Changes in Treatment of Very Elderly Patients Six Weeks after Discharge from Geriatrics Department
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Population
2.2.1. Sampling Process
2.2.2. Inclusion Criteria
2.2.3. Exclusion Criteria
2.2.4. Exclusion Criteria at Six Weeks
2.3. Collected Data
2.4. Main Outcomes
2.5. Statistical Analyses
3. Results
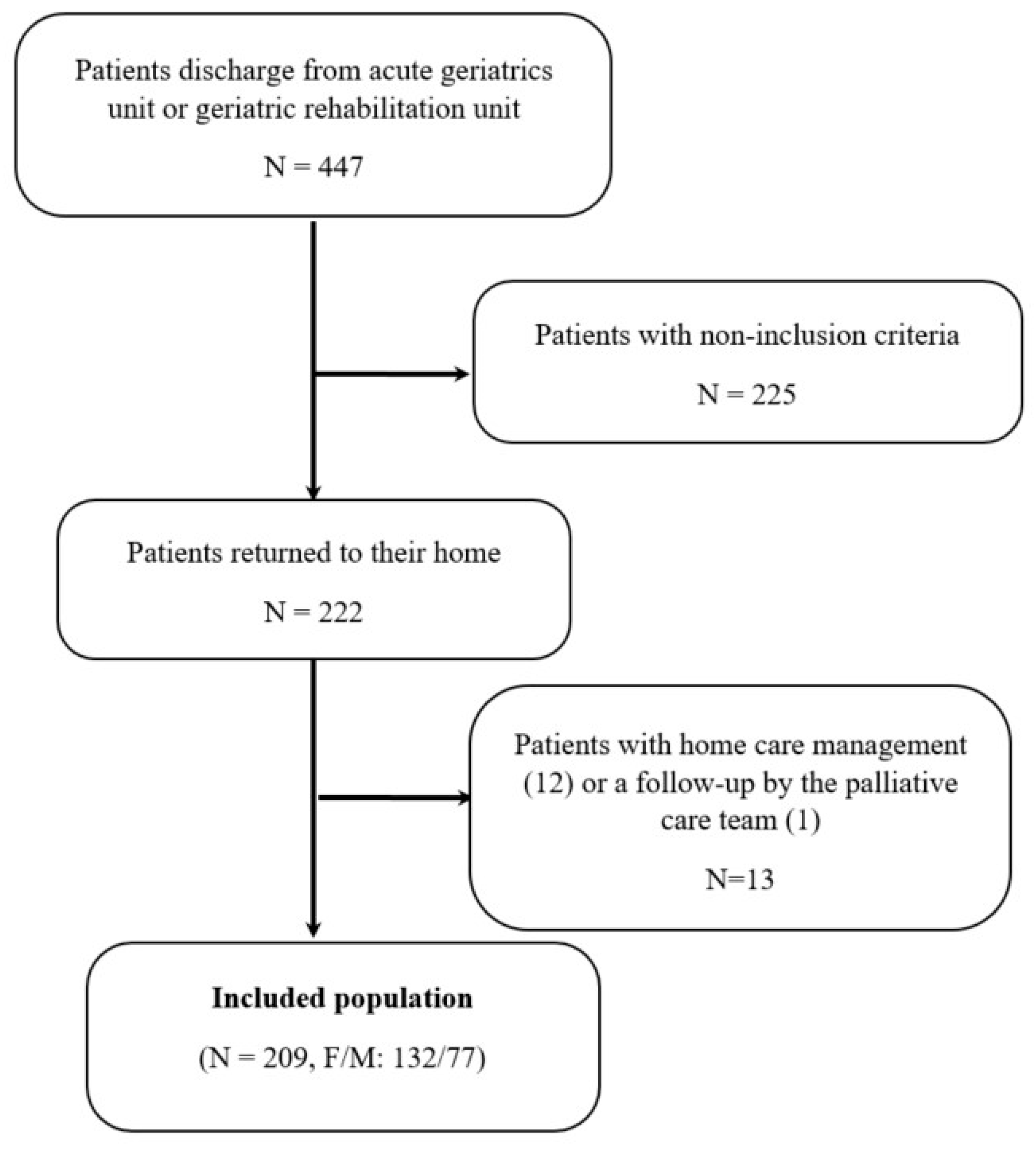
3.1. Patients Included
3.2. Characteristics of Patients at Admission
3.3. Patient Characteristics Six Weeks after Hospital Discharge
3.4. Analysis of Prescription Modifications
3.4.1. Prescription Changes during Hospitalization
Initiations
Discontinuations
Adjustments
3.4.2. Prescription Changes Six Weeks after Hospital Discharge
Initiations
Discontinuations
Dosage Adjustment
Continuation of In-Hospital Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ornstein, S.M.; Nietert, P.J.; Jenkins, R.G.; Litvin, C.B. The prevalence of chronic diseases and multimorbidity in primary care practice: A PPRNet report. J. Am. Board. Fam. Med. 2013, 26, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Sinnott, C.; Bradley, C.P. Multimorbidity or polypharmacy: Two sides of the same coin? J. Comorb. 2015, 5, 29–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajjar, E.R.; Cafiero, A.C.; Hanlon, J.T. Polypharmacy in elderly patients. Am. J. Geriatr. Pharmacother. 2007, 5, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.A.; Poon, I.; Nair, M. Clinical consequences of polypharmacy in elderly: Expect the unexpected, think the unthinkable. Expert. Opin. Drug. Saf. 2007, 6, 695–704. [Google Scholar] [CrossRef]
- Mulhem, E.; Lick, D.; Varughese, J.; Barton, E.; Ripley, T.; Haveman, J. Adherence to medications after hospital discharge in the elderly. Int. J. Fam. Med. 2013, 2013, 901845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laroche, M.L.; Charmes, J.P.; Nouaille, Y.; Fourrier, A.; Merle, L. Impact of hospitalisation in an acute medical geriatric unit on potentially inappropriate medication use. Drugs Aging 2006, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Nauta, K.J.; Groenhof, F.; Schuling, J.; Hugtenburg, J.G.; van Hout, H.P.J.; Haaijer-Ruskamp, F.M.; Denig, P. Application of the STOPP/START criteria to a medical record database. Pharmacoepidemiol. Drug. Saf. 2017, 26, 1242–1247. [Google Scholar] [CrossRef]
- Ponson, I.; Pechu, A. Prescription médicamenteuse chez le sujet âgé: Étude des modifications thérapeutiques au cours d’un séjour en gériatrie chez 221 patients hospitalisés dans la région lyonnaise. Rev. Prat. 2013, 63, 1215–1221. [Google Scholar]
- Leguelinel-Blache, G.; Arnaud, F.; Bouvet, S.; Dubois, F.; Castelli, C.; Roux-Marson, C.; Ray, V.; Sotto, A.; Kinowski, J.M. Impact of admission medication reconciliation performed by clinical pharmacists on medication safety. Eur. J. Intern. Med. 2014, 25, 808–814. [Google Scholar] [CrossRef]
- Rouch, L.; Farbos, F.; Cool, C.; McCambridge, C.; Hein, C.; Elmalem, S.; Rolland, Y.; Vellas, B.; Cestac, P. Hospitalization Drug Regimen Changes in Geriatric Patients and Adherence to Modifications by General Practitioners in Primary Care. J. Nutr. Health Aging 2018, 22, 328–334. [Google Scholar] [CrossRef]
- Strehlau, A.G.; Larsen, M.D.; Søndergaard, J.; Almarsdóttir, A.B.; Rosholm, J.U. General practitioners’ continuation and acceptance of medication changes at sectorial transitions of geriatric patients—a qualitative interview study. BMC Fam. Pract. 2018, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- European Pharmaceutical Market Research Association. 2011. Available online: http://www.ephmra.org/ (accessed on 2 February 2017).
- Lavan, A.H.; Gallagher, P.F.; O’Mahony, D. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin. Interv. Aging 2016, 11, 857–866. [Google Scholar] [PubMed] [Green Version]
- Dramé, M.; Jovenin, N.; Novella, J.L.; Lang, P.O.; Somme, D.; Laniece, I.; Voisin, T.; Blanc, P.; Couturier, P.; Gauvain, J.B.; et al. Predicting early mortality among elderly patients hospitalised in medical wards via emergency department: The SAFES cohort study. J. Nutr. Health Aging 2008, 12, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, R.A.; Mandal, A.R.; Ledger-Scott, M.; Waler, R. Changes in drug treatment after discharge from hospital in geriatric patients. BMJ 1992, 305, 694–696. [Google Scholar] [CrossRef] [Green Version]
- Himmel, W.; Kochen, M.M.; Sorns, U.; Hummers-Pradier, E. Drug changes at the interface between primary and secondary care. Int. J. Clin. Pharmacol. Ther. 2004, 42, 103–109. [Google Scholar] [CrossRef]
- Mansur, N.; Weiss, A.; Beloosesky, Y. Relationship of in-hospital medication modifications of elderly patients to postdischarge medications, adherence, and mortality. Ann. Pharmacother. 2008, 42, 783–789. [Google Scholar] [CrossRef]
- Guyonnet, S.; Rolland, Y. Screening for Malnutrition in older people. Clin. Geriatr. Med. 2015, 31, 429–437. [Google Scholar] [CrossRef]
- Barry, P.J.; Gallagher, P.F.; Ryan, C.; O’mahony, D. START (Screening Tool to Alert doctors to the Right Treatment)—an evidence-based screening tool to detect prescribing omissions in elderly patients. Age Ageing 2007, 36, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Beers, M.H. Explicit criteria for determining inappropriate medication use by the elderly. An update. Arch. Intern. Med. 1997, 157, 1531–1536. [Google Scholar] [CrossRef]
- Patel, K.V.; Guralnik, J.M.; Dansie, E.J.; Turk, D.C. Prevalence and impact of pain among older adults in the United States: Findings from the 2011 National Health and Aging Trends Study. Pain 2013, 154, 2649–2657. [Google Scholar] [CrossRef] [Green Version]
- Baffy, N.; Foxx-Orenstein, A.E.; Harris, L.A.; Sterler, S. Intractable constipation in the elderly. Curr. Treat. Options. Gastroenterol. 2017, 15, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Morandi, A.; Bellelli, G.; Vasilevskis, E.E.; Turco, R.; Guerini, F.; Torpilliesi, T.; Speciale, S.; Emiliani, V.; Gentile, S.; Schnelle, J.; et al. Predictors of rehospitalization among elderly patients admitted to a rehabilitation hospital: The role of polypharmacy, functional status, and length of stay. J. Am. Med. Dir. Assoc. 2013, 14, 761–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekerstad, N.; Bylin, K.; Karlson, B.W. Early rehospitalizations of frail elderly patients—the role of medications: A clinical, prospective, observational trial. Drug. Healthc. Patient. Saf. 2017, 9, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putot, A.; Jeanmichel, M.; Chague, F.; Manckoundia, P.; Cottin, Y.; Zeller, M. Type 2 myocardial infarction: A geriatric population-based model of pathogenesis. Aging Dis. 2020, 11, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Kripalani, S.; LeFevre, F.; Phillips, C.O.; Williams, M.V.; Basaviah, P.; Baker, D.W. Deficits in communication and information transfer between hospital-based and primary care physicians: Implications for patient safety and continuity of care. JAMA 2007, 297, 831–841. [Google Scholar] [CrossRef]
- Gurwitz, J.H.; Field, T.S.; Harrold, L.R.; Rothschild, J.; Debellis, K.; Seger, A.C.; Cadoret, C.; Fish, L.S.; Garber, L.; Kelleher, M.; et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003, 289, 1107–1116. [Google Scholar] [CrossRef] [Green Version]
- Kardas, P.; Lewek, P.; Matyjaszczyk, M. Determinants of patient adherence: A review of systematic reviews. Front. Pharmacol. 2013, 4, 91. [Google Scholar] [CrossRef] [Green Version]
- Gathright, E.C.; Dolansky, M.A.; Gunstad, J.; Redle, J.D.; Josephson, R.A.; Moore, S.M.; Hughes, J.W. The impact of medication nonadherence on the relationship between mortality risk and depression in heart failure. Health Psychol. 2017, 36, 839–847. [Google Scholar] [CrossRef]
- Smith, D.; Lovell, J.; Weller, C.; Kennedy, B.; Winbolt, M.; Young, C.; Ibrahim, J. A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLoS ONE 2017, 12, e0170651. [Google Scholar] [CrossRef] [Green Version]
- Burnier, M.; Polychronopoulou, E.; Wuerzner, G. Hypertension and drug adherence in the elderly. Front. Cardiovasc. Med. 2020, 7, 49. [Google Scholar] [CrossRef]
- Gallo, P.; De Vincentis, A.; Pedone, C.; Nobili, A.; Tettamanti, M.; Gentilucci, U.V.; Picardi, A.; Mannucci, P.M.; Incalzi, R.A.; REPOSI Investigators. Drug-drug interactions involving CYP3A4 and p-glycoprotein in hospitalized elderly patients. Eur. J. Intern. Med. 2019, 65, 51–57. [Google Scholar] [PubMed]
| Drug Classes | Number of Initiations | % |
|---|---|---|
| Oral nutritional supplements | 103 | 16.0 |
| Laxatives | 79 | 12.3 |
| Paracetamol | 71 | 11 |
| Alimentary tract and metabolism | 52 | 8.1 |
| Short acting benzodiazepines | 43 | 6.7 |
| Vitamin D | 31 | 4.8 |
| Other antidepressants | 23 | 3.6 |
| Selective serotonin reuptake inhibitors | 21 | 3.3 |
| Proton pump inhibitors | 17 | 2.6 |
| Calcium | 16 | 2.5 |
| Angiotensin-converting enzyme inhibitors | 16 | 2.5 |
| Antiplatelet medications | 14 | 2.2 |
| Insulin | 14 | 2.2 |
| Strong opioid analgesics | 12 | 1.9 |
| Calcium-channel blockers | 12 | 1.9 |
| Antipsychotic drugs | 9 | 1.4 |
| Vitamin K antagonist | 8 | 1.2 |
| Cholinesterase inhibitors | 8 | 1.2 |
| Angiotensin II receptor blockers | 7 | 1.1 |
| Direct oral anticoagulants | 7 | 1.1 |
| Hypnotics | 7 | 1.1 |
| Beta-blockers | 7 | 1.1 |
| Loop diuretics | 6 | 0.9 |
| Beta-2 mimetics | 6 | 0.9 |
| Antiepileptic medications | 5 | 0.8 |
| Glinides | 4 | 0.6 |
| Heparins | 4 | 0.6 |
| Statins | 4 | 0.6 |
| Genito-urinary system and sex hormones | 4 | 0.6 |
| Nervous system | 4 | 0.6 |
| Corticosteroids | 3 | 0.5 |
| Digitalis | 3 | 0.5 |
| Antiemetics | 2 | 0.3 |
| Other oral antidiabetic drugs | 2 | 0.3 |
| Dopamine | 2 | 0.3 |
| Cardiovascular system | 2 | 0.3 |
| Musculoskeletal system | 2 | 0.3 |
| Mild opioid analgesics | 1 | 0.2 |
| Antihistamines | 1 | 0.2 |
| Centrally acting antihypertensive drugs | 1 | 0.2 |
| Antispasmodics | 1 | 0.2 |
| Antivertigo drugs | 1 | 0.2 |
| Long acting benzodiazepines | 1 | 0.2 |
| Biguanides | 1 | 0.2 |
| Inhaled corticosteroids | 1 | 0.2 |
| Nitrates | 1 | 0.2 |
| Systemic hormonal preparation (excluding sex hormones and insulins) | 1 | 0.2 |
| Thyroid hormones | 1 | 0.2 |
| Blood and blood-forming organs | 1 | 0.2 |
| Respiratory system | 1 | 0.2 |
| Drug Classes | Number of Discontinuations | % |
|---|---|---|
| Alimentary tract and metabolism | 35 | 7.3 |
| Loop diuretics | 34 | 7.1 |
| Calcium-channel blockers | 21 | 4.4 |
| Beta-blockers | 21 | 4.4 |
| Short acting benzodiazepines | 19 | 4.0 |
| Antiplatelet medications | 18 | 3.8 |
| Mild opioid analgesics | 17 | 3.6 |
| Antihistamines | 16 | 3.3 |
| Statins | 16 | 3.3 |
| Vitamin K antagonist | 15 | 3.1 |
| Angiotensin II receptor blockers | 14 | 2.9 |
| Selective serotonin reuptake inhibitors | 14 | 2.9 |
| Angiotensin-conversion enzyme inhibitors | 12 | 2.5 |
| Proton pump inhibitors | 12 | 2.5 |
| Antipsychotic drugs | 12 | 2.5 |
| Vitamin D | 12 | 2.5 |
| Paracetamol | 11 | 2.3 |
| Genito-urinary system and sex hormones | 11 | 2.3 |
| Biguanides | 9 | 1.9 |
| Amiodarone | 9 | 1.9 |
| Hypoglycemic sulfamides | 8 | 1.7 |
| Allopurinol | 7 | 1.5 |
| Antivertigo drugs | 7 | 1.5 |
| Beta-2 mimetics | 7 | 1.5 |
| Digitalis | 7 | 1.5 |
| Thiazide diuretics | 7 | 1.5 |
| Hypnotics | 7 | 1.5 |
| Calcium | 6 | 1.3 |
| Laxatives | 6 | 1.3 |
| Strong opioid analgesics | 5 | 1.0 |
| Centrally acting antihypertensive drugs | 5 | 1.0 |
| Other oral antidiabetic drugs | 5 | 1.0 |
| Bisphosphonates | 5 | 1.0 |
| Insulins | 5 | 1.0 |
| Cardiovascular system | 5 | 1.0 |
| Aldosterone receptor antagonists | 4 | 0.8 |
| Cholinesterase inhibitors | 4 | 0.8 |
| Antispasmodics | 4 | 0.8 |
| Other antiparkinson drugs | 4 | 0.8 |
| Long acting benzodiazepines | 4 | 0.8 |
| Oral nutritional supplements | 4 | 0.8 |
| Antiepileptic medications | 3 | 0.6 |
| Corticosteroids | 3 | 0.6 |
| Imipramine | 3 | 0.6 |
| Musculoskeletal system | 3 | 0.6 |
| Nervous system | 3 | 0.6 |
| Antiemetics | 3 | 0.6 |
| Non-steroidal anti-inflammatory drugs | 2 | 0.4 |
| Direct oral anticoagulants | 2 | 0.4 |
| Other antidepressants (mianserin, mirtazapine, …) | 2 | 0.4 |
| Nitrates | 2 | 0.4 |
| Glinides | 2 | 0.4 |
| Heparins | 2 | 0.4 |
| Various | 1 | 0.2 |
| Thyroid hormones | 1 | 0.2 |
| Monoamine oxidase inhibitors | 1 | 0.2 |
| Respiratory system | 1 | 0.2 |
| Drug Classes | Number of Dose Adjustments | % |
|---|---|---|
| Beta-blockers | 20 | 20.8 |
| Loop diuretics | 13 | 13.5 |
| Angiotensin-converting enzyme inhibitors | 13 | 13.5 |
| Angiotensin II receptor blockers | 8 | 8.3 |
| Proton pump inhibitors | 8 | 8.3 |
| Short acting benzodiazepines | 7 | 7.3 |
| Thyroid hormones | 5 | 5.2 |
| Dopamine | 3 | 3.1 |
| Selective serotonin reuptake inhibitors | 3 | 3.1 |
| Strong opioid analgesics | 2 | 2.1 |
| Antiplatelet medications | 2 | 2.1 |
| Other antidepressants (mianserin, mirtazapine, …) | 2 | 2.1 |
| Calcium-channel blockers | 2 | 2.1 |
| Statins | 2 | 2.1 |
| Cholinesterase inhibitors | 1 | 1.0 |
| Antiepileptic medications | 1 | 1.0 |
| Corticosteroids | 1 | 1.0 |
| Glinides | 1 | 1.0 |
| Insulins | 1 | 1.0 |
| Antipsychotic drugs | 1 | 1.0 |
| Analyzed Factors | p | p * | p ** | OR (95% CI) | |
|---|---|---|---|---|---|
| Age | 0.18 | 0.426 | |||
| Gender | 0.40 | 0.502 | |||
| Residential status before hospitalization | 0.35 | 0.264 | |||
| Medical history | Cognitive impairment | 0.04 | 0.037 | 0.718 | 1.19 (0.16–9.02) |
| Depression | 0.17 | 0.089 | 0.350 | 2.94 (0.26–33.79) | |
| Hypertension | 0.68 | 0.425 | |||
| Heart failure | 0.18 | 0.404 | |||
| Atrial fibrillation | 0.32 | 0.246 | |||
| Diabetes | 0.60 | 0.485 | |||
| Thyroid dysfunction | 0.58 | 0.835 | |||
| Chronic nephropathy | 0.08 | 0.019 | 0.565 | 0.62 (0.06–6.72) | |
| Number of drugs at admission | 0.86 | 0.638 | |||
| Patient provenance | 0.58 | 0.408 | |||
| Type of stay | 0.89 | 0.632 | |||
| Unit | 0.07 | 0.049 | |||
| Length of stay | 0.75 | 0.732 | |||
| Residential status after hospitalization | 0.08 | 0.148 | |||
| Number of drugs/prescription at discharge | 0.30 | 0.419 | |||
| Changes in prescription during hospitalization | 0.21 | <0.001 | <0.001 | 104 (167.99–106) | |
| Changes reported on discharge prescription | 0.17 | 0.159 | |||
| In-hospital modification(s) notified on hospital record | 0.007 | 0.005 | 0.571 | 10−4 (10−5–105) | |
| Drug Classes | Number of Initiations | % |
|---|---|---|
| Short acting benzodiazepines | 5 | 8.6 |
| Loop diuretics | 5 | 8.6 |
| Selective serotonin reuptake inhibitors | 4 | 6.9 |
| Alimentary tract and metabolism drugs | 4 | 6.9 |
| Beta-2 mimetics | 3 | 5.2 |
| Angiotensin-converting enzyme-inhibitors | 3 | 5.2 |
| Antipsychotic drugs | 3 | 5.2 |
| Mild opioid analgesics | 2 | 3.4 |
| Antiplatelet medications | 2 | 3.4 |
| Thiazide diuretics | 2 | 3.4 |
| Proton pump inhibitors | 2 | 3.4 |
| Vitamin D | 2 | 3.4 |
| Angiotensin II receptor blockers | 1 | 1.7 |
| Vitamin K antagonist | 1 | 1.7 |
| Allopurinol | 1 | 1.7 |
| Paracetamol | 1 | 1.7 |
| Strong opioid analgesics | 1 | 1.7 |
| Antihistamines | 1 | 1.7 |
| Antivertigo drugs | 1 | 1.7 |
| Antiepileptic medications | 1 | 1.7 |
| Other antidepressants (mianserin, mirtazapine, …) | 1 | 1.7 |
| Long acting benzodiazepines | 1 | 1.7 |
| Beta-blockers | 1 | 1.7 |
| Oral nutritional supplements | 1 | 1.7 |
| Dopamine | 1 | 1.7 |
| Nitrates | 1 | 1.7 |
| Hypnotics | 1 | 1.7 |
| Calcium-channel blockers | 1 | 1.7 |
| Insulin | 1 | 1.7 |
| Laxatives | 1 | 1.7 |
| Hypoglycemic sulfamides | 1 | 1.7 |
| Genito-urinary system and sex hormones | 1 | 1.7 |
| Musculoskeletal system drugs | 1 | 1.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dipanda, M.; Barben, J.; Nuémi, G.; Vadot, L.; Nuss, V.; Vovelle, J.; Putot, A.; Manckoundia, P. Changes in Treatment of Very Elderly Patients Six Weeks after Discharge from Geriatrics Department. Geriatrics 2020, 5, 44. https://doi.org/10.3390/geriatrics5030044
Dipanda M, Barben J, Nuémi G, Vadot L, Nuss V, Vovelle J, Putot A, Manckoundia P. Changes in Treatment of Very Elderly Patients Six Weeks after Discharge from Geriatrics Department. Geriatrics. 2020; 5(3):44. https://doi.org/10.3390/geriatrics5030044
Chicago/Turabian StyleDipanda, Mélanie, Jérémy Barben, Gilles Nuémi, Lucie Vadot, Valentine Nuss, Jérémie Vovelle, Alain Putot, and Patrick Manckoundia. 2020. "Changes in Treatment of Very Elderly Patients Six Weeks after Discharge from Geriatrics Department" Geriatrics 5, no. 3: 44. https://doi.org/10.3390/geriatrics5030044
APA StyleDipanda, M., Barben, J., Nuémi, G., Vadot, L., Nuss, V., Vovelle, J., Putot, A., & Manckoundia, P. (2020). Changes in Treatment of Very Elderly Patients Six Weeks after Discharge from Geriatrics Department. Geriatrics, 5(3), 44. https://doi.org/10.3390/geriatrics5030044






