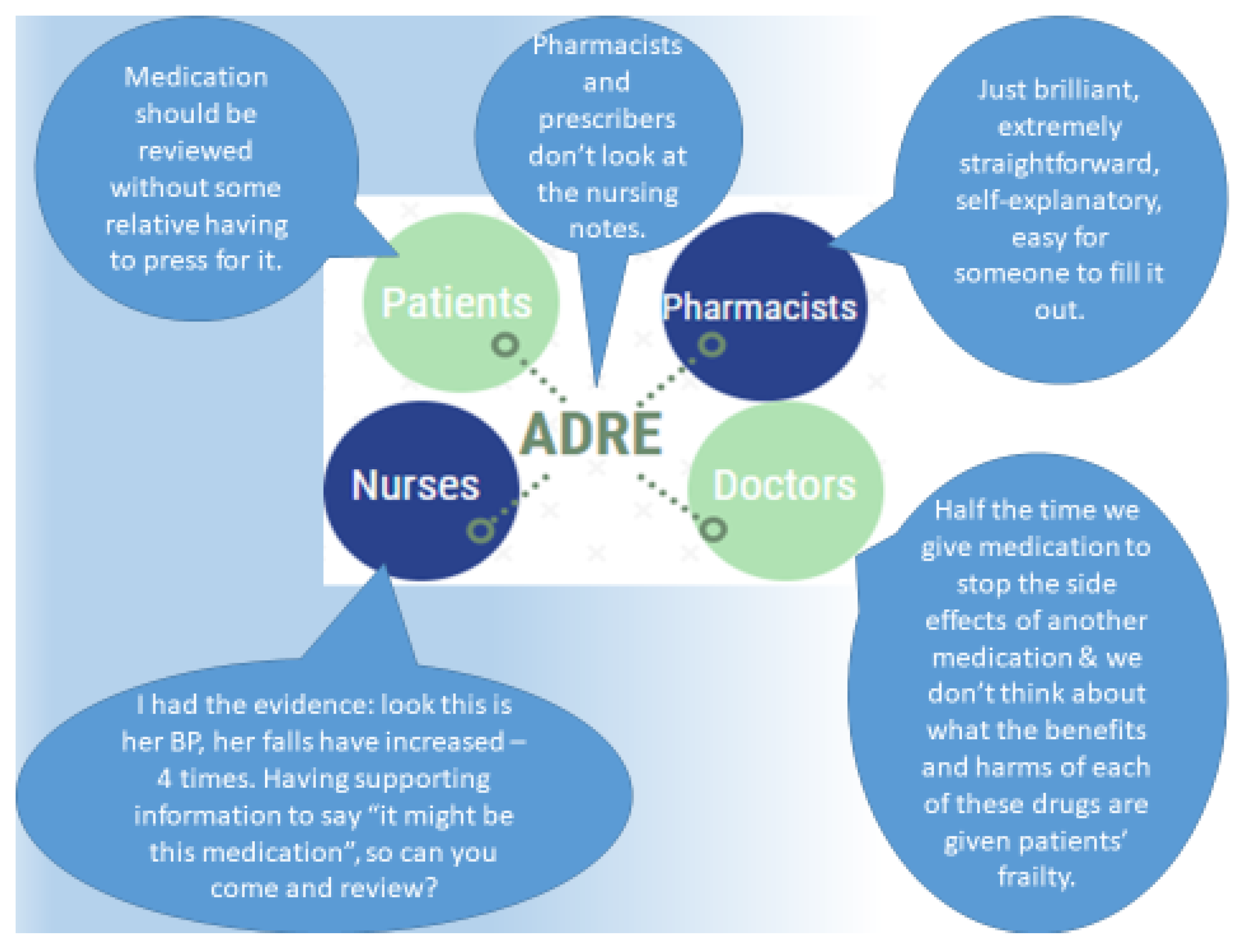
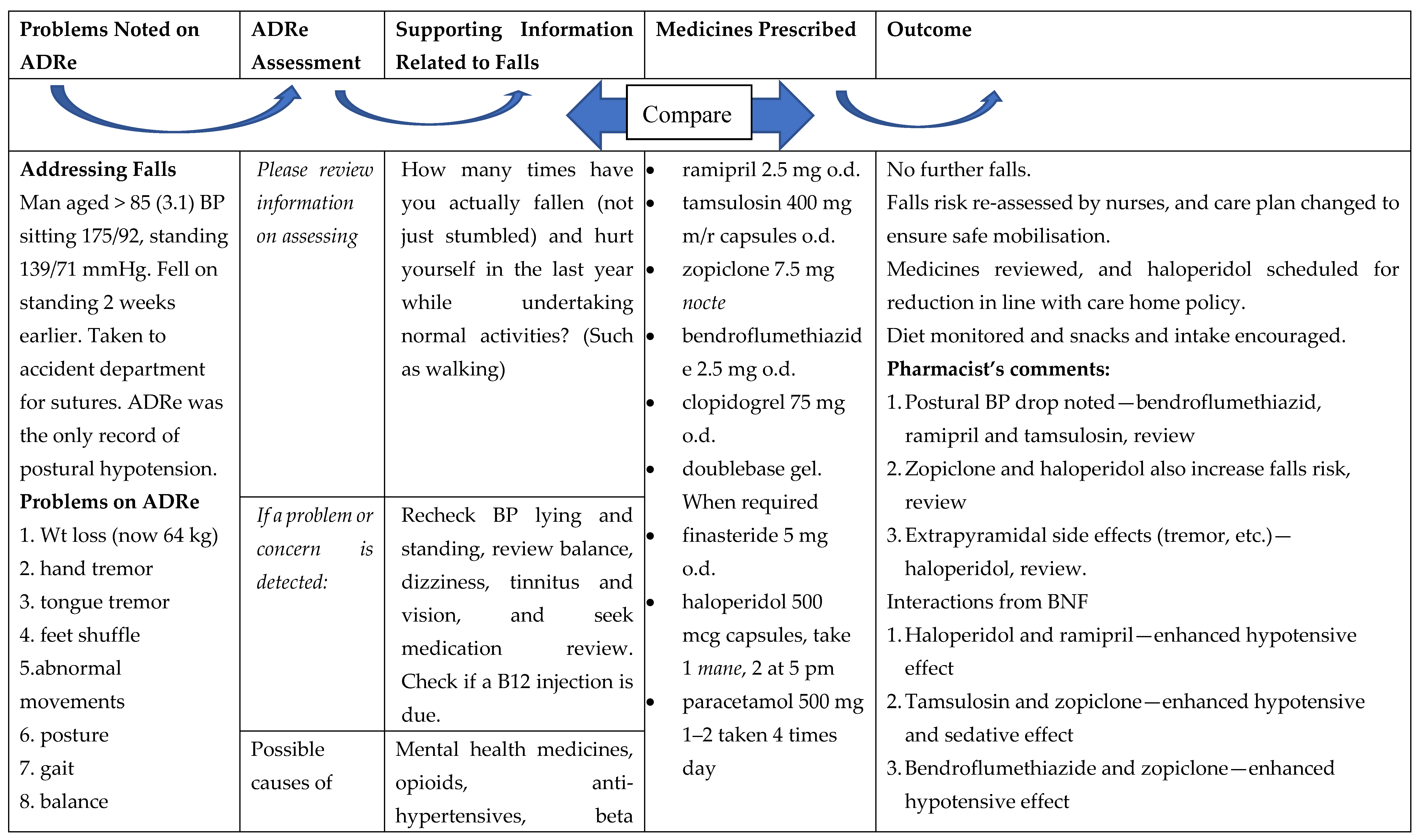
“The CPN started a resident on lurasidone and after a week, we noticed her behaviour had escalated: she was restless, constipated, dehydrated, confused, disorientated, far worse than she had been, (…) I made some notes on it [ADRe], to lead me in the right directions, really to go down the right path. (…) Rather than just, trying to put my opinion across, I had the evidence in front of me, the things to say ‘actually, it might be this medication, so can you come and review?’ The CPN might have said ‘carry on’, but she said ‘ok, fair enough’, and came back and we had a good result. It’s useful, definitely useful, but quite complex to work on. She’s still quite challenging but we haven’t got all these issues, side effects, any more.” (Care home lead nurse, N6)
“Trying to get hold of the GPs in the first place... We have no direct line of communication with them. Some surgeries are extremely difficult to get hold of to get to the actual receptionist to get request a conversation with the GPs. That is a major barrier. When you can get hold of them, they [GPs] are absolutely fine. (…) A person could be absolutely fine when the nurse comes to visit and then for the rest of the day, they could be exhibiting some sort of symptom or side effect. So if the carers were able to use this [ADRe] it would be extremely valuable (…) and if they need help, to come to us rather than the GP.” (Retail pharmacist)
7. ADRe in the Context of Regulatory and Policy Interventions
8. A Professional Blind Spot: Out of Sight, Out of Mind
9. ADRs and the Health Divide
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sampson, C.; O’Neill, P.; Lorgelly, P. The Impact of New Medicines in the NHS: 70 Years of Innovation. OHE Consulting Report. 2018. Available online: https://www.ohe.org/publications/impact-new-medicines-nhs-70-years-innovation (accessed on 25 September 2020).
- Weinshilboum, R.M. The therapeutic revolution. Clin. Pharm. Ther. 1987, 42, 481–484. [Google Scholar] [CrossRef]
- D’Arcy, P.F. Iatrogenic disease: A hazard of multiple drug therapy. R. Soc. Health J. 1976, 96, 277–283. [Google Scholar] [PubMed]
- Stolley, J.M.; Buckwalter, K.C.; Fjordbak, B.; Bush, S. Iatrogenesis in the elderly. Drug-related problems. J. Gerontol. Nurs. 1991, 17, 12–17. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Guideline on Good Pharmacovigilance (GVP)-Module VI–Collection, Management and Submission of Reports of Suspected Adverse Reactions to Medicinal Products (Rev 2). 2017, p. 6. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2017/08/WC500232767.pdf (accessed on 25 September 2020).
- Jordan, S.; Banner, T.; Gabe-Walters, M.; Mikhail, J.M.; Panes, G.; Round, J.; Snelgrove, S.; Storey, M.; Hughes, D. Nurse-led medicines’ monitoring in care homes, implementing the Adverse Drug Reaction (ADRe) Profile improvement initiative for mental health medicines: An observational and interview study. PLoS ONE 2019, 14, e0220885. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; Banner, T.; Gabe-Walters, M.; Mikhail, J.; Round, J.; Snelgrove, S.; Storey, M.; Wilson, D.W.; Hughes, D. Nurse-led medicines’ monitoring in care homes study protocol: A process evaluation of the impact and sustainability of the adverse drug reaction (ADRe) profile for mental health medicines. BMJ Open 2018, 8, e023377. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; Hughes, D. Bioscience knowledge and the health professions: Has professional monopoly created a care gap? Soc. Sci. Health 1996, 2, 80–92. [Google Scholar]
- Abrahams, J. Pharmaceuticalization of society in context: Theoretical, empirical and health dimensions. Sociology 2010, 44, 603–622. [Google Scholar] [CrossRef]
- Williams, S.J.; Martin, P.; Gabe, J. The pharmaceuticalisation of society? A framework for analysis. Sociol. Health Illn. 2011, 33, 710–725. [Google Scholar] [CrossRef]
- Bell, S.E.; Figert, A.E. Medicalization and pharmaceuticalization at the intersections: Looking backward, sideways and forward. Soc. Sci. Med. 2012, 75, 775–783. [Google Scholar] [CrossRef]
- Conrad, P. The Medicalization of Society; John Hopkins University Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Jordan, S.; Logan, P.A.; Panes, G.; Vaismoradi, M.; Hughes, D. Adverse drug reactions, power, harm reduction, regulation and the ADRe profiles. Pharmacy 2018, 6, 102. [Google Scholar] [CrossRef]
- Banerjee, S. The Use of Antipsychotic Medication for People with Dementia: Time for Action. A Report for the Minister of State for Care Services. An Independent Report Commissioned for the Department of Health. 2009. Available online: https://www.jcpmh.info/wp-content/uploads/time-for-action.pdf (accessed on 25 September 2020).
- Sherlaw-Johnson, C.; Crump, H.; Curry, N.; Paddison, C.; Meaker, R. Transforming Health Care in Nursing Homes: An Evaluation of a Dedicated Primary Care Service in Outer East London. 2018. Available online: https://www.nuffieldtrust.org.uk/research/transforming-health-care-in-nursing-homes-an-evaluation-of-a-dedicated-primary-care-service-in-outer-east-london (accessed on 25 September 2020).
- Szczepura, A.; Clay, D.; Nelson, S.; Wild, D.; Spilsbury, K. Improving Care in Residential Care Homes: A Literature Review. 2008. Available online: https://www.jrf.org.uk/report/improving-care-residential-care-homes-literature-review (accessed on 25 September 2020).
- Roberts, M.L.A.; Schiavenato, M. Othering in the nursing context: A concept analysis. Nurs. Open 2017, 4, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.C.; Del Mar, C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: A systematic review. JAMA Intern. Med. 2015, 175, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.C.; Del Mar, C. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: A systematic review. JAMA Intern. Med. 2017, 177, 407–419. [Google Scholar] [CrossRef]
- Hakkarainen, K.M.; Andersson Sundell, K.; Petzold, M.; Hägg, S. Prevalence and perceived preventability of self-reported adverse drug events—A population-based survey of 7099 adults. PLoS ONE 2013, 8, e73166. [Google Scholar] [CrossRef]
- Kanagaratnam, L.; Dramé, M.; Trenque, T.; Oubaya, N.; Nazeyrollas, P.; Novella, J.L.; Jolly, D.; Mahmoudi, R. Adverse drug reactions in elderly patients with cognitive disorders: A systematic review. Maturitas 2016, 85, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Alhawassi, T.M.; Krass, I.; Bajorek, B.V.; Pont, L.G. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin. Interv. Aging 2014, 9, 2079–2086. [Google Scholar] [CrossRef]
- Piggott, K.L.; Mehta, N.; Wong, C.L.; Rochon, P.A. Using a clinical process map to identify prescribing cascades in your patient. BMJ 2020, 368, m261. [Google Scholar] [CrossRef]
- Tangiisuran, B.; Wright, J.; Van der Cammen, T.; Rajkumar, C. Adverse drug reactions in elderly: Challenges in identification and improving preventative strategies. Age Ageing 2009, 38, 358–359. [Google Scholar] [CrossRef]
- Bouvy, J.C.; De Bruin, M.L.; Koopmanschap, M.A. Epidemiology of adverse drug reactions in Europe: A review of recent observational studies. Drug Saf. 2015, 38, 437–453. [Google Scholar] [CrossRef]
- Deilkås, E.T.; Risberg, M.B.; Haugen, M.; Lindstrøm, J.C.; Nylén, U.; Rutberg, H.; Michael, S. Exploring similarities and differences in hospital adverse event rates between Norway and Sweden using Global Trigger Tool. BMJ Open 2017, 7, e012492. [Google Scholar] [CrossRef]
- Vaismoradi, M.; Vizcaya Moreno, F.; Sletvold, H.; Jordan, S. PRN Medicines’ management for psychotropic medicines in long-term care settings: A systematic review. Pharmacy 2019, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Vaismoradi, M.; Amaniyan, S.; Jordan, S. Patient safety and pro re nata prescription and administration: A systematic review. Pharmacy 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; Gabe-Walters, M.E.; Watkins, A.; Humphreys, I.; Newson, L.; Snelgrove, S.; Dennis, M.S. Nurse-led medicines’ monitoring for patients with dementia in care homes: A pragmatic cohort stepped wedge cluster randomised trial. PLoS ONE 2015, 10, e0140203. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, J.E.; Meo, N.; Kassebaum, N.J.; Collison, M.L.; Mokdad, A.H.; Naghavi, M. Association of adverse effects of medical treatment with mortality in the United States: A secondary analysis of the global burden of diseases, injuries, and risk factors study. JAMA Netw. Open 2019, 2, e187041. [Google Scholar] [CrossRef]
- Panagioti, M.; Khan, K.; Keers, R.N.; Abuzour, A.; Phipps, D.; Kontopantelis, E.; Bower, P.; Campbell, S.; Haneef, R.; Avery, A.J.; et al. Prevalence, severity, and nature of preventable patient harm across medical care settings: Systematic review and meta-analysis. BMJ 2019, 366, l4185. [Google Scholar] [CrossRef]
- NICE Medicines and Prescribing Centre. Medicines Optimisation: The Safe and Effective Use of Medicines to Enable the Best Possible Outcomes; NICE Guideline 5; NICE. 2015. Available online: https://www.nice.org.uk/guidance/ng5/resources/medicines-optimisation-the-safe-and-effective-use-of-medicines-to-enable-the-best-possible-outcomes-pdf-51041805253 (accessed on 25 September 2020).
- Pirmohamed, M.; James, S.; Meakin, S.; Green, C.; Scott, A.K.; Walley, T.J.; Farrar, K.; Park, B.K.; Breckenridge, A.M. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ 2004, 329, 15–19. [Google Scholar] [CrossRef]
- Kongkaew, C.; Noyce, P.R.; Ashcroft, D.M. Hospital admissions associated with adverse drug reactions: A systematic review of prospective observational studies. Ann. Pharm. 2008, 42, 1017–1025. [Google Scholar] [CrossRef]
- Elliott, R.A.; Camacho, E.; Jankovic, D.; Sculpher, M.J.; Faria, R. Economic analysis of the prevalence and clinical and economic burden of medication error in England. BMJ Qual. Saf. 2020. [Google Scholar] [CrossRef]
- Frontier Economics. Exploring the Costs of Unsafe Care in the NHS: A Report Prepared for the Department of Health; Frontier Economics: London, UK, 2014; Available online: https://www.frontier-economics.com/media/2459/exploring-the-costs-of-unsafe-care-in-the-nhs-frontier-report-2-2-2-2.pdf (accessed on 25 September 2020).
- Thomas, R.E.; Nguyen, L.T.; Jackson, D.; Naugler, C. Potentially inappropriate prescribing and potential prescribing omissions in 82,935 older hospitalised adults: Association with hospital readmission and mortality within six months. Geriatrics 2020, 5, 37. [Google Scholar] [CrossRef]
- Stewart, R.; Hotopf, M.; Dewey, M.; Ballard, C.; Bisla, J.; Calem, M.; Fahmy, V.; Hockley, J.; Kinley, J.; Pearce, H.; et al. Current prevalence of dementia, depression and behavioural problems in the older adult care home sector: The South East London Care Home Survey. Age Ageing 2014, 43, 562–567. [Google Scholar] [CrossRef]
- Sköldunger, A.; Fastbom, J.; Wimo, A.; Fratiglioni, L.; Johnell, K. Impact of inappropriate drug use on hospitalizations, mortality, and costs in older persons and persons with dementia: Findings from the SNAC study. Drugs Aging 2015, 32, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Beijer, H.J.; de Blaey, C.J. Hospitalisations caused by adverse drug reactions (ADR): A meta-analysis of observational studies. Pharm World Sci. 2002, 24, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S.; Detz, A.; López, A.; Horton, C.; Sarkar, U. Signal and noise: Applying a laboratory trigger tool to identify adverse drug events among primary care patients. BMJ Qual. Saf. 2012, 21, 670–675. [Google Scholar] [CrossRef]
- Forster, A.J.; Murff, H.J.; Peterson, J.F.; Gandhi, T.K.; Bates, D.W. Adverse drug events occurring following hospital discharge. J. Gen. Intern. Med. 2005, 20, 317–323. [Google Scholar] [CrossRef]
- Gurwitz, J.H.; Field, T.S.; Judge, J.; Rochon, P.; Harrold, L.R.; Cadoret, C.; Lee, M.; White, K.; LaPrino, J.; Erramuspe-Mainard, J.; et al. The incidence of adverse drug events in two large academic long-term care facilities. Am. J. Med. 2005, 118, 251–258. [Google Scholar] [CrossRef]
- Steinman, M.A.; Handler, S.M.; Gurwitz, J.H.; Schiff, G.D.; Covinsky, K.E. Beyond the prescription: Medication monitoring and adverse drug events in older adults. J. Am. Geriatr. Soc. 2011, 59, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; Vaismoradi, M.; Griffiths, P. Adverse Drug Reactions, Nursing and policy: A narrative review. Ann. Nurs. Pract. 2016, 3, 1–7. [Google Scholar]
- Cooper, A.; Edwards, A.; Williams, H.; Evans, H.P.; Avery, A.; Hibbert, P.; Makeham, M.; Sheikh, A.; Donaldson, L.J.; Carson-Stevens, A. Sources of unsafe primary care for older adults: A mixed-methods analysis of patient safety incident reports. Age Ageing 2017, 46, 833–839. [Google Scholar] [CrossRef]
- Vaismoradi, M.; Vizcaya-Moreno, F.; Jordan, S.; Gåre Kymre, I.; Kangasniemi, M. Disclosing and reporting practice errors by nurses in residential long-term care settings: A systematic review. Sustainability 2020, 12, 2630. [Google Scholar] [CrossRef]
- Jordan, S.; Tunnicliffe, C.; Sykes, A. Minimizing side-effects: The clinical impact of nurse-administered ‘side-effect’ checklists. J. Adv. Nurs. 2002, 37, 155–165. [Google Scholar] [CrossRef]
- Gabe, M.E.; Davies, G.A.; Murphy, F.; Davies, M.; Johnstone, L.; Jordan, S. Adverse drug reactions: Treatment burdens and nurse-led medication monitoring. J. Nurs. Manag. 2011, 19, 377–392. [Google Scholar] [CrossRef]
- Jones, R.; Moyle, C.; Jordan, S. Nurse-led medicines monitoring: A study examining the effects of the West Wales Adverse Drug Reaction Profile. Nurs. Stand. 2016, 31, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Hazell, L.; Shakir, S.A. Under-reporting of adverse drug reactions: A systematic review. Drug Saf. 2006, 29, 385–396. [Google Scholar] [CrossRef] [PubMed]
- De Boissieu, P.; Kanagaratnam, L.; Abou Taam, M.; Roux, M.P.; Dramé, M.; Trenque, T. Notoriety bias in a database of spontaneous reports: The example of osteonecrosis of the jaw under bisphosphonate therapy in the French national pharmacovigilance database. Pharm. Drug Saf. 2014, 23, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Pariente, A.; Gregoire, F.; Fourrier-Reglat, A.; Haramburu, F.; Moore, N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: The notoriety bias. Drug Saf. 2007, 30, 891–898. [Google Scholar] [CrossRef]
- Older People’s Commissioner for Wales. A Place to Call Home. 2014. Available online: https://www.olderpeoplewales.com/Libraries/Uploads/A_Place_to_Call_Home_-_A_Review_into_the_Quality_of_Life_and_Care_of_Older_People_living_in_Care_Homes_in_Wales.sflb.ashx (accessed on 25 September 2020).
- Older People’s Commissioner for Wales. A Place to Call Home: Impact and Analysis. 2018. Available online: http://www.olderpeoplewales.com/Libraries/chrfollowupreport/A_Place_to_Call_Home_-_Impact_Analysis.sflb.ashx (accessed on 25 September 2020).
- Finnegan, G. HTA Bodies and Regulators Have a Shared Interest in Understanding Patient Experience, Patient Focused Medicines Development. 2020. Available online: https://patientfocusedmedicine.org/hta-bodies-and-regulators-have-a-shared-interest-in-understanding-patient-experiences/ (accessed on 25 September 2020).
- Jensen, J.D.; King, A.J.; Guntzviller, L.M.; Davis, L.A. Patient-provider communication and low-income adults: Age, race, literacy, and optimism predict communication satisfaction. Patient Educ. Couns 2010, 79, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Verlinde, E.; De Laender, N.; De Maesschalck, S.; Deveugele, M.; Willems, S. The social gradient in doctor-patient communication. Int. J. Equity Health 2012, 11, 12. [Google Scholar] [CrossRef]
- Willems, S.; De Maesschalck, S.; Deveugele, M.; Derese, A.; De Maeseneer, J. Socio-economic status of the patient and doctor-patient communication: Does it make a difference? Patient Educ. Couns 2005, 56, 139–146. [Google Scholar] [CrossRef]
- Beisecker, A.E. Older persons’ medical encounters and their outcomes. Res. Aging 1996, 18, 9–31. [Google Scholar] [CrossRef]
- Macrae, H. “My opinion is that doctors prefer younger people”: Older women, physicians and ageism. Ageing Soc. 2018, 38, 240–266. [Google Scholar] [CrossRef]
- Peck, B.M. Age-related differences in doctor-patient interaction and patient satisfaction. Curr. Gerontol. Geriatr. Res. 2011, 2011, 137492. [Google Scholar] [CrossRef] [PubMed]
- Tudor Hart, J. The inverse care law. Lancet 1971, 297, 405–412. [Google Scholar] [CrossRef]
- Pedersen, A.F.; Vedsted, P. Understanding the inverse care law: A register and survey-based study of patient deprivation and burnout in general practice. Int. J. Equity Health 2014, 13, 121. [Google Scholar] [CrossRef]
- Marston, L.; Nazareth, I.; Petersen, I.; Walters, K.; Osborn, D.P.J. Prescribing of antipsychotics in UK primary care: A cohort study. BMJ Open 2014, 4, e006135. [Google Scholar] [CrossRef]
- Guthrie, B.; Makubate, B.; Hernandez-Santiago, V.; Dreischulte, T. The rising tide of polypharmacy and drug-drug interactions: Population database analysis 1995–2010. BMC Med. 2015, 13, 74. [Google Scholar] [CrossRef]
- Welsh Government. Chief Medical Officer for Wales Annual Report 2015–2016. 2016. Available online: https://www.childreninwales.org.uk/policy-document/chief-medical-officer-wales-annual-report-2015-2016-111116-w/ (accessed on 25 September 2020).
- Ryan, A.M.; Krinsky, S.; Kontopantelis, E.; Doran, T. Long-term evidence for the effect of pay-for-performance in primary care on mortality in the UK: A population study. Lancet 2016, 388, 268–274. [Google Scholar] [CrossRef]
- Department of Health. Meeting the Challenge: A Strategy for the Allied Health Professions. 2000. Available online: http://www.nursingleadership.org.uk/publications/meetingthechallenge.pdf (accessed on 25 September 2020).
- Drennan, V.M.; Grant, R.L.; Harris, R. Trends over time in prescribing by English primary care nurses: A secondary analysis of a national prescription database. BMC Health Serv. Res. 2014, 14, 54. [Google Scholar] [CrossRef]
- Lesho, E.P.; Myers, C.P.; Ott, M.; Winslow, C.; Brown, J.E. Do clinical practice guidelines improve processes or outcomes in primary care? Mil. Med. 2005, 170, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, J.; Glasziou, P.; Westbrook, J. The three numbers you need to know about healthcare: The 60-30-10 Challenge. BMC Med. 2020, 18, 102. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, D.; Jordan, M.; Logan, P.A.; Willson, A.; Snelgrove, S.; Storey, M.; Vaismoradi, M.; Jordan, S. Looking for the “Little Things”: A Multi-Disciplinary Approach to Medicines Monitoring for Older People Using the ADRe Resource. Geriatrics 2020, 5, 79. https://doi.org/10.3390/geriatrics5040079
Hughes D, Jordan M, Logan PA, Willson A, Snelgrove S, Storey M, Vaismoradi M, Jordan S. Looking for the “Little Things”: A Multi-Disciplinary Approach to Medicines Monitoring for Older People Using the ADRe Resource. Geriatrics. 2020; 5(4):79. https://doi.org/10.3390/geriatrics5040079
Chicago/Turabian StyleHughes, David, Meirion Jordan, Patricia A. Logan, Alan Willson, Sherrill Snelgrove, Melanie Storey, Mojtaba Vaismoradi, and Sue Jordan. 2020. "Looking for the “Little Things”: A Multi-Disciplinary Approach to Medicines Monitoring for Older People Using the ADRe Resource" Geriatrics 5, no. 4: 79. https://doi.org/10.3390/geriatrics5040079
APA StyleHughes, D., Jordan, M., Logan, P. A., Willson, A., Snelgrove, S., Storey, M., Vaismoradi, M., & Jordan, S. (2020). Looking for the “Little Things”: A Multi-Disciplinary Approach to Medicines Monitoring for Older People Using the ADRe Resource. Geriatrics, 5(4), 79. https://doi.org/10.3390/geriatrics5040079







