Implications of Intra-Individual Variability in Motor Performance on Functional Mobility in Stroke Survivors
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Experimental Procedures
2.4. Goal-Directed Movements
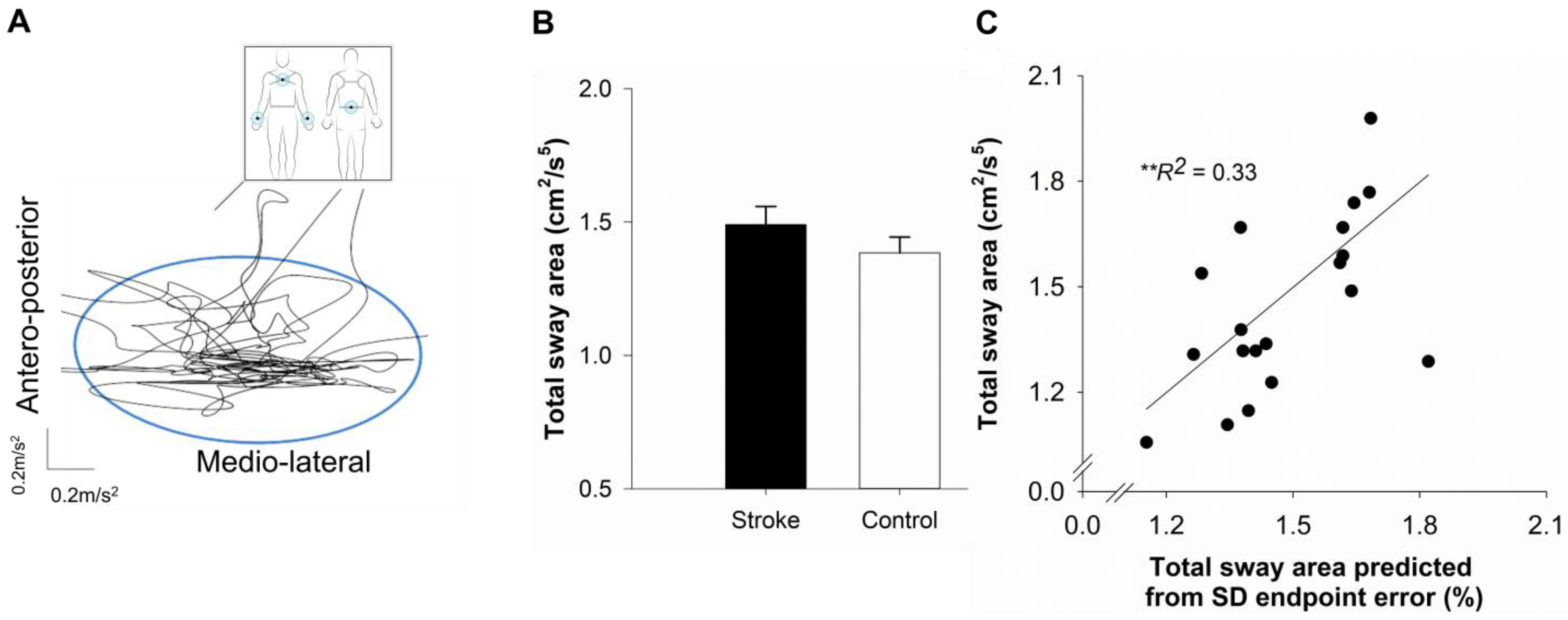
2.5. Postural Balance
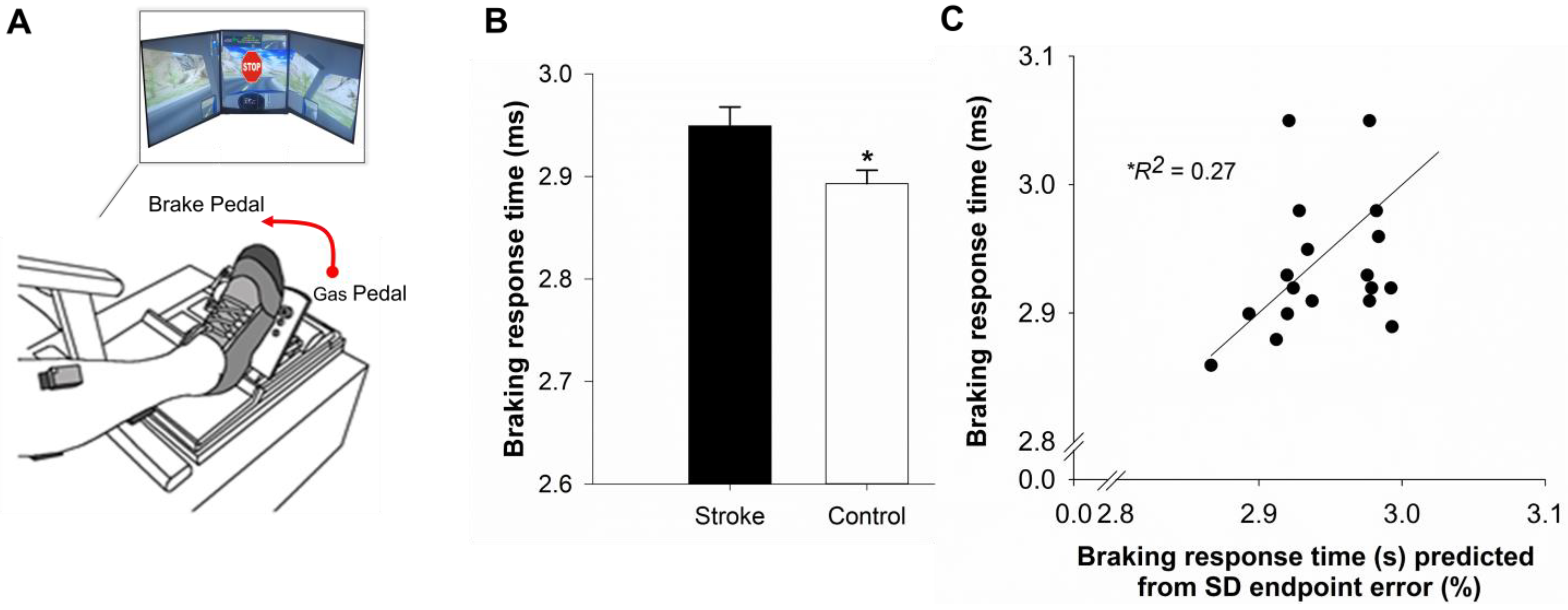
2.6. Simulated Driving
3. Statistical Analysis
4. Results
4.1. Clinical Characteristics
4.2. Performance on Goal-Directed Task
4.3. Performance on Functional Mobility Tasks
4.4. Association Between the Functional Mobility Outcomes, Mean Endpoint Error, and IIV of Endpoint Error
4.5. Predicting Sway Area Using Hierarchical Regression
4.6. Predicting Braking Response Time Using Hierarchical Regression
5. Discussion
5.1. Stroke Amplifies Intra-Individual Variability in Motor Performance
5.2. Stroke-Related Increase in IIV in Motor Performance Relates to Postural Balance
5.3. Stroke-Related Increase in IIV in Motor Performance Relates to Delayed Braking
5.4. Considerations and Clinical Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| cm | centimeters |
| DHQ | Driving Habits Questionnaire |
| FMA | Fugl–Meyer Assessment |
| IIV | intra-individual variability |
| LL | lower limit |
| MoCA | Montreal Cognitive Assessment |
| ms | milliseconds |
| s | seconds |
| SD | standard deviation |
| UL | upper limit |
References
- Patel, A.T.; Duncan, P.W.; Lai, S.M.; Studenski, S. The relation between impairments and functional outcomes poststroke. Arch. Phys. Med. Rehab. 2000, 81, 1357–1363. [Google Scholar]
- Rafsten, L.; Meirelles, C.; Danielsson, A.; Sunnerhagen, K.S. Impaired Motor Function in the Affected Arm Predicts Impaired Postural Balance After Stroke: A Cross Sectional Study. Front. Neurol. 2019, 10, 912. [Google Scholar]
- Eng, J.J.; Kim, C.M.; Macintyre, D.L. Reliability of lower extremity strength measures in persons with chronic stroke. Arch. Phys. Med. Rehabil. 2002, 83, 322–328. [Google Scholar]
- Rohrer, B.; Fasoli, S.; Krebs, H.I.; Hughes, R.; Volpe, B.; Frontera, W.R.; Stein, J.; Hogan, N. Movement smoothness changes during stroke recovery. J. Neurosci. 2002, 22, 8297–8304. [Google Scholar]
- Lam, T.K.; Binns, M.A.; Honjo, K.; Dawson, D.R.; Ross, B.; Stuss, D.T.; Black, S.E.; Chen, J.J.; Fujioka, T.; Chen, J.L. Variability in stroke motor outcome is explained by structural and functional integrity of the motor system. Sci. Rep. 2018, 8, 9480. [Google Scholar] [CrossRef] [PubMed]
- Nesselroade, J.R.; Salthouse, T.A. Methodological and theoretical implications of intraindividual variability in perceptual-motor performance. J. Gerontol. B Psychol. Sci. Soc. Sci. 2004, 59, P49–P55. [Google Scholar]
- Yacoubi, B.; Christou, E.A. Motor Output Variability in Movement Disorders: Insights from Essential Tremor. Exerc. Sport Sci. Rev. 2024, 52, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Munsell, E.G.S.; Bui, Q.; Kaufman, K.J.; Tomazin, S.E.; Regan, B.A.; Lenze, E.J.; Lee, J.M.; Mohr, D.C.; Fong, M.W.M.; Metts, C.L.; et al. Intraindividual variability in post-stroke cognition and its relationship with activities of daily living and social functioning: An ecological momentary assessment approach. Top. Stroke Rehabil. 2024, 31, 564–575. [Google Scholar]
- Jackson, J.D.; Balota, D.A.; Duchek, J.M.; Head, D. White matter integrity and reaction time intraindividual variability in healthy aging and early-stage Alzheimer disease. Neuropsychologia 2012, 50, 357–366. [Google Scholar]
- MacDonald, S.W.; Nyberg, L.; Backman, L. Intra-individual variability in behavior: Links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006, 29, 474–480. [Google Scholar]
- Fjell, A.M.; Westlye, L.T.; Amlien, I.K.; Walhovd, K.B. Reduced White Matter Integrity Is Related to Cognitive Instability. J. Neurosci. 2011, 31, 18060–18072. [Google Scholar] [CrossRef]
- Stuss, D.T.; Pogue, J.; Buckle, L.; Bondar, J. Characterization of Stability of Performance in Patients with Traumatic Brain Injury: Variability and Consistency on Reaction Time Tests. Neuropsychology 1994, 8, 316–324. [Google Scholar]
- Burton, C.L.; Hultsch, D.F.; Strauss, E.; Hunter, M.A. Intraindividual variability in physical and emotional functioning: Comparison of adults with traumatic brain injuries and healthy adults. Clin. Neuropsychol. 2002, 16, 264–279. [Google Scholar] [CrossRef]
- Burton, C.L.; Strauss, E.; Hultsch, D.F.; Moll, A.; Hunter, M.A. Intraindividual variability as a marker of neurological dysfunction: A comparison of Alzheimer’s disease and Parkinson’s disease. J. Clin. Exp. Neuropsyc. 2006, 28, 67–83. [Google Scholar]
- Davis, J.J.; Sivaramakrishnan, A.; Rolin, S.; Subramanian, S. Intra-individual variability in cognitive performance predicts functional decline in Parkinson’s disease. Appl. Neuropsych.-Adul. 2023. [Google Scholar] [CrossRef]
- Wojtowicz, M.; Berrigan, L.I.; Fisk, J.D. Intra-individual Variability as a Measure of Information Processing Difficulties in Multiple Sclerosis. Int. J. MS Care 2012, 14, 77–83. [Google Scholar]
- Mazerolle, E.L.; Wojtowicz, M.A.; Omisade, A.; Fisk, J.D. Intra-individual variability in information processing speed reflects white matter microstructure in multiple sclerosis. Neuroimage Clin. 2013, 2, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.S.; Dogan, I.; Schulz, J.B.; Reetz, K. Going beyond the mean: Intraindividual variability of cognitive performance in prodromal and early neurodegenerative disorders. Clin. Neuropsychol. 2019, 33, 369–389. [Google Scholar] [CrossRef]
- Mumme, R.; Pushpanathan, M.; Donaldson, S.; Weinborn, M.; Rainey-Smith, S.R.; Maruff, P.; Bucks, R.S. Longitudinal Association of Intraindividual Variability with Cognitive Decline and Dementia: A Meta-Analysis. Neuropsychology 2021, 35, 669–678. [Google Scholar] [CrossRef]
- Kawahira, K.; Shimodozono, M.; Ogata, A.; Etoh, S.; Ikeda, S.; Yoshida, A.; Tanaka, N.; Tsujio, S. Impaired visuo-motor skills in the unaffected lower limb of patients with stroke. Int. J. Neurosci. 2005, 115, 1315–1332. [Google Scholar]
- Lindberg, P.G.; Roche, N.; Robertson, J.; Roby-Brami, A.; Bussel, B.; Maier, M.A. Affected and unaffected quantitative aspects of grip force control in hemiparetic patients after stroke. Brain Res. 2012, 1452, 96–107. [Google Scholar]
- Mukherjee, M.; Koutakis, P.; Siu, K.C.; Fayad, P.B.; Stergiou, N. Stroke Survivors Control the Temporal Structure of Variability During Reaching in Dynamic Environments. Ann. Biomed. Eng. 2013, 41, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Sendhilnathan, N.; Basu, D.; Murthy, A. Assessing within-trial and across-trial neural variability in macaque frontal eye fields and their relation to behaviour. Eur. J. Neurosci. 2020, 52, 4267–4282. [Google Scholar]
- Ranganathan, R.; Gebara, R.; Andary, M.; Sylvain, J. Chronic stroke survivors show task-dependent modulation of motor variability during bimanual coordination. J. Neurophysiol. 2019, 121, 756–763. [Google Scholar] [PubMed]
- Lang, C.E.; Wagner, J.M.; Bastian, A.J.; Hu, Q.L.; Edwards, D.F.; Sahrmann, S.A.; Dromerick, A.W. Deficits in grasp versus reach during acute hemiparesis. Exp. Brain Res. 2005, 166, 126–136. [Google Scholar] [CrossRef]
- Roalf, D.R.; Quarmley, M.; Mechanic-Hamilton, D.; Wolk, D.A.; Arnold, S.E.; Moberg, P.J. Alzheimer’s Disease Neuroimaging I: Within-Individual Variability: An Index for Subtle Change in Neurocognition in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2016, 54, 325–335. [Google Scholar] [CrossRef]
- Haynes, B.I.; Bauermeister, S.; Bunce, D. A Systematic Review of Longitudinal Associations Between Reaction Time Intraindividual Variability and Age-Related Cognitive Decline or Impairment, Dementia, and Mortality. J. Int. Neuropsych. Soc. 2017, 23, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, F.X.; Sonuga-Barke, E.J.S.; Scheres, A.; Di Martino, A.; Hyde, C.; Walters, J.R. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol. Psychiat. 2005, 57, 1416–1423. [Google Scholar]
- MacPherson, S.E.; Gillebert, C.R.; Robinson, G.A.; Vallesi, A. Editorial: Intra- and Inter-individual Variability of Executive Functions: Determinant and Modulating Factors in Healthy and Pathological Conditions. Front. Psychol. 2019, 10, 432. [Google Scholar]
- Borges, S.D.; Radanovic, M.; Forlenza, O.V. Correlation between functional mobility and cognitive performance in older adults with cognitive impairment. Aging Neuropsychol. C 2018, 25, 23–32. [Google Scholar]
- Gothe, N.P.; Fanning, J.; Awick, E.; Chung, D.; Wójcicki, T.R.; Olson, E.A.; Mullen, S.P.; Voss, M.; Erickson, K.I.; Kramer, A.F.; et al. Executive Function Processes Predict Mobility Outcomes in Older Adults. J. Am. Geriatr. Soc. 2014, 62, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Webber, S.C.; Porter, M.M.; Menec, V.H. Mobility in older adults: A comprehensive framework. Gerontologist 2010, 50, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.J.; Ivanova, T.D.; Mochizuki, G. Recovery of standing balance and health-related quality of life after mild or moderately severe stroke. Arch. Phys. Med. Rehabil. 2007, 88, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Griffen, J.A.; Rapport, L.J.; Bryer, R.C.; Scott, C.A. Driving status and community integration after stroke. Top. Stroke Rehabil. 2009, 16, 212–221. [Google Scholar] [CrossRef]
- Bielak, A.A.; Hultsch, D.F.; Strauss, E.; Macdonald, S.W.; Hunter, M.A. Intraindividual variability in reaction time predicts cognitive outcomes 5 years later. Neuropsychology 2010, 24, 731–741. [Google Scholar] [CrossRef]
- Lövdén, M.; Li, S.C.; Shing, Y.L.; Lindenberger, U. Within-person trial-to-trial variability precedes and predicts cognitive decline in old and very old age: Longitudinal data from the Berlin Aging Study. Neuropsychologia 2007, 45, 2827–2838. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehab. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Owsley, C.; Stalvey, B.; Wells, J.; Sloane, M.E. Older drivers and cataract: Driving habits and crash risk. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, M203–M211. [Google Scholar]
- Casamento-Moran, A.; Chen, Y.T.; Kwon, M.; Snyder, A.; Subramony, S.H.; Vaillancourt, D.E.; Christou, E.A. Force dysmetria in spinocerebellar ataxia 6 correlates with functional capacity. Front. Hum. Neurosci. 2015, 9, 184. [Google Scholar] [CrossRef]
- Christou, E.A.; Poston, B.; Enoka, J.A.; Enoka, R.M. Different neural adjustments improve endpoint accuracy with practice in young and old adults. J. Neurophysiol. 2007, 97, 3340–3350. [Google Scholar] [CrossRef] [PubMed]
- Casamento-Moran, A.; Chen, Y.T.; Lodha, N.; Yacoubi, B.; Christou, E.A. Motor plan differs for young and older adults during similar movements. J. Neurophysiol. 2017, 117, 1483–1488. [Google Scholar]
- Casamento-Moran, A.; Fleeman, R.; Chen, Y.T.; Kwon, M.; Fox, E.J.; Yacoubi, B.; Christou, E.A. Neuromuscular variability and spatial accuracy in children and older adults. J. Electromyogr. Kinesiol. 2018, 41, 27–33. [Google Scholar] [CrossRef]
- Casamento-Moran, A.; Hunter, S.K.; Chen, Y.T.; Kwon, M.H.; Fox, E.J.; Yacoubi, B.; Christou, E.A. Sex differences in spatial accuracy relate to the neural activation of antagonistic muscles in young adults. Exp. Brain Res. 2017, 235, 2425–2436. [Google Scholar]
- Delmas, S.; Choi, Y.J.; Komer, M.; Weintraub, M.; Yacoubi, B.; Christou, E.A. Older adults use a motor plan that is detrimental to endpoint control. Sci. Rep. 2021, 11, 7562. [Google Scholar] [CrossRef] [PubMed]
- Lodha, N.; Patel, P.; Casamento-Moran, A.; Gauger, K.; Christou, E.A. Endpoint accuracy of goal-directed ankle movements correlates to over-ground walking in stroke. Clin. Neurophysiol. 2019, 130, 1008–1016. [Google Scholar] [PubMed]
- Jung, H.G.; Yamasaki, M. Association of lower extremity range of motion and muscle strength with physical performance of community-dwelling older women. J. Physiol. Anthropol. 2016, 35, 30. [Google Scholar]
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Translating Research into Clinical Practice, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Mancini, M.; Horak, F.B. Potential of APDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert Rev. Med. Devic. 2016, 13, 455–462. [Google Scholar]
- Morris, R.; Stuart, S.; McBarron, G.; Fino, P.C.; Mancini, M.; Curtze, C. Validity of Mobility Lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiol. Meas. 2019, 40, 095003. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Altenburger, P.; Ambike, S.S.; Haddad, J.M. Integrating Motor Variability Evaluation Into Movement System Assessment. Phys. Ther. 2023, 103, pzad075. [Google Scholar]
- Schaefer, S.Y.; Mutha, P.K.; Haaland, K.Y.; Sainburg, R.L. Hemispheric specialization for movement control produces dissociable differences in online corrections after stroke. Cereb. Cortex 2012, 22, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Pohl, P.S. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp. Brain Res. 1995, 105, 163–174. [Google Scholar] [PubMed]
- Hultsch, D.F.; MacDonald, S.W.; Hunter, M.A.; Levy-Bencheton, J.; Strauss, E. Intraindividual variability in cognitive performance in older adults: Comparison of adults with mild dementia, adults with arthritis, and healthy adults. Neuropsychology 2000, 14, 588–598. [Google Scholar] [PubMed]
- Marigold, D.S.; Eng, J.J.; Tokuno, C.D.; Donnelly, C.A. Contribution of muscle strength and integration of afferent input to postural instability in persons with stroke. Neurorehabil. Neural Repair 2004, 18, 222–229. [Google Scholar]
- Garland, S.J.; Gray, V.L.; Knorr, S. Muscle activation patterns and postural control following stroke. Motor Control 2009, 13, 387–411. [Google Scholar]
- Rahimzadeh Khiabani, R.; Mochizuki, G.; Ismail, F.; Boulias, C.; Phadke, C.P.; Gage, W.H. Impact of Spasticity on Balance Control during Quiet Standing in Persons after Stroke. Stroke Res. Treat. 2017, 2017, 6153714. [Google Scholar]
- Bunce, D.; Haynes, B.I.; Lord, S.R.; Gschwind, Y.J.; Kochan, N.A.; Reppermund, S.; Brodaty, H.; Sachdev, P.S.; Delbaere, K. Intraindividual Stepping Reaction Time Variability Predicts Falls in Older Adults with Mild Cognitive Impairment. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 832–837. [Google Scholar]
- Reelick, M.F.; Kessels, R.P.; Faes, M.C.; Weerdesteyn, V.; Esselink, R.A.; Olde Rikkert, M.G. Increased intra-individual variability in stride length and reaction time in recurrent older fallers. Aging Clin. Exp. Res. 2011, 23, 393–399. [Google Scholar] [CrossRef]
- Aufman, E.L.; Bland, M.D.; Barco, P.P.; Carr, D.B.; Lang, C.E. Predictors of return to driving after stroke. Am. J. Phys. Med. Rehabil. 2013, 92, 627–634. [Google Scholar]
- Lodha, N.; Patel, P.; Casamento-Moran, A.; Hays, E.; Poisson, S.N.; Christou, E.A. Strength or Motor Control: What Matters in High-Functioning Stroke? Front. Neurol. 2018, 9, 1160. [Google Scholar]
- Patel, P.; Alam, T.; Tracy, B.L.; Lodha, N. Impaired force control contributes to car steering dysfunction in chronic stroke. Disabil. Rehabil. 2021, 43, 1948–1954. [Google Scholar] [PubMed]
- Devos, H.; Tant, M.; Akinwuntan, A.E. On-road driving impairments and associated cognitive deficits after stroke. Cerebrovasc. Dis. 2014, 38, 226–232. [Google Scholar] [PubMed]
- Hird, M.A.; Vetivelu, A.; Saposnik, G.; Schweizer, T.A. Cognitive, on-road, and simulator-based driving assessment after stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 2654–2670. [Google Scholar] [PubMed]
- Lodha, N.; Patel, P.; Shad, J.M.; Casamento-Moran, A.; Christou, E.A. Cognitive and motor deficits contribute to longer braking time in stroke. J. Neuroeng. Rehabil. 2021, 18, 7. [Google Scholar]
- Perrier, M.J.; Korner-Bitensky, N.; Mayo, N.E. Patient factors associated with return to driving poststroke: Findings from a multicenter cohort study. Arch. Phys. Med. Rehabil. 2010, 91, 868–873. [Google Scholar]
- Hird, M.A.; Vesely, K.A.; Christie, L.E.; Alves, M.A.; Pongmoragot, J.; Saposnik, G.; Schweizer, T.A. Is it safe to drive after acute mild stroke? A preliminary report. J. Neurol. Sci. 2015, 354, 46–50. [Google Scholar]
- Kwon, M.; Christou, E.A. Visual information processing in older adults: Reaction time and motor unit pool modulation. J. Neurophysiol. 2018, 120, 2630–2639. [Google Scholar]
- Kennedy, Q.; Taylor, J.; Heraldez, D.; Noda, A.; Lazzeroni, L.C.; Yesavage, J. Intraindividual variability in basic reaction time predicts middle-aged and older pilots’ flight simulator performance. J. Gerontol. B Psychol. Sci. Soc. Sci. 2013, 68, 487–494. [Google Scholar]
- Duncan, P.W.; Sullivan, K.J.; Behrman, A.L.; Azen, S.P.; Wu, S.S.; Nadeau, S.E.; Dobkin, B.H.; Rose, D.K.; Tilson, J.K.; Cen, S.; et al. Body-weight-supported treadmill rehabilitation after stroke. N. Engl. J. Med. 2011, 364, 2026–2036. [Google Scholar] [CrossRef]
- Hornby, T.G.; Henderson, C.E.; Plawecki, A.; Lucas, E.; Lotter, J.; Holthus, M.; Brazg, G.; Fahey, M.; Woodward, J.; Ardestani, M.; et al. Contributions of Stepping Intensity and Variability to Mobility in Individuals Poststroke. Stroke 2019, 50, 2492–2499. [Google Scholar]
- Shmuelof, L.; Krakauer, J.W.; Mazzoni, P. How is a motor skill learned? Change and invariance at the levels of task success and trajectory control. J. Neurophysiol. 2012, 108, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Mawase, F.; Bar-Haim, S.; Joubran, K.; Rubin, L.; Karniel, A.; Shmuelof, L. Increased Adaptation Rates and Reduction in Trial-by-Trial Variability in Subjects with Cerebral Palsy Following a Multi-session Locomotor Adaptation Training. Front. Hum. Neurosci. 2016, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Hammerbeck, U.; Yousif, N.; Hoad, D.; Greenwood, R.; Diedrichsen, J.; Rothwell, J.C. Chronic Stroke Survivors Improve Reaching Accuracy by Reducing Movement Variability at the Trained Movement Speed. Neurorehabil. Neural Repair 2017, 31, 499–508. [Google Scholar] [CrossRef] [PubMed]

| Participant Characteristics | Stroke (n = 20) | Control (n = 20) |
|---|---|---|
| Age (years) | 62.01 ± 14.39 | 62.01 ± 10.18 |
| Sex (Male/Female), n | 12/8 | 10/10 |
| Fugl–Meyer Assessment-lower extremity (/34) | 26.39 ± 6.33 | n/a |
| Montreal Cognitive Assessment (/30) | 21.90 ± 4.33 | 26.65 ± 2.18 |
| Driving Habits Questionnaire (/15) | 8.25 ± 5.80 | 13.95 ± 4.34 |
| No. of individuals using walking aid | 2 | 0 |
| Hemiparetic side (left/right), n | 11/9 | n/a |
| Time since stroke (years) | 4.85 ± 3.95 | n/a |
| Stroke location | 12 cortical, 3 sub-cortical, 5 unavailable | n/a |
| Stroke (n = 20) | Control (n = 20) | |
|---|---|---|
| Endpoint error (%) | ||
| Mean | 83.66 ± 66.23 | 52.54 ± 30.58 |
| Within-person SD | 53.66 ± 37.29 | 30.16 ± 19.32 |
| Sway area (cm2/s5) | 40.97 ± 40.92 | 29.09 ± 19.59 |
| Braking response time (ms) | 910 ± 200 | 790 ± 110 |
| Model Summary | |||||||
|---|---|---|---|---|---|---|---|
| Variable | B | 95% CI for B | SE B | β | R2 | ΔR2 | |
| LL | UL | ||||||
| Step 1 | 0.327 ** | 0.327 ** | |||||
| Constant | 0.559 | −0.114 | 1.232 | 0.320 | |||
| IIV of endpoint error | 0.571 | 0.165 | 0.977 | 0.193 | 0.572 | ||
| Step 2 | 0.017 * | 0.017 * | |||||
| Constant | 0.719 | −0.137 | 1.575 | 0.406 | |||
| IIV of endpoint error | 0.701 | 0.114 | 1.288 | 0.278 | 0.702 | ||
| Mean endpoint error | −0.204 | −0.855 | 0.448 | 0.309 | −0.184 | ||
| Model Summary | |||||||
|---|---|---|---|---|---|---|---|
| Variable | B | 95% CI for B | SE B | β | R2 | ΔR2 | |
| LL | UL | ||||||
| Step 1 | 0.265 | 0.265 * | |||||
| Constant | 2.725 | 2.531 | 2.919 | 0.092 | |||
| IIV of endpoint error | 0.136 | 0.020 | 0.252 | 0.055 | 0.515 | ||
| Step 2 | 0.296 | 0.031 | |||||
| Constant | 2.663 | 2.412 | 2.915 | 0.119 | |||
| IIV of endpoint error | 0.093 | −0.067 | 0.253 | 0.076 | 0.351 | ||
| Mean endpoint error | 0.072 | −0.110 | 0.254 | 0.086 | 0.240 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lodha, N.; Patel, P.; Christou, E.A.; Tiwari, A.; Diehl, M. Implications of Intra-Individual Variability in Motor Performance on Functional Mobility in Stroke Survivors. Geriatrics 2025, 10, 51. https://doi.org/10.3390/geriatrics10020051
Lodha N, Patel P, Christou EA, Tiwari A, Diehl M. Implications of Intra-Individual Variability in Motor Performance on Functional Mobility in Stroke Survivors. Geriatrics. 2025; 10(2):51. https://doi.org/10.3390/geriatrics10020051
Chicago/Turabian StyleLodha, Neha, Prakruti Patel, Evangelos A. Christou, Anjali Tiwari, and Manfred Diehl. 2025. "Implications of Intra-Individual Variability in Motor Performance on Functional Mobility in Stroke Survivors" Geriatrics 10, no. 2: 51. https://doi.org/10.3390/geriatrics10020051
APA StyleLodha, N., Patel, P., Christou, E. A., Tiwari, A., & Diehl, M. (2025). Implications of Intra-Individual Variability in Motor Performance on Functional Mobility in Stroke Survivors. Geriatrics, 10(2), 51. https://doi.org/10.3390/geriatrics10020051





