Chronic Obstructive Pulmonary Disease and Dysphagia: A Synergistic Review
Abstract
1. Introduction
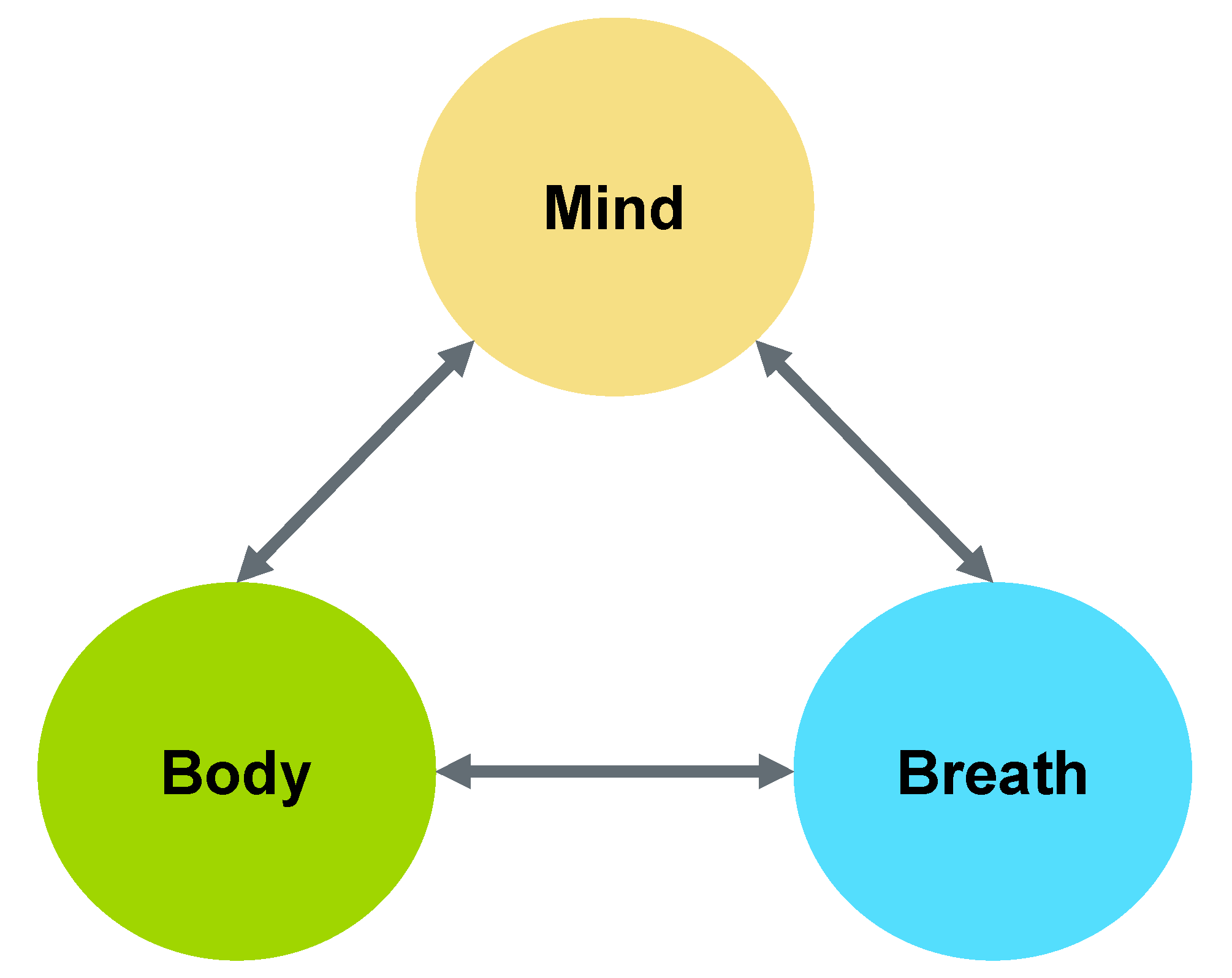
2. Mind-Body-Breath Feedback Loop
3. Dysphagia in COPD—Primary Physiological Impairment
3.1. Respiratory-Swallowing Discoordination
3.2. Oropharyngeal Swallowing Impairments
4. Dysphagia in COPD—Primary Psycho-Emotional Sequalae
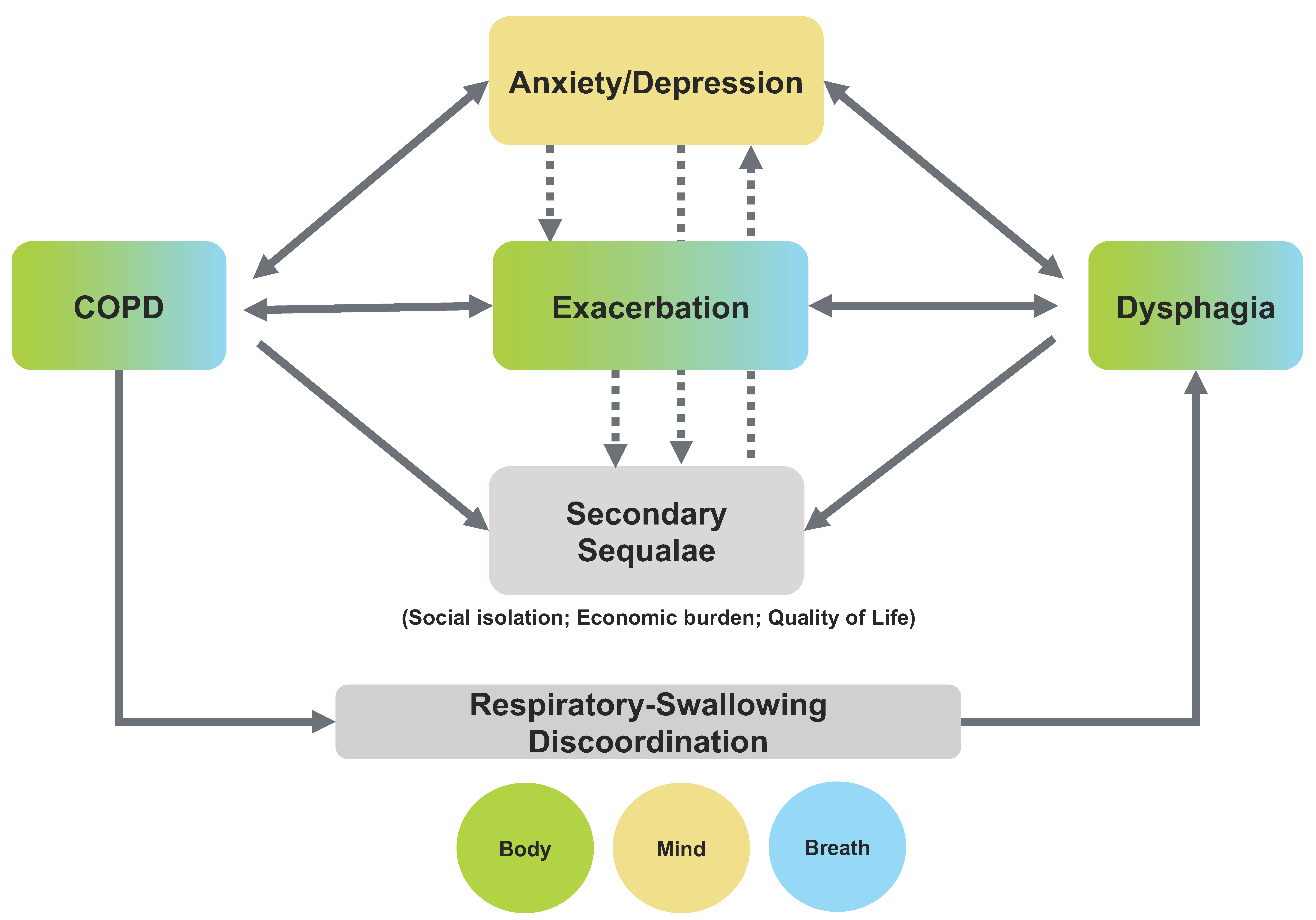
5. Interplay between the Secondary Sequalae of COPD and Dysphagia
6. Discussion and Clinical Implications
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Varmaghani, M.; Dehghani, M.; Heidari, E.; Sharifi, F.; Moghaddam, S.; Farzadfar, F. Global prevalence of chronic obstructive pulmonary disease: Systematic review and meta-analysis. East. Mediterr. Health J. 2019, 25, 58–65. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease: Pocket Guide to COPD Diagnosis, Management, and Prevention. 2019. Available online: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-POCKET-GUIDE-FINAL_WMS.pdf (accessed on 26 October 2019).
- Diaz-Guzman, E.; Mannino, D.M. Epidemiology and Prevalence of Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2014, 35, 7–16. [Google Scholar] [CrossRef]
- World Health Organization (WHO). 2019. Available online: https://www.who.int/respiratory/copd/en (accessed on 26 October 2019).
- Mathers, C.D.; Loncar, D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Schneider, C.; Jick, S.S.; Bothner, U.; Meier, C.R. COPD and the Risk of Depression. CHEST 2010, 137, 341–347. [Google Scholar] [CrossRef]
- Miravitlles, M. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: A 2 year follow up study. Thorax 2004, 59, 387–395. [Google Scholar] [CrossRef]
- Bentsen, S.B.; Miaskowski, C.; Rustøen, T. Demographic and clinical characteristics associated with quality of life in patients with chronic obstructive pulmonary disease. Qual. Life Res. 2013, 23, 991–998. [Google Scholar] [CrossRef]
- Dalal, A.A.; Shah, M.; Lunacsek, O.; Hanania, N.A. Clinical and Economic Burden of Depression/Anxiety in Chronic Obstructive Pulmonary Disease Patients within a Managed Care Population. COPD J. Chronic Obstr. Pulm. Dis. 2011, 8, 293–299. [Google Scholar] [CrossRef]
- Montserrat, J.M.; Godoy, P.; Marsal, J.R.; Barbe, F.; Pifarré, J.; Alsedà, M.; Ortega, M. Overview of the Impact of Depression and Anxiety in Chronic Obstructive Pulmonary Disease. Lung 2017, 195, 77–85. [Google Scholar] [CrossRef]
- Zareifopoulos, N.; Bellou, A.; Spiropoulou, A.; Spiropoulos, K. Prevalence, Contribution to Disease Burden and Management of Comorbid Depression and Anxiety in Chronic Obstructive Pulmonary Disease: A Narrative Review. COPD J. Chronic Obstr. Pulm. Dis. 2019, 16, 406–417. [Google Scholar] [CrossRef]
- Pooler, A.; Beech, R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: A systematic review. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 315–330. [Google Scholar] [CrossRef]
- Hynninen, K.M.J.; Breitve, M.H.; Wiborg, A.B.; Pallesen, S.; Nordhus, I.H. Psychological characteristics of patients with chronic obstructive pulmonary disease: A review. J. Psychosom. Res. 2005, 59, 429–443. [Google Scholar] [CrossRef]
- Yohannes, A.M.; Willgoss, T.G.; Baldwin, R.C.; Connolly, M.J. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: Prevalence, relevance, clinical implications and management principles. Int. J. Geriatr. Psychiatry 2010, 25, 1209–1221. [Google Scholar] [CrossRef]
- Fumagalli, G.; Fabiani, F.; Forte, S.; Napolitano, M.; Balzano, G.; Bonini, M.; De Simone, G.; Fuschillo, S.; Pentassuglia, A.; Pasqua, F.; et al. INDACO project: COPD and link between comorbidities, lung function and inhalation therapy. Multidiscip. Respir. Med. 2015, 10, 4. [Google Scholar] [CrossRef]
- O’Kane, L.; Groher, M. Oropharyngeal dysphagia in patients with chronic obstructive pulmonary disease: A systematic review. Rev. CEFAC 2009, 11, 449–506. [Google Scholar] [CrossRef][Green Version]
- Cvejic, L.; Bardin, P.G. Swallow and Aspiration in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1122–1129. [Google Scholar] [CrossRef]
- Shaker, R.; Li, Q.; Ren, J.; Townsend, W.F.; Dodds, W.J.; Martin, B.J.; Kern, M.K.; Rynders, A. Coordination of deglutition and phases of respiration: Effect of aging, tachypnea, bolus volume, and chronic obstructive pulmonary disease. Am. J. Physiol. Gastrointest. Liver Physiol. 1992, 263, G750–G755. [Google Scholar] [CrossRef]
- Gross, R.D.; Atwood, C.W.; Ross, S.B.; Olszewski, J.W.; Eichhorn, K.A. The Coordination of Breathing and Swallowing in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2009, 179, 559–565. [Google Scholar] [CrossRef]
- Ohta, K.; Murata, K.; Takahashi, T.; Minatani, S.; Sako, S.; Kanada, Y. Evaluation of swallowing function by two screening tests in primary COPD. Eur. Respir. J. 2009, 34, 280–281. [Google Scholar] [CrossRef]
- Garand, K.L.; Strange, C.; Paoletti, L.; Hopkins-Rossabi, T.; Martin-Harris, B. Oropharyngeal swallow physiology and swallowing-related quality of life in underweight patients with concomitant advanced chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2663–2671. [Google Scholar] [CrossRef]
- Langmore, S.E.; Skarupski, K.A.; Park, P.S.; Fries, B.E. Predictors of Aspiration Pneumonia in Nursing Home Residents. Dysphagia 2002, 17, 298–307. [Google Scholar] [CrossRef]
- Patel, D.A.; Krishnaswami, S.; Steger, E.; Conover, E.; Vaezi, M.F.; Ciucci, M.R.; Francis, D.O. Economic and survival burden of dysphagia among inpatients in the United States. Dis. Esophagus 2018, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shune, S.E.; Karnell, L.H.; Karnell, M.P.; van Daele, D.J.; Funk, G.F. Association between severity of dysphagia and survival in patients with head and neck cancer. Head Neck 2012, 34, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Karvonen-Gutierrez, C.A.; Ronis, D.L.; Fowler, K.E.; Terrell, J.E.; Gruber, S.B.; Duffy, S.A. Quality of Life Scores Predict Survival Among Patients with Head and Neck Cancer. J. Clin. Oncol. 2008, 26, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Speyer, R.; Kertscher, B.; Denman, D.; Swan, K.; Cordier, R. Health-Related Quality of Life and Oropharyngeal Dysphagia: A Systematic Review. Dysphagia 2018, 33, 141–172. [Google Scholar] [CrossRef]
- Eslick, G.D.; Talley, N.J. Dysphagia: Epidemiology, risk factors and impact on quality of life—A population-based study: DYSPHAGIA—A POPULATION-BASED STUDY. Aliment. Pharmacol. Ther. 2008, 27, 971–979. [Google Scholar] [CrossRef]
- Plowman-Prine, E.K.; Sapienza, C.M.; Okun, M.S.; Pollock, S.L.; Jacobson, C.; Wu, S.S.; Rosenbek, J.C. The relationship between quality of life and swallowing in Parkinson’s disease. Mov. Disord. 2009, 24, 1352–1358. [Google Scholar] [CrossRef]
- Cichero, J.A. Thickening agents used for dysphagia management: Effect on bioavailability of water, medication and feelings of satiety. Nutr. J. 2013, 12, 1–54. [Google Scholar] [CrossRef]
- Marik, P.E. Aspiration pneumonitis and aspiration pneumonia. N. Engl. J. Med. 2001, 344, 665–671. [Google Scholar] [CrossRef]
- Allen, J.; Greene, M.; Sabido, I.; Stretton, M.; Miles, A. Economic costs of dysphagia among hospitalized patients. Laryngoscope 2020, 130, 974–979. [Google Scholar] [CrossRef]
- Ekberg, O.; Hamdy, S.; Woisard, V.; Wuttge-Hannig, A.; Ortega, P. Social and Psychological Burden of Dysphagia: Its Impact on Diagnosis and Treatment. Dysphagia 2002, 17, 139–146. [Google Scholar] [CrossRef]
- Namasivayam-MacDonald, A.; Shune, S. The Burden of Dysphagia on Family Caregivers of the Elderly: A Systematic Review. Geriatrics 2018, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Nund, R.L.; Scarinci, N.A.; Cartmill, B.; Ward, E.C.; Kuipers, P.; Porceddu, S.V. Third-party disability in carers of people with dysphagia following non-surgical management for head and neck cancer. Disabil. Rehabil. 2015, 38, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Philippot, P.; Chapelle, G.; Blairy, S. Respiratory feedback in the generation of emotion. Cogn. Emot. 2002, 16, 605–627. [Google Scholar] [CrossRef]
- Boiten, F.A.; Frijda, N.H.; Wientjes, C.J.E. Emotions and respiratory patterns: Review and critical analysis. Int. J. Psychophysiol. 1994, 17, 103–128. [Google Scholar] [CrossRef]
- Arch, J.J.; Craske, M.G. Mechanisms of mindfulness: Emotion regulation following a focused breathing induction. Behav. Res. Ther. 2006, 44, 1849–1858. [Google Scholar] [CrossRef]
- Kaushik, R.M.; Kaushik, R.; Mahajan, S.K.; Rajesh, V. Effects of mental relaxation and slow breathing in essential hypertension. Complement. Ther. Med. 2006, 14, 120–126. [Google Scholar] [CrossRef]
- Carnaby, G.D.; Harenberg, L. What is ‘Usual Care’ in Dysphagia Rehabilitation: A Survey of USA Dysphagia Practice Patterns. Dysphagia 2013, 28, 567–574. [Google Scholar] [CrossRef]
- Crary, M.; Sura, L.; Madhavan, A.; Carnaby-Mann, G. Dysphagia in the elderly: Management and nutritional considerations. Clin. Interv. Aging 2012, 7, 287–298. [Google Scholar] [CrossRef]
- Hillemacher, T.; Gräßel, E.; Tigges, S.; Bleich, S.; Neundörfer, B.; Kornhuber, J.; Hecht, M. Depression and bulbar involvement in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2004, 5, 245–249. [Google Scholar] [CrossRef]
- Chow, E.S.L.; Kong, B.M.H.; Wong, M.T.P.; Draper, B.; Lin, K.L.; Ho, S.K.S.; Wong, C.P. The prevalence of depressive symptoms among elderly Chinese private nursing home residents in Hong Kong. Int. J. Geriatr. Psychiatry 2004, 19, 734–740. [Google Scholar] [CrossRef]
- Santos, M.; Richards, C.S.; Bleckley, M.K. Comorbidity between depression and disordered eating in adolescents. Eat. Behav. 2007, 8, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, E.M.; Bradley, C.; Moeller, J.; Harouni, L.; Nandamudi, D.; Brackett, M.A. Promoting Mental Health and Psychological Thriving in University Students: A Randomized Controlled Trial of Three Well-Being Interventions. Front. Psychiatry 2020, 11, 590. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.R.; Lewin, R.K.; Allen, J.J.B. Improvements in well-being and cardiac metrics of stress following a yogic breathing workshop: Randomized controlled trial with active comparison. J. Am. Coll. Health 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dudley, D.L.; Glaser, E.M.; Jorgenson, B.N.; Logan, D.L. Psychosocial Concomitants to Rehabilitation in Chronic Obstructive Pulmonary Disease. CHEST 1980, 77, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Clayton, N.A.; Carnaby, G.D.; Peters, M.J.; Ing, A.J. Impaired laryngopharyngeal sensitivity in patients with COPD: The association with swallow function. Int. J. Speech Lang. Pathol. 2014, 16, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Martin-Harris, B.; Brodsky, M.B.; Price, C.C.; Michel, Y.; Walters, B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: Single liquid swallows. J. Appl. Physiol. 2003, 94, 1735–1743. [Google Scholar] [CrossRef]
- Martin-Harris, B.; Brodsky, M.B.; Michel, Y.; Ford, C.L.; Walters, B.; Heffner, J. Breathing and swallowing dynamics across the adult lifespan. Arch. Otolaryngol. Neck Surg. 2005, 131, 762–770. [Google Scholar] [CrossRef]
- Martin-Harris, B.; McFarland, D.; Hill, E.G.; Strange, C.B.; Focht, K.L.; Wan, Z.; Blair, J.; McGrattan, K. Respiratory-Swallow Training in Patients with Head and Neck Cancer. Arch. Phys. Med. Rehabil. 2014, 96, 885–893. [Google Scholar] [CrossRef]
- Martin, B.J.W.; Logemann, J.A.; Shaker, R.; Dodds, W.J. Coordination between respiration and swallowing: Respiratory phase relationships and temporal integration. J. Appl. Physiol. 1994, 76, 714–723. [Google Scholar] [CrossRef]
- Cvejic, L.; Churchward, T.; Harding, R.; Turton, A.; Finlay, P.; Massey, D.; Bardin, P.G.; Guy, P. Laryngeal penetration and aspiration in individuals with stable COPD: Aspiration in COPD. Respirology 2011, 16, 269–275. [Google Scholar] [CrossRef]
- Huff, A.; Reed, M.D.; Smith, B.K.; Brown, E.H.; Ovechkin, A.V.; Pitts, T. Strategies for the Integration of Cough and Swallow to Maintain Airway Protection in Humans. Lung 2018, 196, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Hopkins-Rossabi, T.; Curtis, P.; Temenak, M.; Miller, C.; Martin-Harris, B. Respiratory Phase and Lung Volume Patterns During Swallowing in Healthy Adults: A Systematic Review and Meta-Analysis. J. Speech Lang. Hear. Res. 2019, 62, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Drulia, T. The Effects of Lung Volume on Swallowing in Chronic Obstructive Pulmonary Disease. Available online: https://commons.lib.jmu.edu/cgi/viewcontent.cgi?article=1134&context=diss201019 (accessed on 10 August 2020).
- Good-Fratturelli, M.D.; Curlee, R.F.; Holle, J.L. Prevalence and nature of dysphagia in va patients with copd referred for videofluoroscopic swallow examination. J. Commun. Disord. 2000, 33, 93–110. [Google Scholar] [CrossRef]
- Mokhlesi, B.; Logemann, J.A.; Rademaker, A.W.; Stangl, C.A.; Corbridge, T.C. Oropharyngeal deglutition in stable COPD. CHEST 2002, 121, 361–369. [Google Scholar] [CrossRef]
- Coelho, C.A. Preliminary findings on the nature of dysphagia in patients with chronic obstructive pulmonary disease. Dysphagia 1987, 2, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Williams, A.J.; Grossman, F.; Weinberg, A.S.; Zuckerbraun, L. Cricopharyngeal dysfunction in chronic obstructive pulmonary disease. CHEST 1990, 97, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Kubo, H.; Yanai, M. Impairment of the swallowing reflex in exacerbations of COPD. Thorax 2007, 62, 1017. [Google Scholar] [CrossRef]
- Terada, K.; Muro, S.; Ohara, T.; Kudo, M.; Ogawa, E.; Hoshino, Y.; Hirai, T.; Niimi, A.; Chin, K.; Mishima, M. Abnormal Swallowing Reflex and COPD Exacerbations. CHEST 2010, 137, 326–332. [Google Scholar] [CrossRef]
- Steidl, E.; Ribeiro, C.; Gonçalves, B.; Fernandes, N.; Antunes, V.; Mancopes, R. Relationship between Dysphagia and Exacerbations in Chronic Obstructive Pulmonary Disease: A Literature Review. Int. Arch. Otorhinolaryngol. 2014, 19, 074–079. [Google Scholar] [CrossRef]
- Schroedl, C.J.; Yount, S.E.; Szmuilowicz, E.; Hutchison, P.J.; Rosenberg, S.R.; Kalhan, R. A Qualitative Study of Unmet Healthcare Needs in Chronic Obstructive Pulmonary Disease. A Potential Role for Specialist Palliative Care? Ann. Am. Thorac. Soc. 2014, 11, 1433–1438. [Google Scholar] [CrossRef]
- Seamark, D.A.; Blake, S.D.; Seamark, C.J.; Halpin, D.M. Living with severe chronic obstructive pulmonary disease (COPD): Perceptions of patients and their carers: An interpretative phenomenological analysis. Palliat. Med. 2004, 18, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Schane, R.E.; Woodruff, P.G.; Dinno, A.; Covinsky, K.E.; Walter, L.C. Prevalence and Risk Factors for Depressive Symptoms in Persons with Chronic Obstructive Pulmonary Disease. J. Gen. Intern. Med. 2008, 23, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Vögele, C.; Von Leupoldt, A. Mental disorders in chronic obstructive pulmonary disease (COPD). Respir. Med. 2008, 102, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, A.M.; Müllerová, H.; Hanania, N.A.; Lavoie, K.; Tal-Singer, R.; Vestbo, J.; Rennard, S.I.; Wouters, E.F.; Mülerova, H. Long-term course of depression trajectories in patients with COPD: A 3-year follow-up analysis of the evaluation of COPD longitudinally to identify predictive surrogate endpoints cohort. CHEST 2016, 149, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Atlantis, E.; Fahey, P.; Cochrane, B.; Smith, S. Bidirectional Associations Between Clinically Relevant Depression or Anxiety and COPD. CHEST 2013, 144, 766–777. [Google Scholar] [CrossRef] [PubMed]
- von Leupoldt, A.; Taube, K.; Henkhus, M.; Dahme, B.; Magnussen, H. The impact of affective states on the perception of dyspnea in patients with chronic obstructive pulmonary disease. Biol. Psychol. 2010, 84, 129–134. [Google Scholar] [CrossRef]
- Iyer, A.S.; Bhatt, S.P.; Garner, J.J.; Wells, J.M.; Trevor, J.L.; Patel, N.M.; Kirkpatrick, D.; Williams, J.C.; Dransfield, M.T. Depression is Associated with Readmission due to Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2015, 13, 197–203. [Google Scholar] [CrossRef]
- Yohannes, A.M.; Kaplan, A.; Hanania, N.A. Anxiety and Depression in Chronic Obstructive Pulmonary Disease: Recognition and Management. Cleve. Clin. J. Med. 2018, 85 (Suppl. 1), S11–S18. [Google Scholar] [CrossRef]
- Nguyen, N.P.; Frank, C.; Moltz, C.C.; Vos, P.; Smith, H.J.; Karlsson, U.; Dutta, S.; Midyett, A.; Barloon, J.; Sallah, S. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int. J. Radiat. Oncol. 2005, 61, 772–778. [Google Scholar] [CrossRef]
- McHorney, C.A.; Robbins, J.; Lomax, K.; Rosenbek, J.C.; Chignell, K.; Kramer, A.E.; Bricker, D.E. The SWAL-QOL and SWAL-CARE Outcomes Tool for Oropharyngeal Dysphagia in Adults: III. Documentation of Reliability and Validity. Dysphagia 2002, 17, 97–114. [Google Scholar] [CrossRef]
- Mintz, S.W.; Bois, C.M.D. The Anthropology of Food and Eating, Annu. Rev. Anthropol. 2002, 31, 99–119. [Google Scholar] [CrossRef]
- Plastow, N.A.; Atwal, A.; Gilhooly, M. Food activities and identity maintenance in old age: A systematic review and meta-synthesis. Aging Ment. Health 2014, 19, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.; McLaren, S. Coping and adaptation at six months after stroke: Experiences with eating disabilities. Int. J. Nurs. Stud. 2003, 40, 185–195. [Google Scholar] [CrossRef]
- Klinke, M.E.; Wilson, M.E.; Hafsteinsdóttir, T.B.; Jónsdóttir, H. Recognizing new perspectives in eating difficulties following stroke: A concept analysis. Disabil. Rehabil. 2013, 35, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Murphy, L.B.; Khavjou, O.; Giles, W.H.; Holt, J.B.; Croft, J.B. Total and State-Specific Medical and Absenteeism Costs of COPD Among Adults Aged 18 Years in the United States for 2010 and Projections Through 2020. CHEST 2015, 147, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Rogus-Pulia, N.M.; Rusche, N.; Hind, J.A.; Zielinski, J.; Gangnon, R.E.; Safdar, N.; Robbins, J. Effects of Device-Facilitated Isometric Progressive Resistance Oropharyngeal Therapy on Swallowing and Health-Related Outcomes in Older Adults with Dysphagia. J. Am. Geriatr. Soc. 2016, 64, 417–424. [Google Scholar] [CrossRef]
- Verdonschot, R.J.; Baijens, L.W.; Vanbelle, S.; van de Kolk, I.; Kremer, B.; Leue, C. Affective symptoms in patients with oropharyngeal dysphagia: A systematic review. J. Psychosom. Res. 2017, 97, 102–110. [Google Scholar] [CrossRef]
- Verdonschot, R.J.; Baijens, L.W.; Serroyen, J.L.; Leue, C.; Kremer, B. Symptoms of anxiety and depression assessed with the Hospital Anxiety and Depression Scale in patients with oropharyngeal dysphagia. J. Psychosom. Res. 2013, 75, 451–455. [Google Scholar] [CrossRef]
- Patterson, J.M.; McColl, E.; Carding, P.N.; Hildreth, A.J.; Kelly, C.; Wilson, J.A. Swallowing in the first year after chemoradiotherapy for head and neck cancer: Clinician-and patient-reported outcomes: Swallowing Outcomes after Chemoradiotherapy for Head and Neck Cancer. Head Neck 2014, 36, 352–358. [Google Scholar] [CrossRef]
- Leder, S.B.; Suiter, D.M.; Agogo, G.O.; Cooney, L.M. An Epidemiologic Study on Ageing and Dysphagia in the Acute Care Geriatric-Hospitalized Population: A Replication and Continuation Study. Dysphagia 2016, 31, 619–625. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-f.; Shune, S. Chronic Obstructive Pulmonary Disease and Dysphagia: A Synergistic Review. Geriatrics 2020, 5, 45. https://doi.org/10.3390/geriatrics5030045
Lin T-f, Shune S. Chronic Obstructive Pulmonary Disease and Dysphagia: A Synergistic Review. Geriatrics. 2020; 5(3):45. https://doi.org/10.3390/geriatrics5030045
Chicago/Turabian StyleLin, Ting-fen, and Samantha Shune. 2020. "Chronic Obstructive Pulmonary Disease and Dysphagia: A Synergistic Review" Geriatrics 5, no. 3: 45. https://doi.org/10.3390/geriatrics5030045
APA StyleLin, T.-f., & Shune, S. (2020). Chronic Obstructive Pulmonary Disease and Dysphagia: A Synergistic Review. Geriatrics, 5(3), 45. https://doi.org/10.3390/geriatrics5030045




