Feasibility of Using Strength Measures, Including Peak Inspiratory Flow, for Routine Monitoring in Case Management Patients Aged 65 and over
Abstract
1. Introduction
2. Methods
3. Results
3.1. Participant Characteristics
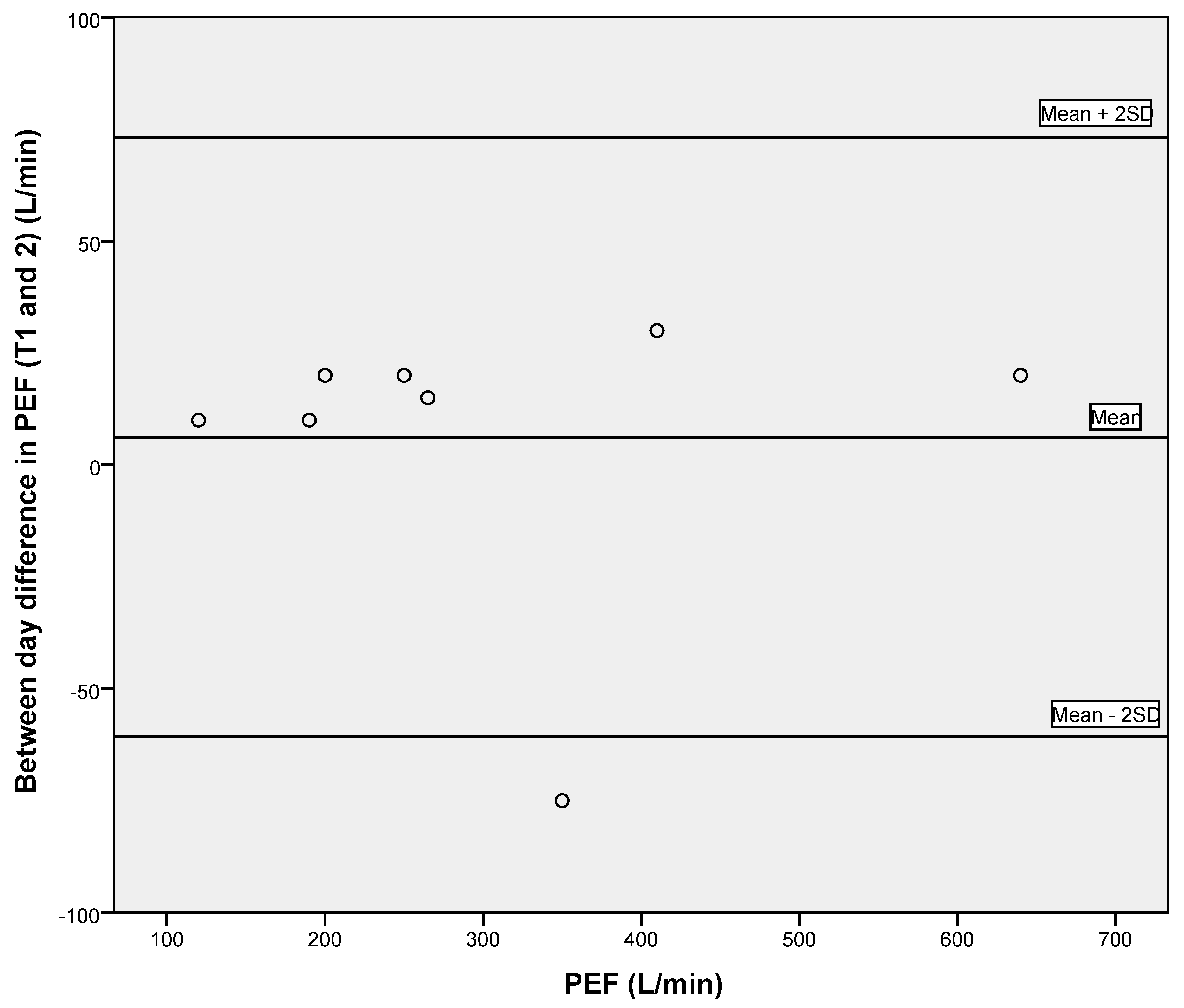
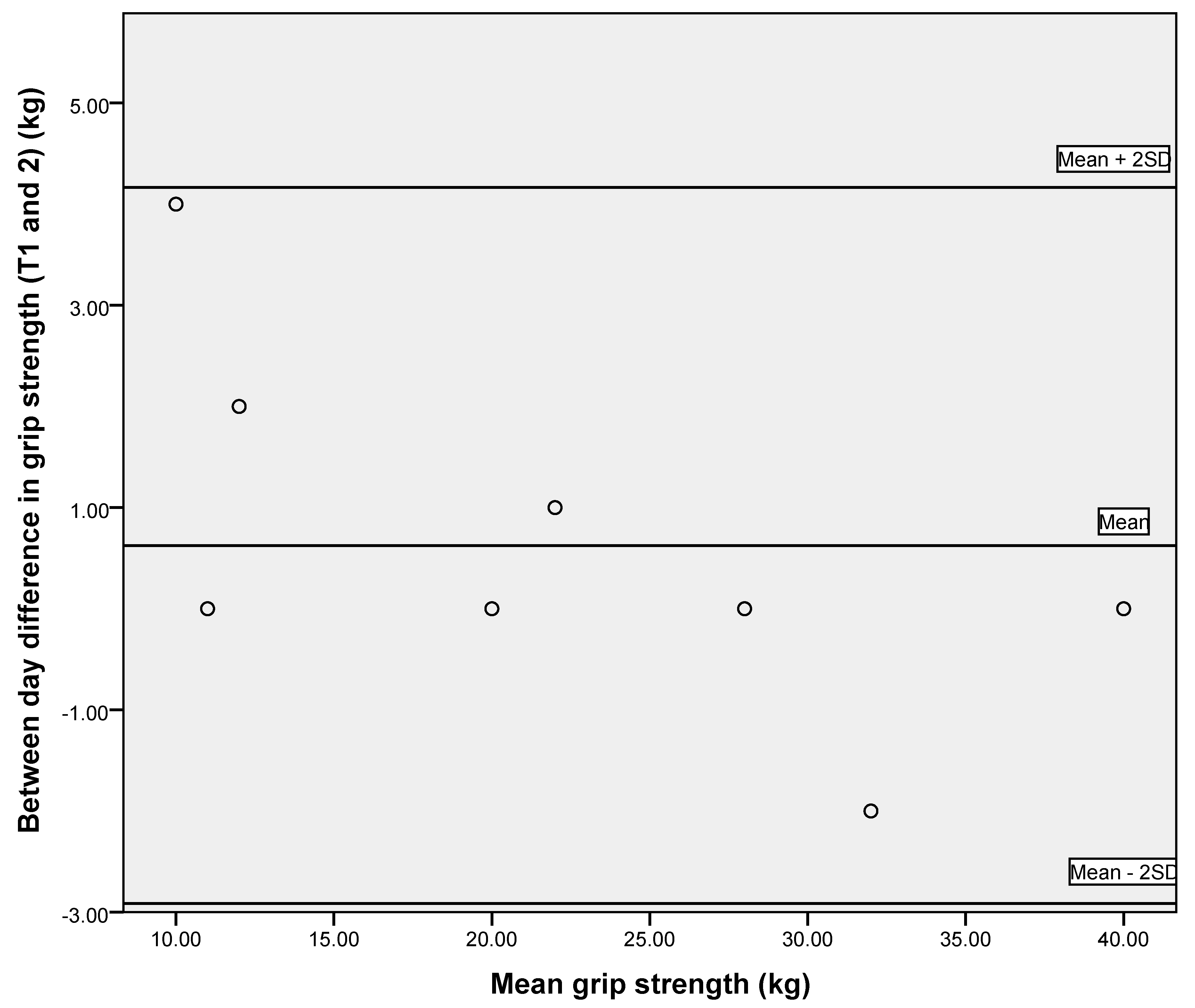
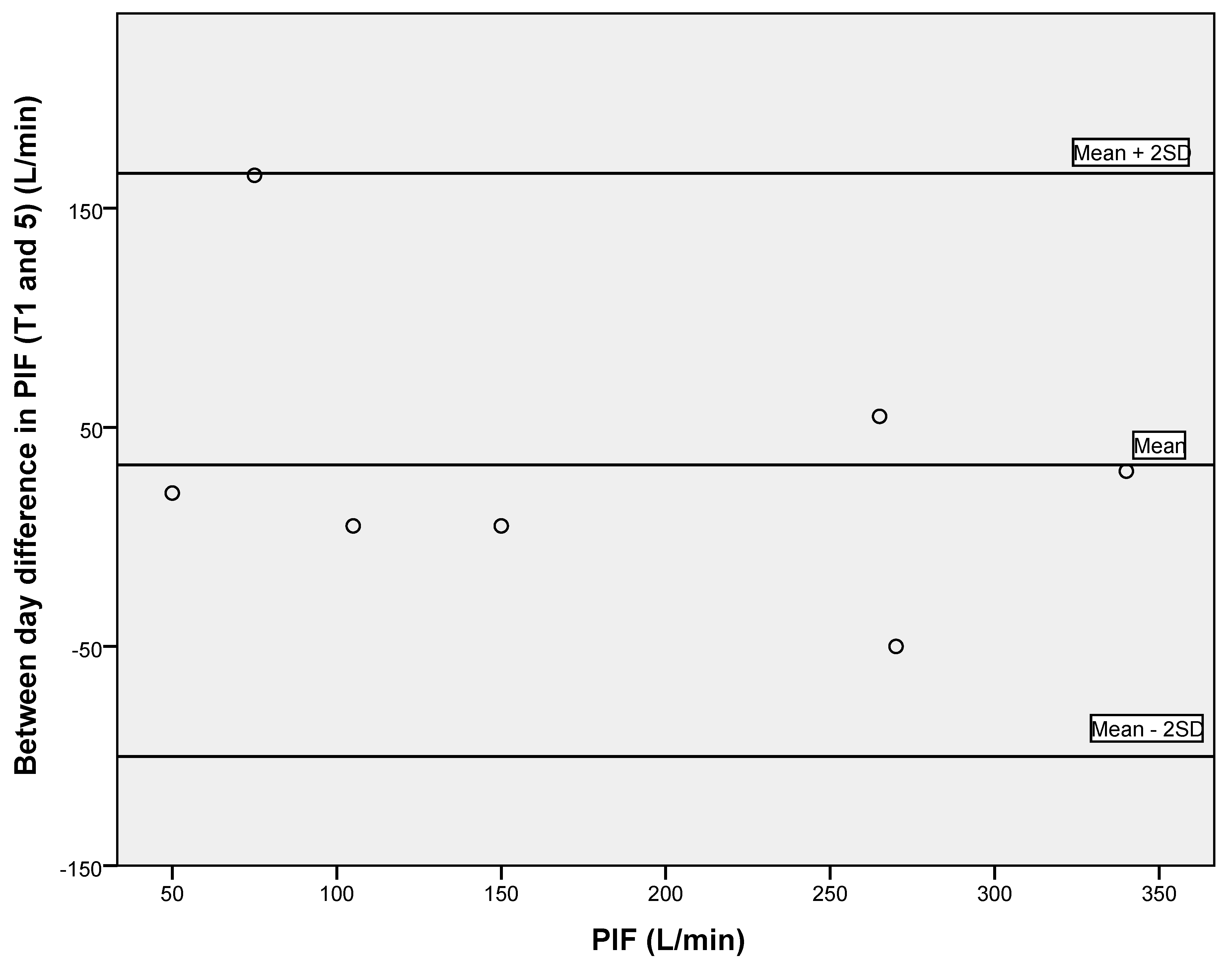
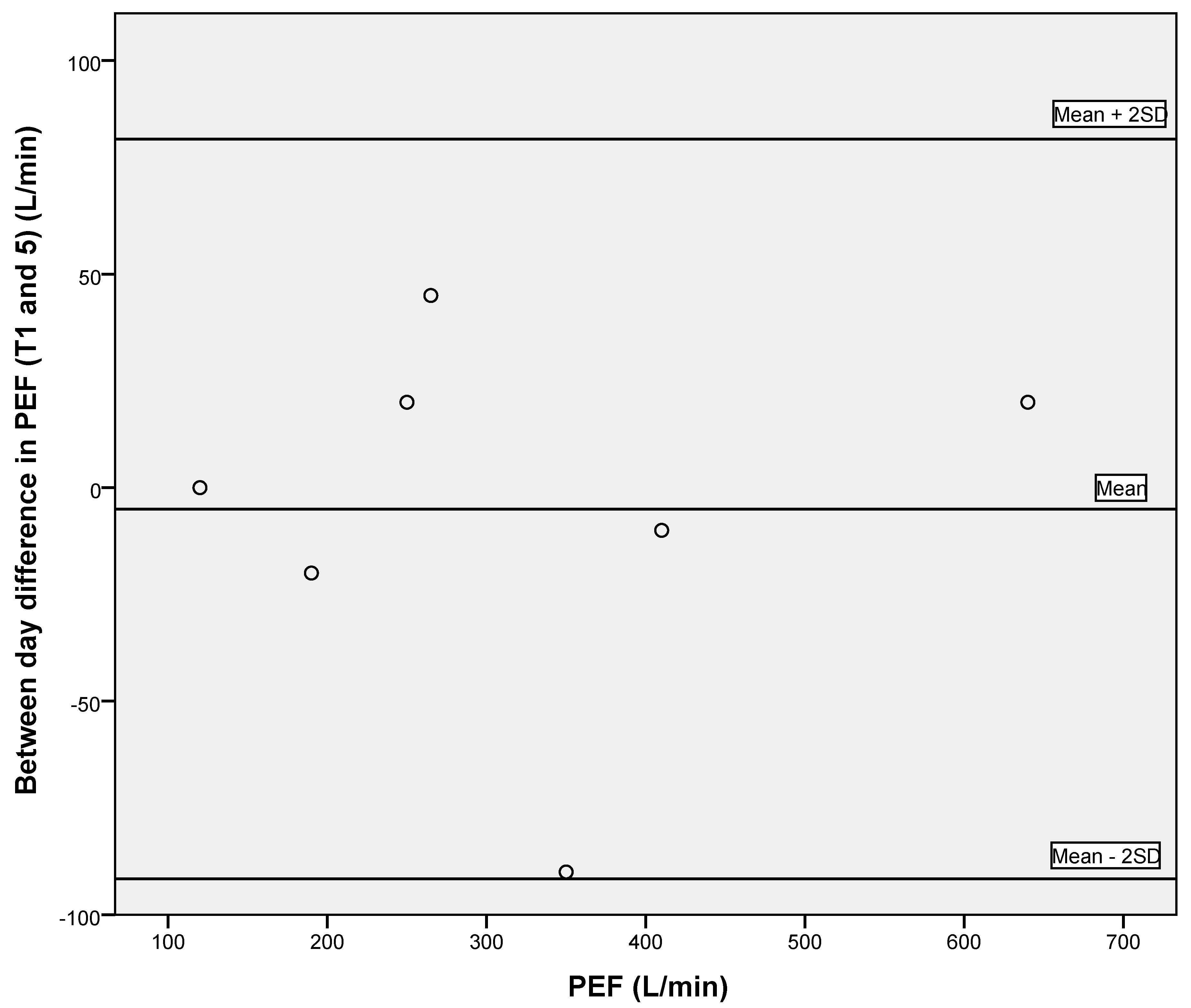
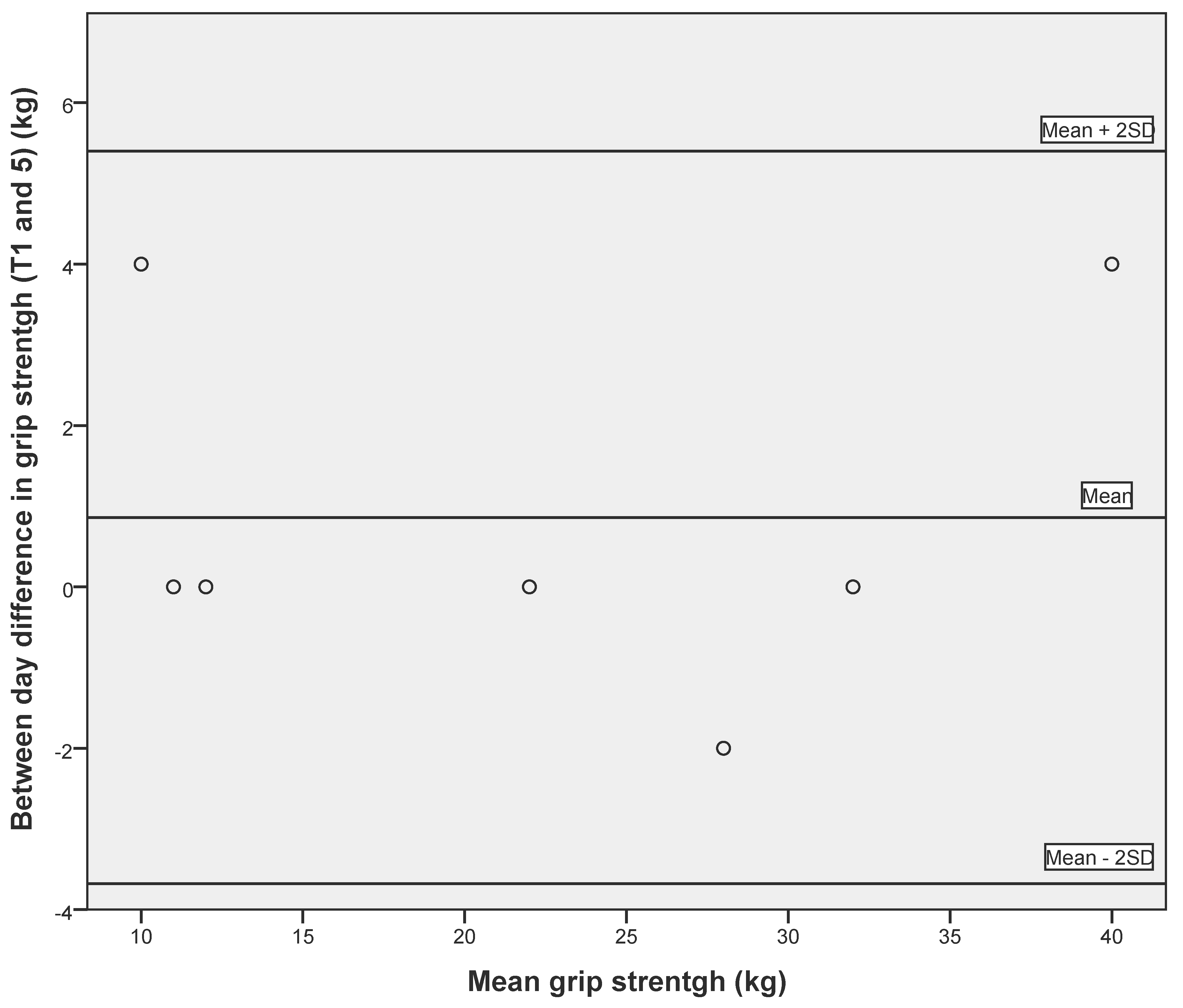
3.2. Reliability of Respiratory Measures and Grip Strength
3.3. Acceptability of Measures of Strength
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LTCs | Long term conditions |
| PEF | peak expiratory flow |
| PIF | peak inspiratory flow |
| COPD | chronic obstructive pulmonary disease |
| PASE | Physical Activity Scale for the Elderly |
| BI | Barthel Index |
| VES | Vulnerable Elders Scale |
| ICC | intra-class correlation coefficient |
| SEM | standard error of measurement |
| MDC | minimal detectable change |
| T | time point |
References
- Bassey, E.J. Longitudinal changes in selected physical capabilities: Muscle strength, flexibility and body size. Age Ageing 1998, 27, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence of phenotype. J. Gerontol. 2001, 56, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Arnold, A.M.; Sachs, M.C.; Ives, D.G.; Cushman, M.; Strotmeyer, E.S.; Ding, J.; Kritchevsky, S.B.; Chaves, P.H.M.; Fried, L.P.; et al. Long-term function in an older cohort—The Cardiovascular Health Study All Stars Study. J. Am. Geriatr. Soc. 2009, 57, 432–440. [Google Scholar]
- Esteban, C.; Quintana, J.M.; Aburto, M.; Moraza, J.; Eggurola, M.; Perez-Izquierdo, J.; Aizpiri, S.; Aguirre, U.; Capelastegui, A. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur. Respir. J. 2010, 36, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Letts, L.; Chan, D.; Officer, A.; Wojkowski, S.; Oliver, D.; Moore, A.; McCarthy, L.; Price, D.; Kinzie, S. Monitoring physical functioning as the sixth vital sign: Evaluating patient and practice engagement in chronic illness care in a primary care setting—A quasi-experimental design. BMC Fam. Pract. 2012, 13, 29–41. [Google Scholar]
- Hunt, K.J.; Walsh, B.M.; Voegeli, D.; Roberts, H. Inflammation in Aging Part 2: Implications for the Health of Older People and Recommendations for Nursing Practice. Biol. Res. Nurs. 2009, 11, 253–260. [Google Scholar] [CrossRef]
- Challis, D.; Hughes, J.; Berzins, K.; Reilly, S.; Abell, J.; Stewart, K. Self-Care and Case Management in Long-Term Conditions: The Effective Management of Critical Interfaces; NIHR Service Delivery and Organisation Programme, SDO Project 08/1715/201; NETSCC: Southampton, UK, 2010; 257p. [Google Scholar]
- Skelton, D.A.; Greig, C.A.; Davies, J.M.; Young, A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing 1994, 23, 371–377. [Google Scholar] [CrossRef]
- Forrest, K.Y.Z.; Zmuda, J.M.; Cauley, J.A. Patterns and correlates of muscle strength loss in older women. Gerontology 2007, 53, 140–147. [Google Scholar] [CrossRef]
- Visser, M.; Deeg, D.J.H.; Lips, P.; Harris, T.B.; Bouter, L.M. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J. Am. Geriatr. Soc. 2000, 48, 381–386. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchesvsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the Health, Aging and Body Composition Study Cohort. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef]
- Dourado, V.Z.; de Oliveira Antunes, L.C.; Tanni, S.E.; de Paiva, S.A.R.; Padovani, C.R.; Godoy, R. Relationship of upper-limb and thoracic muscle strength to 6-min walk distance in COPD patients. Chest 2006, 129, 551–557. [Google Scholar] [CrossRef]
- Sayer, A.A.; Syddall, H.E.; Martin, H.J.; Dennison, E.M.; Roberts, H.C.; Cooper, C. Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing 2006, 35, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.-I.; Beamer, B.A.; Chaves, P.H.M.; Guralnik, J.M.; Fried, L.P. Heterogenicity in rate of decline in grip, hip, and knee strength and the risk of all-cause mortality: The women’s health and aging study II. J. Am. Geriatr. Soc. 2010, 58, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscle and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Hairi, N.N.; Cumming, R.G.; Naganathan, V.; Handelsman, D.J.; Le Couteur, D.G.; Creasey, H.; Waite, L.M.; Seibel, M.J.; Sambrook, P.N. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: The Concord health and aging in men project. J. Am. Geriatr. Soc. 2010, 58, 2055–2062. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Fox, K.M.; Gandra, S.R.; Delmonico, M.J.; Chiou, C.F.; Anthony, M.S.; Sewall, A.; Goodpaster, B.; Satterfield, S.; Cummings, S.R.; et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J. Am. Geriatr. Soc. 2009, 57, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Kuh, D.; Cooper, C.; Gale, C.R.; Lawlor, D.A.; Matthews, F.; Hardy, R.; FALcon and HAYcon Study Teams. Objective measures of physical capability and subsequent health: A systematic review. Age Ageing 2011, 40, 14–23. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Stevens, P.; Syddall, H.E.; Patel, H.P.; Martin, H.J.; Cooper, D.; Sayer, A.A. Is grip strength a good marker of physical performance among community-dwelling older people? J. Nutr. Health Aging 2012, 16, 769–774. [Google Scholar] [CrossRef]
- Watsford, M.L.; Murphy, A.J.; Pine, M.J. The effects of ageing on respiratory muscle function and performance in older adults. J. Sci. Med. Sport 2007, 10, 36–44. [Google Scholar] [CrossRef]
- Buchman, A.S.; Boyle, P.A.; Leurgans, S.E. Pulmonary Function, Muscle Strength, and Incident Mobility Disability in Elders. Proc. Am. Thorac. Soc. 2009, 6, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Evans, D.A.; Scherr, P.A.; Speizer, F.E.; Taylor, J.O.; Hennekens, C.H. Peak expiratory flow and 5-year mortality in an elderly population. Am. J. Epidemiol. 1991, 133, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Vaz Fragoso, C.A.; Gahbauer, E.A.; Van Ness, P.H.; Gill, T.M. Reporting peak expiratory flow in older persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.; Agyapong-Badu, S.; Walsh, B.; Stokes, M.; Samuel, D. Reliability and acceptability of measuring sniff nasal inspiratory pressure (SNIP) and peak inspiratory flow (PIF) to assess respiratory muscle strength in older adults: A preliminary study. Aging Clin. Exp. Res. 2014, 26, 171–176. [Google Scholar] [CrossRef]
- Vaswani, R.; Moy, R.; Vaswani, S.K. Evaluation of factors affecting peak expiratory flow in healthy adults: Is it necessary to stand up? J. Asthma 2005, 42, 793–794. [Google Scholar] [CrossRef]
- McCoy, E.K.; Thomas, J.L.; Sowell, R.S.; George, C.; Finch, C.K.; Tolley, E.A.; Self, T.H. An evaluation of peak expiratory flow monitoring: A comparison of sitting versus standing measurements. J. Am. Board Fam. Med. 2010, 23, 166–170. [Google Scholar] [CrossRef]
- Washburn, R.A.; Smith, K.W.; Jette, A.M.; Janney, C.A. The Physical Activity Scale for the Elderly (PASE): Development and Evaluation. J. Clin. Epidemiol. 1993, 46, 153–162. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index: A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md. State Med. J. 1965, 14, 56–61. [Google Scholar]
- Saliba, D.; Elliott, M.M.; Rubenstein, L.Z.; Solomon, D.H.; Young, R.T.; Kamberg, C.J.; Roth, C.; MacLean, C.H.; Shekelle, P.G.; Sloss, E.M.; et al. The Vulnerable Elders Survey: A Tool for Identifying Vulnerable Older People in the Community. J. Am. Geriatr. Soc. 2001, 49, 1691–1699. [Google Scholar] [CrossRef]
- Gupta, A. Barthel Index. In Measurement Scales Used in Elderly Care; Radcliffe Publishing Ltd.: Abingdon, UK, 2008; pp. 42–49. [Google Scholar]
- Rockwood, K.; Mitnitski, A. Frailty in Relation to the Accumulation of Deficits. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef]
- Depledge, M.H. Peak inspiratory flow: Measurement using a modified mini Wright peak flow meter. Thorax 1985, 40, 205–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mathiowetz, V.; Kashman, N.; Volland, G.; Weber, K.; Dowe, M.; Rogers, S. Grip and pinch strength: Normative data for adults. Arch. Phys. Med. Rehabil. 1985, 66, 69–74. [Google Scholar] [PubMed]
- Nunn, A.J.; Gregg, I. New regression equations for predicting peak expiratory flow in adults. Br. Med. J. 1989, 298, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice; Prentice Hall: Upper Saddle River, NJ, USA, 2000. [Google Scholar]
- Innes, E. Handgrip strength testing: A review of the literature. Aust. Occup. Ther. J. 1999, 46, 120–140. [Google Scholar] [CrossRef]
- Fonseca, J.; Costa-Pereira, A.; Delgado, L.; Nogueria-Silva, L.; Ferreria-Magalhaes, M.; Castel-Branco, M.G.; Vaz, M.G. Pulmonary function electronic monitoring devices. Chest 2005, 128, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Abizanda, P.; Navarro, J.L.; García-Tomás, M.I.; López-Jiménez, E.; Martínez-Sánchez, E.; Paterna, G. Validity and usefulness of hand-held dynamometry for measuring muscle strength in community-dwelling older persons. Arch. Gerontol. Geriatr. 2012, 54, 21–27. [Google Scholar] [CrossRef]
- McMurdo, M.E.T.; Roberts, H.; Parker, S.; Wyatt, N.; May, H.; Goodman, C.; Jackson, S.; Gladman, J.; O’Mahony, S.; Ali, K.; et al. Improving recruitment of older people to research through good practice. Age Ageing 2011, 40, 659–665. [Google Scholar] [CrossRef]

| Characteristics | Males (n = 5) Mean ± Standard Deviation (SD) | Females (n = 3) Mean ± SD |
|---|---|---|
| Age (years) | 82.8 ± 8.8 | 79.3 ± 6.4 |
| BMI (kg/m2) | 27.3 ± 1.8 | 29.2 ± 4 |
| No. of reported regular prescribed medication | 4.4 ± 2.9 | 5.7 ± 3.9 |
| No. of reported diagnosed LTCs | 2.2 ± 1.2 | 2.3 ± 1.9 |
| Grip strength (kg) | 29.2 ± 11.5 | 16.7 ± 3.1 |
| PEF (L/min) | 410.0 ± 163.6 | 188.3 ± 46.5 |
| PIF (L/min) | 283.0 ± 76.9 | 116.7 ± 35.1 |
| BI | 89.5 ± 18.5 | 64.2 ± 34.3 |
| VES-13 | 5.8 ± 3.6 | 6.2 ± 1.2 |
| PASE | 81.3 ± 57.7 | 33.5 ± 4.4 |
| Measures | Mean ± SD | ICC (95%Confidence Interval (CI)) | SEM | MDC |
|---|---|---|---|---|
| Grip strength (kg) | 22.4 ± 10.6 | 0.991 (0.954–0.998) | 1.01 | 2.80 |
| PEF (L/min) | 306.3 ± 165.6 | 0.980 (0.902–0.996) | 23.42 | 64.92 |
| PIF (L/min) | 180.3 ± 102.8 | 0.967 (0.847–0.993) | 18.67 | 51.75 |
| Measures | Mean ± SD | ICC (95%CI) | SEM | MDD |
|---|---|---|---|---|
| Grip strength (kg) | 23.2 ± 10.7 | 0.988 (0.963–0.998) | 1.17 | 3.24 |
| PEF (L/min) | 313.0 ± 164.4 | 0.988 (0.964–0.998) | 18.01 | 49.92 |
| PIF (L/min) | 194.7 ± 100.0 | 0.923 (0.794–0.984) | 27.75 | 76.92 |
| Questions 1–4 Likert Scale 1–5, Where 1 Is Strongly Disagree, to 5 Strongly Agree (Mean Score) | PEF | PIF | Grip Strength |
|---|---|---|---|
| 1. “It was easy to understand what I had to do” | 4.0 | 3.9 | 5.0 |
| 2. “It was easy to do” | 3.9 | 3.6 | 4.5 |
| 3. “It was comfortable to do” | 4.9 | 5.0 | 4.4 |
| 4. “I would recommend the test to anyone” | 5.0 | 5.0 | 5.0 |
| 5. Tests’ ranking in order of preference (1 to 3, where 1 is most preferred to 3 is least preferred): | |||
| Mean | 1.8 | 1.9 | 1.8 |
| Median | 2 | 2 | 1 |
| Mode | 2 | 2 | 1 |
| Time taken, range in minutes, to complete 3 repetitions for PEF, PIF and grip strength | 0–6 | 0–4 | 0–5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnes, N.; Walsh, B.; Samuel, D. Feasibility of Using Strength Measures, Including Peak Inspiratory Flow, for Routine Monitoring in Case Management Patients Aged 65 and over. Geriatrics 2020, 5, 59. https://doi.org/10.3390/geriatrics5030059
Barnes N, Walsh B, Samuel D. Feasibility of Using Strength Measures, Including Peak Inspiratory Flow, for Routine Monitoring in Case Management Patients Aged 65 and over. Geriatrics. 2020; 5(3):59. https://doi.org/10.3390/geriatrics5030059
Chicago/Turabian StyleBarnes, Nicola, Bronagh Walsh, and Dinesh Samuel. 2020. "Feasibility of Using Strength Measures, Including Peak Inspiratory Flow, for Routine Monitoring in Case Management Patients Aged 65 and over" Geriatrics 5, no. 3: 59. https://doi.org/10.3390/geriatrics5030059
APA StyleBarnes, N., Walsh, B., & Samuel, D. (2020). Feasibility of Using Strength Measures, Including Peak Inspiratory Flow, for Routine Monitoring in Case Management Patients Aged 65 and over. Geriatrics, 5(3), 59. https://doi.org/10.3390/geriatrics5030059






