Formula for the Cross-Sectional Area of the Muscles of the Third Lumbar Vertebra Level from the Twelfth Thoracic Vertebra Level Slice on Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Variables
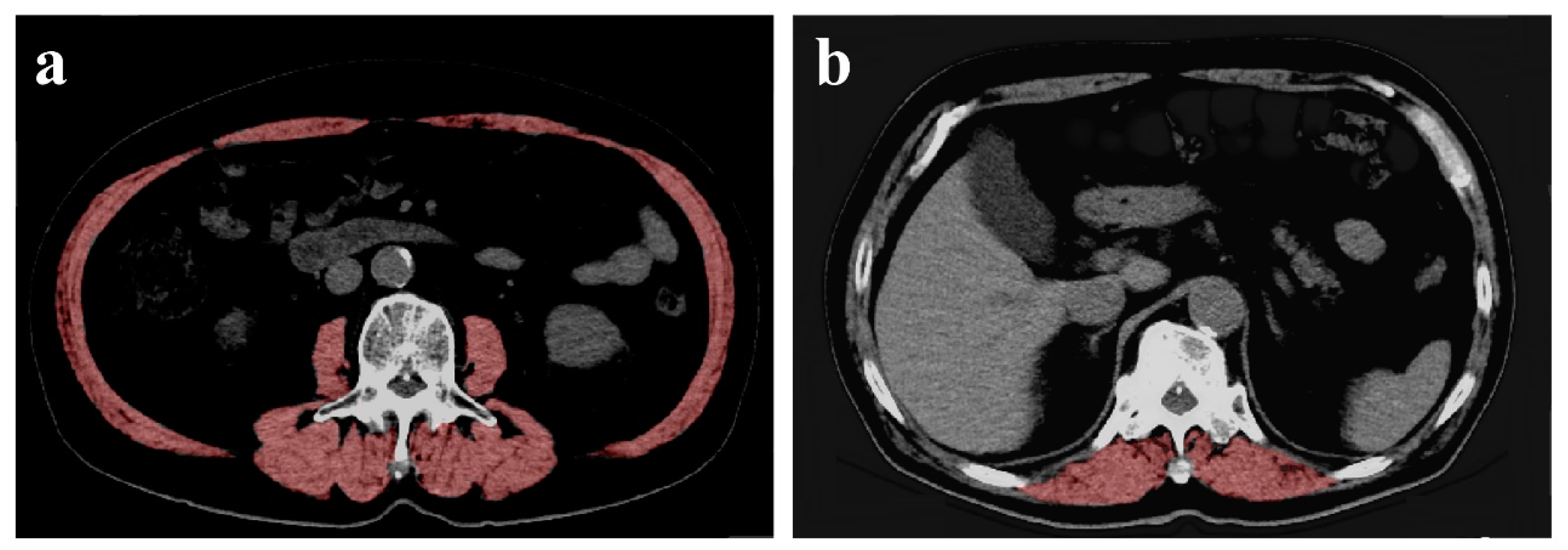
2.3. Measurement of CSA of the Muscles
2.4. Skeletal Muscle Mass Index
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Akagi, J. Muscle Mass Loss Is a Potential Predictor of 90-Day Mortality in Older Adults with Aspiration Pneumonia. J. Am. Geriatr. Soc. 2017, 65, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Adamek, M.; Gonzalez, M.C.; Jia, G.; Thomas, D.M. Assessing skeletal muscle mass: Historical overview and state of the art. J. Cachexia Sarcopenia Muscle 2014, 5, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Janssen, I.; Heymsfield, S.B.; Ross, R. Relation between whole-body and regional measures of human skeletal muscle. Am. J. Clin. Nutr. 2004, 80, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.J.; Lai, J.C.; Wang, C.W.; Dasarathy, S.; Lobach, I.; Montano-Loza, A.J.; Dunn, M.A.; Fitness, Life Enhancement; Exercise in Liver Transplantation Consortium. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017, 23, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Meza-Junco, J.; Prado, C.M.; Tandon, P.; Bain, V.G.; Ma, M.; Beaumont, C.; Esfandiari, N.; Sawyer, M.B.; Baracos, V.E. New cutoff values for sarcopenia for predicting 6-month mortality in cirrhotic patients. J. Hepatol. 2013, 58. [Google Scholar] [CrossRef]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. 2014, 33, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet. Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Sun, G.; Li, Y.; Peng, Y.; Lu, D.; Zhang, F.; Cui, X.; Zhang, Q.; Li, Z. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int. J. Colorectal Dis. 2018, 33, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Harker, J.O.; Salva, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Teigen, L.M.; Kuchnia, A.J.; Nagel, E.; Deuth, C.; Vock, D.M.; Mulasi, U.; Earthman, C.P. Impact of Software Selection and ImageJ Tutorial Corrigendum on Skeletal Muscle Measures at the Third Lumbar Vertebra on Computed Tomography Scans in Clinical Populations. Jpn. J. Parenter Enter. Nutr. 2018, 42, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.E.; Pothen, A.J.; Wegner, I.; Smid, E.J.; Swart, K.M.; de Bree, R.; Leenen, L.P.; Grolman, W. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016, 62, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, J.M.; Ansari, A.Z.; D’Souza, D.M.; Moffatt-Bruce, S.D.; Merritt, R.E.; Kneuertz, P.J. Computed Tomography-Assessed Skeletal Muscle Mass as a Predictor of Outcomes in Lung Cancer Surgery. Ann. Thorac. Surg. 2019, 108, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Hu, J. A K-fold Averaging Cross-validation Procedure. J. Nonparametr. Stat. 2015, 27, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.R.; Roh, J.L.; Kim, J.S.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Efficacy of head and neck computed tomography for skeletal muscle mass estimation in patients with head and neck cancer. Oral Oncol. 2019, 95, 95–99. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, A.; Langius, J.A.E.; de van der Schueren, M.A.E.; Nurmohamed, S.A.; van der Pant, K.; Blauwhoff-Buskermolen, S.; Wierdsma, N.J. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur. J. Clin. Nutr. 2018, 72, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 2018, 8, 11369. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 161) | |
|---|---|
| Age, year | 76.9 ± 6.5 |
| Sex, male (%) | 105 (65.2) |
| Female (%) | 56 (34.8) |
| Weight, kg | 57.0 ± 11.6 |
| Height, cm | 159.8 ± 9.0 |
| Body mass index, kg/m2 | 22.1 ± 3.7 |
| CSA of the muscles mass at L3, cm2 | 94.4 ± 27.3 |
| CSA of the muscles mass at Th12, cm2 | 24.3 ± 6.9 |
| SMI, cm2/m2 | 37.4 ± 8.7 |
| MNA-SF, score | 12 [10–13] |
| Disease related to hospitalization, n (%) | |
| Digestive disease | 71 (44.1) |
| Neoplasms | 40 (24.8) |
| Circulatory system disease | 25 (15.5) |
| Injury | 6 (3.7) |
| Genitourinary system disease | 5 (3.1) |
| Others | 14 (8.7) |
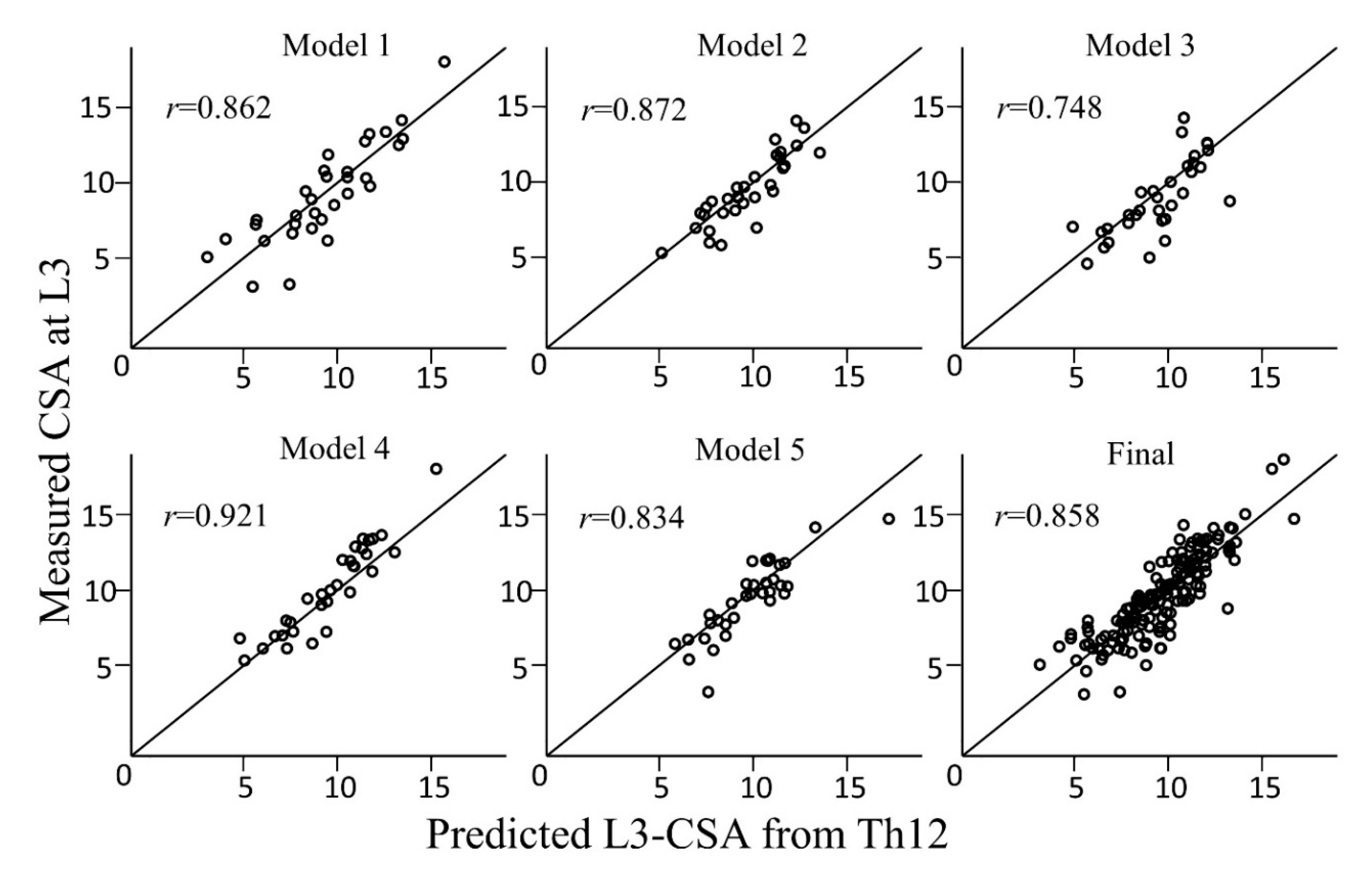
| Preliminary Models | Intercept | Age | Sex (male = 1, female = 0) | Weight | CSA of the Muscles at Th12 | Spearman’s Rank Correlation Coefficient (r) | ICC |
|---|---|---|---|---|---|---|---|
| 1 | −1443.30 | 21.16 | 948.92 | 50.60 | 2.43 | 0.862 [0.733–0.931] | 0.859 [0.732–0.928] |
| 2 | 58.78 | 5.68 | 1352.35 | 55.31 | 2.10 | 0.872 [0.752–0.936] | 0.863 [0.740–0.931] |
| 3 | −1139.18 | 20.93 | 1284.43 | 48.98 | 2.29 | 0.748 [0.540–0.870] | 0.724 [0.509–0.855] |
| 4 | −1707.10 | 20.64 | 1073.13 | 65.51 | 2.12 | 0.921 [0.844–0.961] | 0.897 [0.802–0.948] |
| 5 | −801.11 | 13.05 | 1150.18 | 59.13 | 2.16 | 0.834 [0.688–0.915] | 0.823 [0.673–0.908] |
| Average | −1006.38 | 16.29 | 1161.80 | 55.91 | 2.22 | 0.858 [0.811–0.894] | 0.849 [0.800–0.887] |
| Reported Cutoff | Examined Period | Sensitivity/ | Positive Predictive Value/ | Accuracy | F-Value |
|---|---|---|---|---|---|
| Specificity | Negative Predictive Value | ||||
| Carey et al. | Development | 0.979 [0.939–0.996]/ | 0.926 [0.871–0.962]/ | 0.913 [0.858–0.952] | 0.951 |
| 0.476 [0.257–0.702] | 0.769 [0.462–0.950] | ||||
| Validation | 0.964 [0.817–0.999] | 0.900 [0.735–0.979] | 0.889 [0.739–0.969] | 0.931 | |
| 0.625 [0.245–0.915] | 0.833 [0.359–0.996] | ||||
| Montano et al. | Development | 0.980 [0.942–0.996]/ | 0.960 [0.915–0.985]/ | 0.944 [0.897–0.974] | 0.970 |
| 0.571 [0.289–0.823] | 0.727 [0.390–0.940] | ||||
| Validation | 0.938 [0.792–0.992] | 1.000 [0.833–1.000] | 0.944 [0.813–0.993] | 0.968 | |
| 1.000 [0.284–1.000] | 0.667 [0.223–0.957] | ||||
| ESPEN | Development | 0.986 [0.952–0.998]/ | 0.935 [0.885–0.969]/ | 0.925 [0.873–0.961] | 0.960 |
| 0.286 [0.084–0.581] | 0.667 [0.223-0.957] | ||||
| Validation | 0.966 [0.822–0.999] | 0.903 [0.742-0.980] | 0.889 [0.739–0.969] | 0.933 | |
| 0.571 [0.184–0.901] | 0.800 [0.284–0.995] | ||||
| Prado et al. | Development | 0.986 [0.951–0.998]/ | 0.928 [0.875–0.964]/ | 0.919 [0.866–0.956] | 0.956 |
| 0.353 [0.142–0.617] | 0.750 [0.349–0.968] | ||||
| Validation | 0.966 [0.822–0.999] | 0.903 [0.742–0.980] | 0.889 [0.739–0.969] | 0.933 | |
| 0.571 [0.184–0.901] | 0.800 [0.284–0.995] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, Y.; Maeda, K.; Yamanaka, Y.; Matsuyama, R.; Kato, R.; Yamaguchi, M.; Nonogaki, T.; Shimizu, A.; Ueshima, J.; Murotani, K.; et al. Formula for the Cross-Sectional Area of the Muscles of the Third Lumbar Vertebra Level from the Twelfth Thoracic Vertebra Level Slice on Computed Tomography. Geriatrics 2020, 5, 47. https://doi.org/10.3390/geriatrics5030047
Ishida Y, Maeda K, Yamanaka Y, Matsuyama R, Kato R, Yamaguchi M, Nonogaki T, Shimizu A, Ueshima J, Murotani K, et al. Formula for the Cross-Sectional Area of the Muscles of the Third Lumbar Vertebra Level from the Twelfth Thoracic Vertebra Level Slice on Computed Tomography. Geriatrics. 2020; 5(3):47. https://doi.org/10.3390/geriatrics5030047
Chicago/Turabian StyleIshida, Yuria, Keisuke Maeda, Yosuke Yamanaka, Remi Matsuyama, Ryoko Kato, Makoto Yamaguchi, Tomoyuki Nonogaki, Akio Shimizu, Junko Ueshima, Kenta Murotani, and et al. 2020. "Formula for the Cross-Sectional Area of the Muscles of the Third Lumbar Vertebra Level from the Twelfth Thoracic Vertebra Level Slice on Computed Tomography" Geriatrics 5, no. 3: 47. https://doi.org/10.3390/geriatrics5030047
APA StyleIshida, Y., Maeda, K., Yamanaka, Y., Matsuyama, R., Kato, R., Yamaguchi, M., Nonogaki, T., Shimizu, A., Ueshima, J., Murotani, K., & Mori, N. (2020). Formula for the Cross-Sectional Area of the Muscles of the Third Lumbar Vertebra Level from the Twelfth Thoracic Vertebra Level Slice on Computed Tomography. Geriatrics, 5(3), 47. https://doi.org/10.3390/geriatrics5030047





