Prognosis of Early-Onset vs. Late-Onset Mild Cognitive Impairment: Comparison of Conversion Rates and Its Predictors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Material and Neuropsychological Testing
2.3. Genotyping and CSF biomarkers
2.4. Statistical Analysis
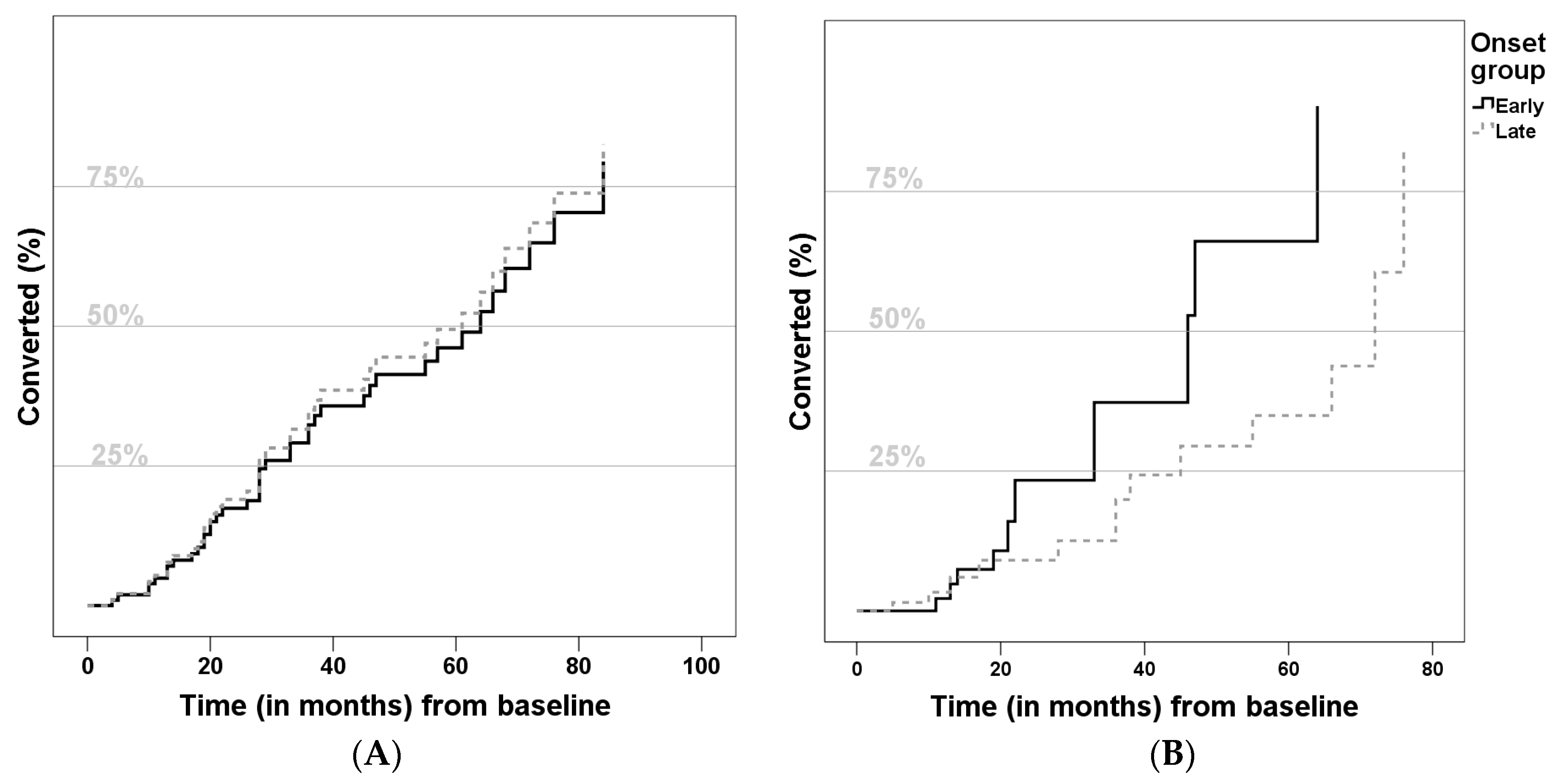
3. Results
4. Discussion
5. Conclusions
Aknowledgments
Author Contributions
Conflicts of Interest
References
- Lobo, A.; Launer, L.J.; Fratiglioni, L.; Andersen, K.; Di Carlo, A.; Breteler, M.M.; Copeland, J.R.; Dartigues, J.F.; Jagger, C.; Martinez-Lage, J.; et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurolo. Dise. Elder. Res. Group Neurol. 2000, 54, S4–S9. [Google Scholar]
- Harvey, R.J.; Skelton-Robinson, M.; Rossor, M.N. The prevalence and causes of dementia in people under the age of 65 years. J. Neurol. Psychiatry 2003, 74, 1206–1209. [Google Scholar] [CrossRef]
- Morris, J.C. Early-stage and preclinical Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2005, 1, 163–165. [Google Scholar]
- Bird, T.D. Alzheimer Disease Overview. In GeneReviews®; Pagon, R.A., Adam, M.P., Bird, T.D., Dolan, C.R., Fong, C.T., Stephens, K., Eds.; GeneReviews: Seattle, WA, USA, 1993. [Google Scholar]
- Sa, F.; Pinto, P.; Cunha, C.; Lemos, R.; Letra, L.; Simoes, M.; Santana, I. Differences between Early and Late-Onset Alzheimer’s Disease in Neuropsychological. Tests. Front. Neurol. 2012, 3, 81. [Google Scholar] [PubMed]
- Licht, E.A.; McMurtray, A.M.; Saul, R.E.; Mendez, M.F. Cognitive differences between early- and late-onset. Am. J. Alzheimer’s Dis. Dement. 2007, 22, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Li, Y.; Aggarwal, N.T.; Barnes, L.L.; McCann, J.J.; Gilley, D.W.; Evans, D.A. Education and the course of cognitive decline in Alzheimer disease. Neurology 2004, 63, 1198–2202. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.S.; Seo, S.W.; Lee, Y.; Kim, S.Y.; Choi, S.H.; Lee, Y.M.; Kim, H.; Han, H.J.; Na, D.L.; Kim, E.J. Neuropsychological performance and conversion to Alzheimer’s disease in early- compared to late-onset amnestic mild cognitive impairment: CREDOS study. Dement. Geriatric. Cogn. Disord. 2012, 34, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.; Teri, L.; Kukull, W.; McCormick, W.; McCurry, S.M.; Larson, E.B. Progression to dementia in patients with isolated memory loss. Lancet 1997, 349, 763–765. [Google Scholar] [CrossRef]
- Morris, J.C.; Cummings, J. Mild cognitive impairment (MCI) represents early-stage Alzheimer’s disease. J. Alzheimer’s Dis. 2005, 7, 235–239. [Google Scholar]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Cummings, J.L.; DeKosky, S.T.; Barberger-Gateau, P.; Delacourte, A.; Frisoni, F.; Fox, N.C.; Galasko, D.; et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010, 9, 1118–1127. [Google Scholar] [CrossRef]
- Tierney, M.C.; Szalai, J.P.; Dunn, E.; Geslani, D.; McDowell, I. Prediction of probable Alzheimer disease in patients with symptoms suggestive of memory impairment: Value of the Mini-Mental State Examination. Arch. Fam. Med. 2000, 9, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Smith, G.E.; Ivnik, R.J.; Tangalos, E.G.; Schaid, D.J.; Thibodeau, S.N.; Kokmen, E.; Waring, S.C.; Kurland, L.T. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. J. Am. Med. Assoc. 1995, 273, 1274–1278. [Google Scholar] [CrossRef]
- Risacher, S.L.; Saykin, A.J.; West, J.D.; Shen, L.; Firpi, H.A.; McDonald, B. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr. Alzheimer Res. 2009, 6, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, L.G.; Di, L.J.; Duffy, E.L.; Brook, J.; Elashoff, D.; Tseng, C.H.; Fairbanks, L.; Cummings, J.L. Risk factors for behavioral abnormalities in mild cognitive impairment and mild Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2014, 37, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Sachs-Ericsson, N.; Moxley, J.H.; Corsentino, E.; Rushing, N.C.; Sheffler, J.; Selby, E.A.; Gotlib, I.; Steffens, D.C. Melancholia in later life: Late and early onset differences in presentation, course, and dementia risk. Int. J. Geriatr. Psychiatry 2014, 29, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Rossor, M.N.; Fox, N.C.; Mummery, C.J.; Schott, J.M.; Warren, J.D. The diagnosis of young-onset dementia. Lancet Neurol. 2010, 9, 793–806. [Google Scholar] [CrossRef]
- Anstey, K.J.; Cherbuin, N.; Eramudugolla, R.; Sargent-Cox, K.; Easteal, S.; Kumar, R.; Sachdev, P. Characterizing mild cognitive disorders in the young-old over 8 years: Prevalence, estimated incidence, stability of diagnosis, and impact on IADLs. Alzheimer’s Demen. 2013, 9, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, M.; Lee, C.W.; Snitz, B.E.; Hughes, T.F.; McDade, E.; Chang, C.C. Rates and risk factors for progression to incident dementia vary by age in a population cohort. Neurology 2015, 84, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Rusted, J.M.; Evans, S.L.; King, S.L.; Dowell, N.; Tabet, N.; Tofts, P.S. APOE e4 polymorphism in young adults is associated with improved attention and indexed by distinct neural signatures. NeuroImage 2013, 65, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Marchant, N.L.; King, S.L.; Tabet, N.; Rusted, J.M. Positive effects of cholinergic stimulation favor young APOE epsilon4 carriers. Neuropsychopharmacology 2010, 35, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Devanand, D.P.; Richards, M.; Miller, L.W.; Marder, K.; Bell, K.; Dooneief, G.; Bylsma, F.W.; Lafleche, G.; Albert, M.; et al. A standardized technique for establishing onset and duration of symptoms of Alzheimer’s disease. Archives Neurol. 1995, 52, 961–966. [Google Scholar] [CrossRef]
- Diagnostic and Statistical Manual of Mental Disorders, 5nd ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013.
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.C.; Tatemichi, T.K.; Erkinjuntti, T.; Cummings, J.L.; Masdeu, J.C.; Garcia, J.H.; Amaducci, L.; Orgogozo, J.-M.; Brun, A.; Hofman, A.; et al. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993, 43, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, M.S.; Botelho, P.; Leitao, M.; Adaptaçao, O. A populaçao portuguesa da traduçao do "Mini Mental State Examination" (MMSE). Rev. Port. Neurologia 1994, 1, 9–10. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatric Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Freitas, S.; Simoes, M.R.; Alves, L.; Santana, I. Montreal Cognitive Assessment (MoCA): Normative study for the Portuguese population. J. Clin. Exp. Neuropsychol. 2011, 33, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatrics Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, M.; Fonseca, S.; Barreto, J.; Garcia, C. Escalas e Testes na Demencia. GEECD 2008, 1, 41–68. [Google Scholar]
- Mohs, R.C.; Rosen, W.G.; Davis, K.L. The Alzheimer’s disease assessment scale: an instrument for assessing treatment efficacy. Psychopharmacol. Bull. 1983, 19, 448–450. [Google Scholar] [PubMed]
- Guerreiro, M. Contributo da Neuropsicologia Para o Estudo Das Demencias [Contribution of Neuropsychology to the study of dementia], Faculdade de Medicina da Universidade de Lisboa, Lisboa, Portugal, 2008.
- Garcia, C.; Silva, G.M.; Botelho, A.P.; Leitão, M.A.; Castro, A.; Caldas, A. Avaliação Breve do Estado Mental: Escalas e Testes na Demência. GEECD 2008, 1, 33–39. [Google Scholar]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- Garrett, C.; Santos, F.; Tracana, I.; Barreto, J.; Sobral, M.; Fonseca, R. Avaliação Clínica da Demência. Escalas e Testes na Demência. GEECD 2008, 1, 18–32. [Google Scholar]
- Leitão, O. Escalas e Testes na Demência. GEECD 2008, 1, 107–110. [Google Scholar]
- Lino, V.T.; Pereira, S.R.; Camacho, L.A.; Ribeiro Filho, S.T.; Buksman, S. Cross-cultural adaptation of the Independence in Activities of Daily Living Index (Katz Index). Cad Saude Publ. 2008, 24, 103–112. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatric Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Barreto, J.L.A.; Santos, F.; Sobral, M. Escala de Depressão Geriátrica [Geriatric Depression Scale] Escalas e Testes na Demência. GEECD 2008, 1, 69–72. [Google Scholar]
- Leitão, O.; Nina, A. Escalas e Testes na Demência. GEECD 2008, 1, 7–97. [Google Scholar]
- Mendes, T.; Gino, S.; Ribeiro, F.; Guerreiro, M.; de Sousa, G.; Ritchie, K.; de Mendonca, A. Memory complaints in healthy young and elderly adults: Reliability of memory reporting. Aging Ment. Health 2008, 12, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wechsler. In WMS-III: Escala de Memória de Wechsler, 3th ed.; Manual de administração e cotação: Cegoc, Lisboa, Portugal, 2008.
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 4, 939–944. [Google Scholar] [CrossRef]
- Crook, R.; Hardy, J.; Duff, K. Single-day apolipoprotein E genotyping. J. Neurosci. Methods 1994, 53, 125–127. [Google Scholar]
- Mattsson, N.; Andreasson, U.; Persson, S.; Arai, H.; Batish, S.D.; Bernardini, S.; Bocchio-Chiavetto, L.; Blankenstein, M.A.; Carrillo, M.C.; Chalbot, S.; et al. The Alzheimer’s Association external quality control program for cerebrospinal fluid biomarkers. Alzheimer’s Dement. 2011, 7, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Baldeiras, I.E.; Ribeiro, M.H.; Pacheco, P.; Machado, A.; Santana, I.; Cunha, L.; Oliveira, C.R. Diagnostic value of CSF protein profile in a Portuguese population of sCJD patients. J. Neurol. 2009, 256, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Kapaki, E.; Kilidireas, K.; Paraskevas, G.P.; Michalopoulou, M.; Patsouris, E. Highly increased CSF Tau protein and decreased beta-amyloid (1-42) in sporadic CJD: A discrimination from Alzheimer’s disease? J. Neurol. Neurosurg. Psychiatry 2001, 71, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Hulstaert, F.; Blennow, K.; Ivanoiu, A.; Schoonderwaldt, H.C.; Riemenschneider, M.; De Deyn, P.P.; Bancher, C.; Cras, P.; Wiltfang, J.; Mehta, P.D.; et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology 1999, 52, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.; Minthon, L.; Vanmechelen, E.; Vanderstichele, H.; Davidsson, P.; Winblad, B.; Blennow, K. Cerebrospinal fluid tau and Abeta42 as predictors of development of Alzheimer’s disease in patients with mild cognitive impairment. Neurosci. Lett. 1999, 273, 5–8. [Google Scholar] [CrossRef]
- Visser, P.J. Use of biomarkers to select the target population for clinical trials in subjects with mild cognitive impairment. J. Nutr. Health Aging 2009, 13, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Seo, S.W.; Yoon, D.S.; Chin, J.; Lee, B.H.; Cheong, H.K.; Han, S.H.; Na, D.L. Comparison of neuropsychological and FDG-PET findings between early- versus late-onset mild cognitive impairment: A five-year longitudinal study. Dement. Geriatr. Cogn. Disord. 2010, 29, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.G.; Kontaxis, T.; Bonakis, A.; Kalfakis, N.; Vassilopoulos, D. Frequency and causes of early-onset dementia in a tertiary referral center in Athens. Alzheimer Dis. Assoc. Disord. 2009, 23, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, S.; Ikeda, M.; Toyota, Y.; Matsumoto, T.; Matsumoto, N.; Mori, T.; Ishikawa, T.; Fukuhara, R.; Komori, K.; Hokoishi, K.; et al. Frequency and clinical characteristics of early-onset dementia in consecutive patients in a memory clinic. Dement. Geriatr. Cogn. Disord. 2007, 24, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Castrillo Sanz, A.; Andres Calvo, M.; Repiso Gento, I.; Izquierdo Delgado, E.; Gutierrez Rios, R.; Rodriguez Herrero, R.; Rodriguez Sanz, F.; Tola-Arribas, M.A. Anosognosia in Alzheimer disease: Prevalence, associated factors, and influence on disease progression. Neurologia 2015. S0213-4853(15)00057-2. [Google Scholar]
- McMurtray, A.; Clark, D.G.; Christine, D.; Mendez, M.F. Early-onset dementia: Frequency and causes compared to late-onset dementia. Dement. Geriatr. Cogn. Disord. 2006, 21, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Geda, Y.E.; Roberts, R.O.; Mielke, M.M.; Knopman, D.S.; Christianson, T.J.; Pankratz, V.S.; Boeve, B.F.; Sochor, O.; Tangalos, E.G.; Petersen, R.C.; et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: A population-based study. Am. J. Psychiatry 2014, 171, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, C.; Zhao, K.; Ma, L.; Qiu, X.; Zhang, L.; Xiu, Y.; Chen, L.; Lu, W.; Huang, C.; et al. Depression as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Int. J. Geriatr. Psychiatry 2013, 28, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Monastero, R.; Mangialasche, F.; Camarda, C.; Ercolani, S.; Camarda, R. A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J. Alzheimer’s Dis. 2009, 18, 11–30. [Google Scholar] [CrossRef]
- Cooper, C.; Sommerlad, A.; Lyketsos, C.G.; Livingston, G. Modifiable predictors of dementia in mild cognitive impairment: A systematic review and meta-analysis. Am. J. Psychiatry 2015, 172, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, E.C.; Delano-Wood, L.; Galasko, D.R.; Salmon, D.P.; Bondi, M.W. Alzheimer’s Disease Neuroimaging I. Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. J. Int. Neuropsychol. Soc. 2014, 20, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Wiste, H.J.; Vemuri, P.; Weigand, S.D.; Senjem, M.L.; Zeng, G.; Bernstein, M.A.; Gunter, J.L.; Pankratz, V.S.; Aisen, P.S.; et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain 2010, 133, 3336–3348. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Zetterberg, H.; Buchhave, P.; Londos, E.; Blennow, K.; Minthon, L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006, 5, 228–234. [Google Scholar] [CrossRef]
- Silverberg, G.D.; Miller, M.C.; Messier, A.A.; Majmudar, S.; Machan, J.T.; Donahue, J.E.; Stopa, E.G.; Johanson, C.E. Amyloid deposition and influx transporter expression at the blood-brain barrier increase in normal aging. J. Neuropathol. Exp. Neurol. 2010, 69, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.J.; Verhey, F.; Knol, D.L.; Scheltens, P.; Wahlund, L.O.; Freund-Levi, Y.; Tsolaki, M.; Minthon, L.; Wallin, A.K.; Hampel, H.; et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: A prospective cohort study. Lancet Neurol. 2009, 8, 619–627. [Google Scholar] [CrossRef]
- Buchhave, P.; Minthon, L.; Zetterberg, H.; Wallin, A.K.; Blennow, K.; Hansson, O. Cerebrospinal fluid levels of beta-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch. Gen. Psychiatry 2012, 69, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Stevens, J.C.; Ganguli, M.; Tangalos, E.G.; Cummings, J.L.; DeKosky, S.T. Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001, 56, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.C.; Szalai, J.P.; Snow, W.G.; Fisher, R.H.; Nores, A.; Nadon, G.; Dunn, E.; St George-Hyslop, P.H. Prediction of probable Alzheimer’s disease in memory-impaired patients: A prospective longitudinal study. Neurology 1996, 46, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Llano, D.A.; Laforet, G.; Devanarayan, V. Alzheimer’s Disease Neuroimaging I. Derivation of a new ADAS-cog composite using tree-based multivariate analysis: Prediction of conversion from mild cognitive impairment to Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2011, 25, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Tosto, G.; Zimmerman, M.E.; Carmichael, O.T.; Brickman, A.M. Alzheimer’s Disease Neuroimaging I. Predicting aggressive decline in mild cognitive impairment: the importance of white matter hyperintensities. JAMA Neurol. 2014, 71, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Defrancesco, M.; Marksteiner, J.; Deisenhammer, E.; Kemmler, G.; Djurdjevic, T.; Schocke, M. Impact of white matter lesions and cognitive deficits on conversion from mild cognitive impairment to Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 34, 665–672. [Google Scholar] [CrossRef]
| EOMCI | LOMCI | Total | p | |
|---|---|---|---|---|
| n | 52 | 107 | 159 | |
| Age at onset | 60.0 (±4.99) | 73.2 (±5.31) | 68.5 (±8.15) | <0.001 |
| Age at first evaluation | 64.7 (±5.57) | 76.1 (±5.39) | 72.4 (±7.65) | <0.001 |
| Education (years) | 5.62 (±3.09) | 6.35 (±4.58) | 5.88 (±4.01) | 0.298 |
| Gender (female %) | 59.6 | 62.6 | 61.6 | 0.715 |
| Positive family history (%) | 53.3 | 44.8 | 47.5 | 0.344 |
| ApoE-ε4 carriers (%) | 43.5 | 43.1 | 43.2 | 0.969 |
| Disease duration at first evaluation (years) | 4.57 (±2.90) | 3.31 (±2.45) | 3.76 (±2.69) | 0.008 |
| Time of follow-up (months) | 38.54 (±30.23) | 37.44 (±24.46) | 40.03 (±26.90) | 0.814 |
| Converters (%) | 31.4 | 41.8 | 38.3 | 0.358 |
| EOMCI Mean (±SD) | LOMCI Mean (±SD) | p | |
|---|---|---|---|
| SMC-patient | 9.91 (±3.29) | 7.85 (±3.85) | 0.008 |
| SMC-caregiver | 8.58 (±3.62) | 8.32 (±3.69) | 0.773 |
| MMSE * | 27.81 (±2.48) | 26.51(±2.69) | <0.001 |
| MoCA * | 19.84 (±5.04) | 18.67 (±4.57) | 0.003 |
| ADAS-cog * | 9.17 (±5.16) | 11.12 (±5.04) | <0.001 |
| Blessed * | 1.63 (±1.52) | 2.29 (±2.46) | 0.707 |
| CDR-sob | 1.41 (±0.97) | 1.577 (±1.01) | 0.332 |
| CDR-memory | 0.56 (±0.17) | 0.60 (±0.20) | 0.287 |
| CDR-orientation | 0.21 (±0.29) | 0.30 (±0.34) | 0.100 |
| CDR-judgement and problem-solving | 0.32 (±0.30) | 0.33 (±0.31) | 0.746 |
| CDR-activity | 0.13 (±0.22) | 0.18 (±0.27) | 0.279 |
| CDR-home and hobbies | 0.12 (±0.24) | 0.16 (±0.26) | 0.321 |
| CDR-personal care | 0.00 (±0.00) | 0.02 (±0.14) | 0.158 |
| DAD | 95.12 (±5.26) | 93.92 (±6.18) | 0.391 |
| ADL | 1.57 (±1.55) | 1.446 (±1.79) | 0.819 |
| GDS | 12.21 (±5.75) | 10.33 (±6.84) | 0.200 |
| NPI | 4.56 (±5.72) | 7.44 (±9.06) | 0.058 |
| EOMCI (n = 34) | LOMCI (n = 42) | p | |
|---|---|---|---|
| Aβ42 (pg/mL) | 649.7 (±339.1) | 591.3 (±336.7) | 0.456 |
| t-Tau (pg/mL) | 437.2 (±396.0) | 459.3 (±227.1) | 0.773 |
| p-Tau (pg/mL) | 57.1 (±38.2) | 61.3 (±29.7) | 0.594 |
| CSF-AD profile (%) | 58.8 | 73.8 | 0.167 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tábuas-Pereira, M.; Baldeiras, I.; Duro, D.; Santiago, B.; Ribeiro, M.H.; Leitão, M.J.; Oliveira, C.; Santana, I. Prognosis of Early-Onset vs. Late-Onset Mild Cognitive Impairment: Comparison of Conversion Rates and Its Predictors. Geriatrics 2016, 1, 11. https://doi.org/10.3390/geriatrics1020011
Tábuas-Pereira M, Baldeiras I, Duro D, Santiago B, Ribeiro MH, Leitão MJ, Oliveira C, Santana I. Prognosis of Early-Onset vs. Late-Onset Mild Cognitive Impairment: Comparison of Conversion Rates and Its Predictors. Geriatrics. 2016; 1(2):11. https://doi.org/10.3390/geriatrics1020011
Chicago/Turabian StyleTábuas-Pereira, Miguel, Inês Baldeiras, Diana Duro, Beatriz Santiago, Maria Helena Ribeiro, Maria João Leitão, Catarina Oliveira, and Isabel Santana. 2016. "Prognosis of Early-Onset vs. Late-Onset Mild Cognitive Impairment: Comparison of Conversion Rates and Its Predictors" Geriatrics 1, no. 2: 11. https://doi.org/10.3390/geriatrics1020011
APA StyleTábuas-Pereira, M., Baldeiras, I., Duro, D., Santiago, B., Ribeiro, M. H., Leitão, M. J., Oliveira, C., & Santana, I. (2016). Prognosis of Early-Onset vs. Late-Onset Mild Cognitive Impairment: Comparison of Conversion Rates and Its Predictors. Geriatrics, 1(2), 11. https://doi.org/10.3390/geriatrics1020011






