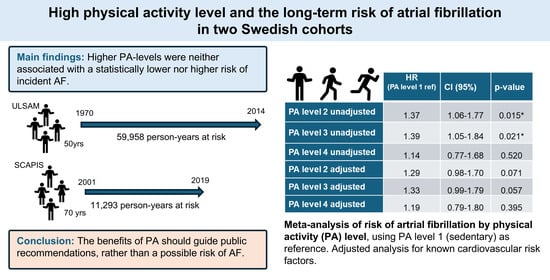
High Physical Activity Level and the Long-Term Risk of Atrial Fibrillation in Two Swedish Cohorts
Abstract
1. Introduction
2. Methods
2.1. Study Samples
2.1.1. The Uppsala Longitudinal Study of Adult Men (ULSAM)
2.1.2. The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS)
2.2. Traditional Risk Factors
2.3. Physical Activity
2.4. Outcomes
2.5. Statistics
3. Results
3.1. General Results
3.2. Risk of AF at Different PA Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [PubMed]
- Morseth, B.; Lochen, M.L.; Ariansen, I.; Myrstad, M.; Thelle, D.S. The ambiguity of physical activity, exercise and atrial fibrillation. Eur. J. Prev. Cardiol. 2018, 25, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Newman, W.; Parry-Williams, G.; Wiles, J.; Edwards, J.; Hulbert, S.; Kipourou, K.; Papadakis, M.; Sharma, R.; O’Driscoll, J. Risk of atrial fibrillation in athletes: A systematic review and meta-analysis. Br. J. Sports Med. 2021, 55, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Zhou, Y.; Zhu, W.; Liu, X. Sex-Specific Exposure-Effect Relationship Between Physical Activity and Incident Atrial Fibrillation in the General Population: A Dose-Response Meta-Analysis of 16 Prospective Studies. Front. Cardiovasc. Med. 2021, 8, 710071. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Cameli, M.; Ciccone, M.M.; Maiello, M.; Modesti, P.A.; Mondillo, S.; Muiesan, M.L.; Scicchitano, P.; Novo, S.; Palmiero, P.; et al. The controversial relationship between exercise and atrial fibrillation: Clinical studies and pathophysiological mechanisms. J. Cardiovasc. Med. 2015, 16, 802–810. [Google Scholar] [CrossRef]
- Pavlik, G.; Major, Z.; Csajagi, E.; Jeserich, M.; Kneffel, Z. The athlete’s heart. Part II: Influencing factors on the athlete’s heart: Types of sports and age (review). Acta Physiol. Hung. 2013, 100, 1–27. [Google Scholar] [CrossRef]
- Elliott, A.D.; Linz, D.; Verdicchio, C.V.; Sanders, P. Exercise and Atrial Fibrillation: Prevention or Causation? Heart Lung Circ. 2018, 27, 1078–1085. [Google Scholar] [CrossRef]
- Ostojic, M.; Ostojic, M.; Petrovic, O.; Nedeljkovic-Arsenovic, O.; Perone, F.; Banovic, M.; Stojmenovic, T.; Stojmenovic, D.; Giga, V.; Beleslin, B.; et al. Endurance Sports and Atrial Fibrillation: A Puzzling Conundrum. J. Clin. Med. 2024, 13, 7691. [Google Scholar] [CrossRef]
- Helmersson, J.; Vessby, B.; Larsson, A.; Basu, S. Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation 2004, 109, 1729–1734. [Google Scholar] [CrossRef]
- Lind, L.; Fors, N.; Hall, J.; Marttala, K.; Stenborg, A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2368–2375. [Google Scholar] [CrossRef]
- Lind, L.; Zethelius, B.; Byberg, L. Self-reported physical activity and different cardiovascular diseases-Results from updated measurements over 40 years. PLoS ONE 2022, 17, e0269402. [Google Scholar] [CrossRef] [PubMed]
- Lochen, M.L.; Rasmussen, K. The Tromso study: Physical fitness, self reported physical activity, and their relationship to other coronary risk factors. J. Epidemiol. Community Health 1992, 46, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.; Wilhelmsen, L. Physical activity protects against coronary death and deaths from all causes in middle-aged men. Evidence from a 20-year follow-up of the primary prevention study in Goteborg. Ann. Epidemiol. 1997, 7, 69–75. [Google Scholar] [CrossRef]
- Jette, M.; Sidney, K.; Blumchen, G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef]
- Lind, L.; Zethelius, B.; Lindberg, E.; Pedersen, N.L.; Byberg, L. Changes in leisure-time physical activity during the adult life span and relations to cardiovascular risk factors-Results from multiple Swedish studies. PLoS ONE 2021, 16, e0256476. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Fried, L.P.; Burke, G.L.; Fitzpatrick, A.; Siscovick, D.S. Lifestyles of older adults: Can we influence cardiovascular risk in older adults? Am. J. Geriatr. Cardiol. 2004, 13, 153–160. [Google Scholar] [CrossRef]
- Masini, A.; Cherasco, N.; Conti, A.; Pighini, I.; Barone-Adesi, F.; Panella, M. Preventive Pathways for Healthy Ageing: A Systematic Literature Review. Geriatrics 2025, 10, 31. [Google Scholar] [CrossRef]
- Forslund, T.; Wettermark, B.; Wandell, P.; von Euler, M.; Hasselstrom, J.; Hjemdahl, P. Risk scoring and thromboprophylactic treatment of patients with atrial fibrillation with and without access to primary healthcare data: Experience from the Stockholm health care system. Int. J. Cardiol. 2013, 170, 208–214. [Google Scholar] [CrossRef]
- Nielsen, J.R.; Wachtell, K.; Abdulla, J. The Relationship Between Physical Activity and Risk of Atrial Fibrillation-A Systematic Review and Meta-Analysis. J. Atr. Fibrillation 2013, 5, 789. [Google Scholar]
- Mozaffarian, D.; Furberg, C.D.; Psaty, B.M.; Siscovick, D. Physical activity and incidence of atrial fibrillation in older adults: The cardiovascular health study. Circulation 2008, 118, 800–807. [Google Scholar] [CrossRef]
- Mittal, S. Physical Activity and Incidence of Atrial Fibrillation in Older Adults: The Cardiovascular Health Study. J. Atr. Fibrillation 2008, 1, 132. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Mohanty, P.; Tamaki, M.; Natale, V.; Gianni, C.; Trivedi, C.; Gokoglan, Y.; Di Biase, L.; Natale, A. Differential Association of Exercise Intensity With Risk of Atrial Fibrillation in Men and Women: Evidence from a Meta-Analysis. J. Cardiovasc. Electrophysiol. 2016, 27, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Zacher, J.; Filipovic, K.; Predel, G.; Schmidt, T. Exercise and Atrial Fibrillation: The Dose Makes the Poison? A Narrative Review. Int. J. Sports Med. 2024, 45, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Farahmand, B.; Ahlbom, A.; Held, C.; Ljunghall, S.; Michaelsson, K.; Sundstrom, J. Risk of arrhythmias in 52,755 long-distance cross-country skiers: A cohort study. Eur. Heart J. 2013, 34, 3624–3631. [Google Scholar] [CrossRef]
- Svedberg, N.; Sundstrom, J.; James, S.; Hallmarker, U.; Hambraeus, K.; Andersen, K. Long-Term Incidence of Atrial Fibrillation and Stroke Among Cross-Country Skiers. Circulation 2019, 140, 910–920. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Anne, W.; Willems, R.; Adriaenssens, B.; Van de Werf, F.; Ector, H. Endurance sports is a risk factor for atrial fibrillation after ablation for atrial flutter. Int. J. Cardiol. 2006, 107, 67–72. [Google Scholar] [CrossRef]
- Elosua, R.; Arquer, A.; Mont, L.; Sambola, A.; Molina, L.; Garcia-Moran, E.; Brugada, J.; Marrugat, J. Sport practice and the risk of lone atrial fibrillation: A case-control study. Int. J. Cardiol. 2006, 108, 332–337. [Google Scholar] [CrossRef]
- Myrstad, M.; Aaronaes, M.; Graff-Iversen, S.; Nystad, W.; Ranhoff, A.H. Does endurance exercise cause atrial fibrillation in women? Int. J. Cardiol. 2015, 184, 431–432. [Google Scholar] [CrossRef]
- Drca, N.; Larsson, S.C.; Grannas, D.; Jensen-Urstad, M. Elite female endurance athletes are at increased risk of atrial fibrillation compared to the general population: A matched cohort study. Br. J. Sports Med. 2023, 57, 1175–1179. [Google Scholar] [CrossRef]
- Molina, L.; Mont, L.; Marrugat, J.; Berruezo, A.; Brugada, J.; Bruguera, J.; Rebato, C.; Elosua, R. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: A follow-up study. Europace 2008, 10, 618–623. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; Di Paolo, F.M.; Biffi, A.; Quattrini, F.M.; Pisicchio, C.; Roselli, A.; Caselli, S.; Culasso, F. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J. Am. Coll. Cardiol. 2005, 46, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.D.; Ariyaratnam, J.; Howden, E.J.; La Gerche, A.; Sanders, P. Influence of exercise training on the left atrium: Implications for atrial fibrillation, heart failure, and stroke. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H822–H836. [Google Scholar] [CrossRef] [PubMed]
- Gorman, R.A.; Yakobov, S.; Polidovitch, N.; Debi, R.; Sanfrancesco, V.C.; Hood, D.A.; Lakin, R.; Backx, P.H. The effects of daily dose of intense exercise on cardiac responses and atrial fibrillation. J. Physiol. 2024, 602, 569–596. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Almqvist, C.; Bonamy, A.K.; Ljung, R.; Michaelsson, K.; Neovius, M.; Stephansson, O.; Ye, W. Registers of the Swedish total population and their use in medical research. Eur. J. Epidemiol. 2016, 31, 125–136. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Andersson, E.; Ekbom, A.; Feychting, M.; Kim, J.L.; Reuterwall, C.; Heurgren, M.; Olausson, P.O. External review and validation of the Swedish national inpatient register. BMC Public Health 2011, 11, 450. [Google Scholar] [CrossRef]
| Examinations | Age 50 | Age 60 | Age 70 | Age 77 | Age 82 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD)/Proportion | N | Mean (SD)/Proportion | N | Mean (SD)/Proportion | N | Mean (SD)/Proportion | N | Mean (SD)/Proportion | |
| SBP (mmHg) | 2291 | 133.05 (18.01) | 1836 | 142.53 (19.63) | 1214 | 146.77 (18.5) | 834 | 150.52 (19.99) | 525 | 145.06 (17.47) |
| Triglycerides (mmol/L) | 2292 | 1.93 (1.24) | 1836 | 1.66 (0.7) | 1214 | 1.45 (0.77) | 834 | 1.38 (0.69) | 525 | 1.39 (0.68) |
| HDL-cholesterol (mmol/L) | 2125 | 1.36 (0.38) | 1742 | 1.28 (0.24) | 1213 | 1.28 (0.35) | 832 | 1.31 (0.32) | 524 | 1.2 (0.29) |
| LDL-cholesterol (mmol/L) | 2123 | 5.26 (1.19) | 1740 | 4.43 (0.66) | 1213 | 3.89 (0.9) | 832 | 3.47 (0.85) | 524 | 3.39 (0.84) |
| BMI (kg/m2) | 2292 | 25 (3.19) | 1836 | 25.48 (3.28) | 1214 | 26.28 (3.42) | 834 | 26.3 (3.44) | 525 | 26.09 (3.43) |
| Glucose (mmol/L) | 2290 | 5.58 (0.97) | 1836 | 5.58 (1.43) | 1214 | 5.77 (1.47) | 834 | 5.88 (1.38) | 525 | 5.94 (1.24) |
| Antihypertensive medication (%) | 2322 | 4 | 1860 | 19 | 1210 | 35 | 782 | 42 | 477 | 54 |
| Lipid lowering medication (%) | 2322 | 1 | 1860 | 6 | 1151 | 9 | 782 | 17 | 477 | 21 |
| Antidiabetic medication (%) | 2322 | 1 | 1855 | 2 | 1151 | 6 | 782 | 9 | 477 | 10 |
| MetS (%) | 2123 | 12 | 1737 | 12 | 1145 | 15 | 775 | 15 | 474 | 20 |
| MetS components | 2123 | 1.35 (0.97) | 1737 | 1.39 (0.9) | 1145 | 1.52 (0.94) | 775 | 1.57 (0.92) | 474 | 1.71 (0.96) |
| Examinations | N | Mean (SD)/Proportion |
|---|---|---|
| Sex (% females) | 1016 | 50 |
| SBP (mmHg) | 1012 | 149.63 (22.68) |
| Triglycerides (mmol/L) | 1013 | 1.28 (0.6) |
| HDL-cholesterol (mmol/L) | 1013 | 1.51 (0.43) |
| LDL-cholesterol (mmol/L) | 1011 | 3.38 (0.88) |
| BMI (kg/m2) | 1016 | 27.03 (4.33) |
| Glucose (mmol/L) | 1013 | 5.34 (1.61) |
| Antihypertensive medication (%) | 1013 | 31 |
| Lipid lowering medication (%) | 1016 | 15 |
| ULSAM | PIVUS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PA Level | Age 50 | Age 60 | Age 70 | Age 77 | Age 70 | |||||
| N | % | N | % | N | % | N | % | N | % | |
| 1 | 324 | 14.71 | 176 | 10.75 | 43 | 3.92 | 63 | 8.21 | 113 | 11.76 |
| 2 | 800 | 36.33 | 863 | 52.72 | 370 | 33.70 | 271 | 35.33 | 594 | 61.81 |
| 3 | 967 | 43.91 | 536 | 32.74 | 619 | 56.38 | 399 | 52.02 | 208 | 21.64 |
| 4 | 111 | 5.04 | 62 | 3.79 | 66 | 6.01 | 34 | 4.43 | 46 | 4.79 |
| Total | 2202 | 1637 | 1098 | 767 | 961 | |||||
| ULSAM | PIVUS | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| PA level 1 | 1 (ref) | 1 (ref) | ||||
| PA level 2 | 1.22 | 0.84–1.76 | 0.29 | 0.74 | 0.49–1.13 | 0.16 |
| PA level 3 | 1.26 | 0.87–1.81 | 0.22 | 0.75 | 0.46–1.21 | 0.23 |
| PA level 4 | 1.21 | 0.72–2.04 | 0.48 | 1.10 | 0.57–2.13 | 0.78 |
| HR | CI (95%) | p-Value | |
|---|---|---|---|
| PA level 2 unadjusted | 1.37 | 1.06–1.77 | 0.015 |
| PA level 3 unadjusted | 1.39 | 1.05–1.84 | 0.021 |
| PA level 4 unadjusted | 1.14 | 0.77–1.68 | 0.520 |
| PA level 2 adjusted | 1.29 | 0.98–1.70 | 0.071 |
| PA level 3 adjusted | 1.33 | 0.99–1.79 | 0.057 |
| PA level 4 adjusted | 1.19 | 0.79–1.80 | 0.395 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wändell, P.; Enarsson, M.; Feldreich, T.; Lind, L.; Ärnlöv, J.; Carlsson, A.C. High Physical Activity Level and the Long-Term Risk of Atrial Fibrillation in Two Swedish Cohorts. Geriatrics 2025, 10, 80. https://doi.org/10.3390/geriatrics10030080
Wändell P, Enarsson M, Feldreich T, Lind L, Ärnlöv J, Carlsson AC. High Physical Activity Level and the Long-Term Risk of Atrial Fibrillation in Two Swedish Cohorts. Geriatrics. 2025; 10(3):80. https://doi.org/10.3390/geriatrics10030080
Chicago/Turabian StyleWändell, Per, Malin Enarsson, Tobias Feldreich, Lars Lind, Johan Ärnlöv, and Axel Carl Carlsson. 2025. "High Physical Activity Level and the Long-Term Risk of Atrial Fibrillation in Two Swedish Cohorts" Geriatrics 10, no. 3: 80. https://doi.org/10.3390/geriatrics10030080
APA StyleWändell, P., Enarsson, M., Feldreich, T., Lind, L., Ärnlöv, J., & Carlsson, A. C. (2025). High Physical Activity Level and the Long-Term Risk of Atrial Fibrillation in Two Swedish Cohorts. Geriatrics, 10(3), 80. https://doi.org/10.3390/geriatrics10030080