Intra- and Interexaminer Measurement Variability Analysis of an Orthodontic Gauge Device to Determine Incisor Occlusal Surface Angles in the Horse
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cadaver Heads
2.2. Examiners
2.3. Orthodontic Gauge Device
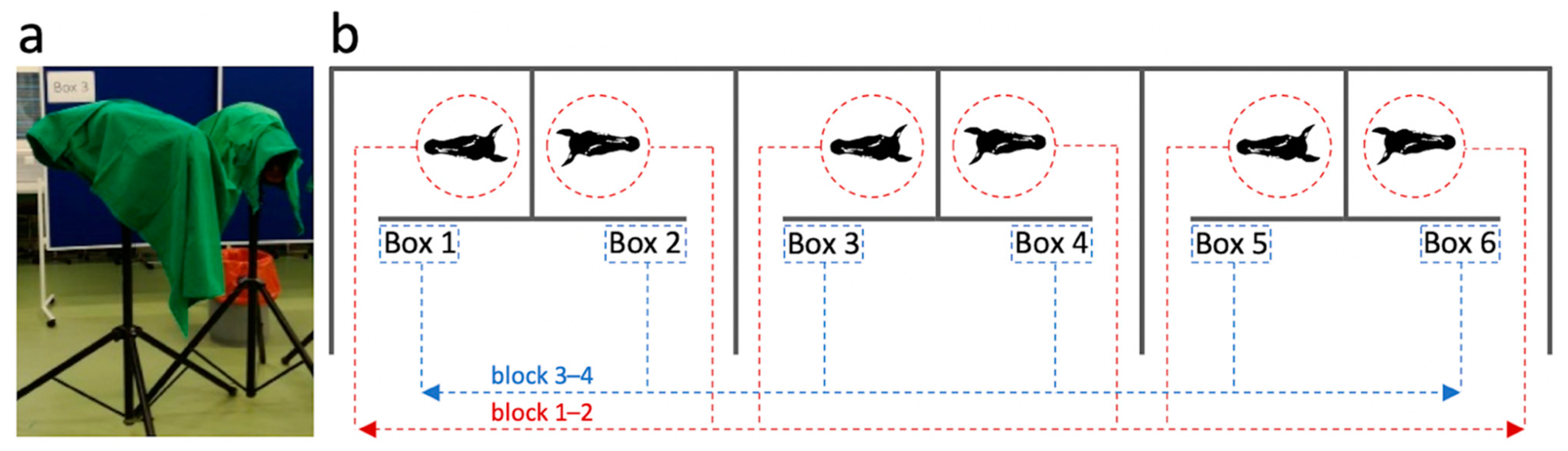
2.4. Experimental Set-Up
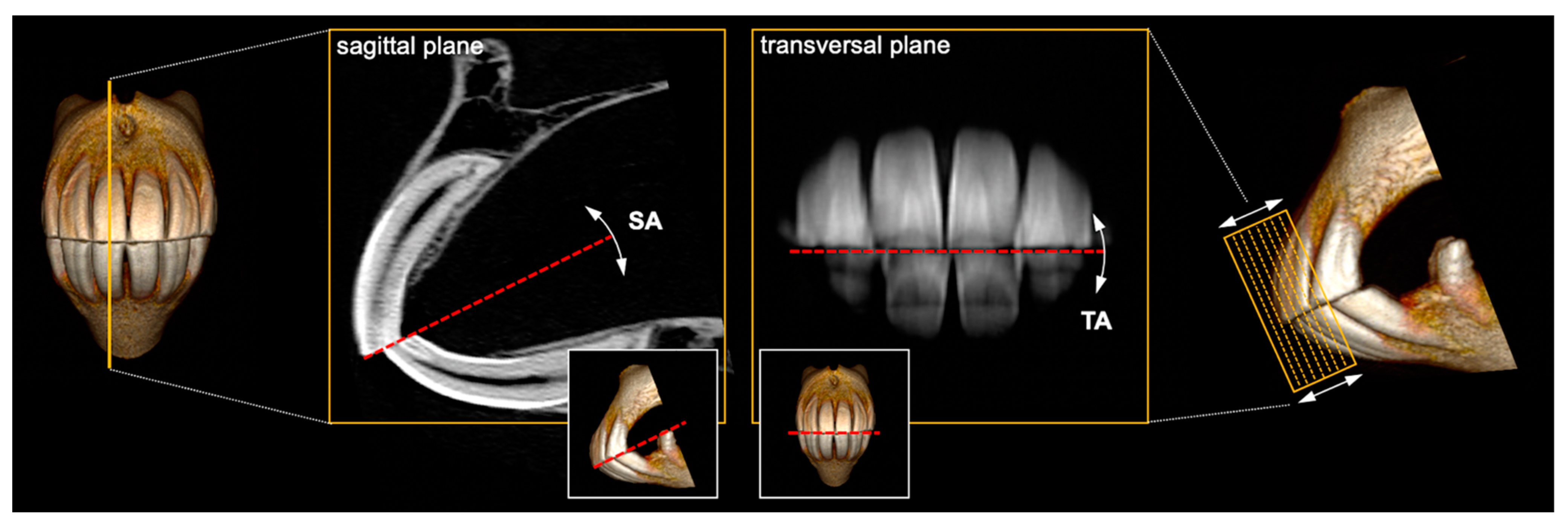
2.5. Angle Measurements
2.6. Time Measurements
2.7. Statistical Analysis
3. Results
3.1. Landmark Identification Proved Difficult with TA but Not SA Measurements
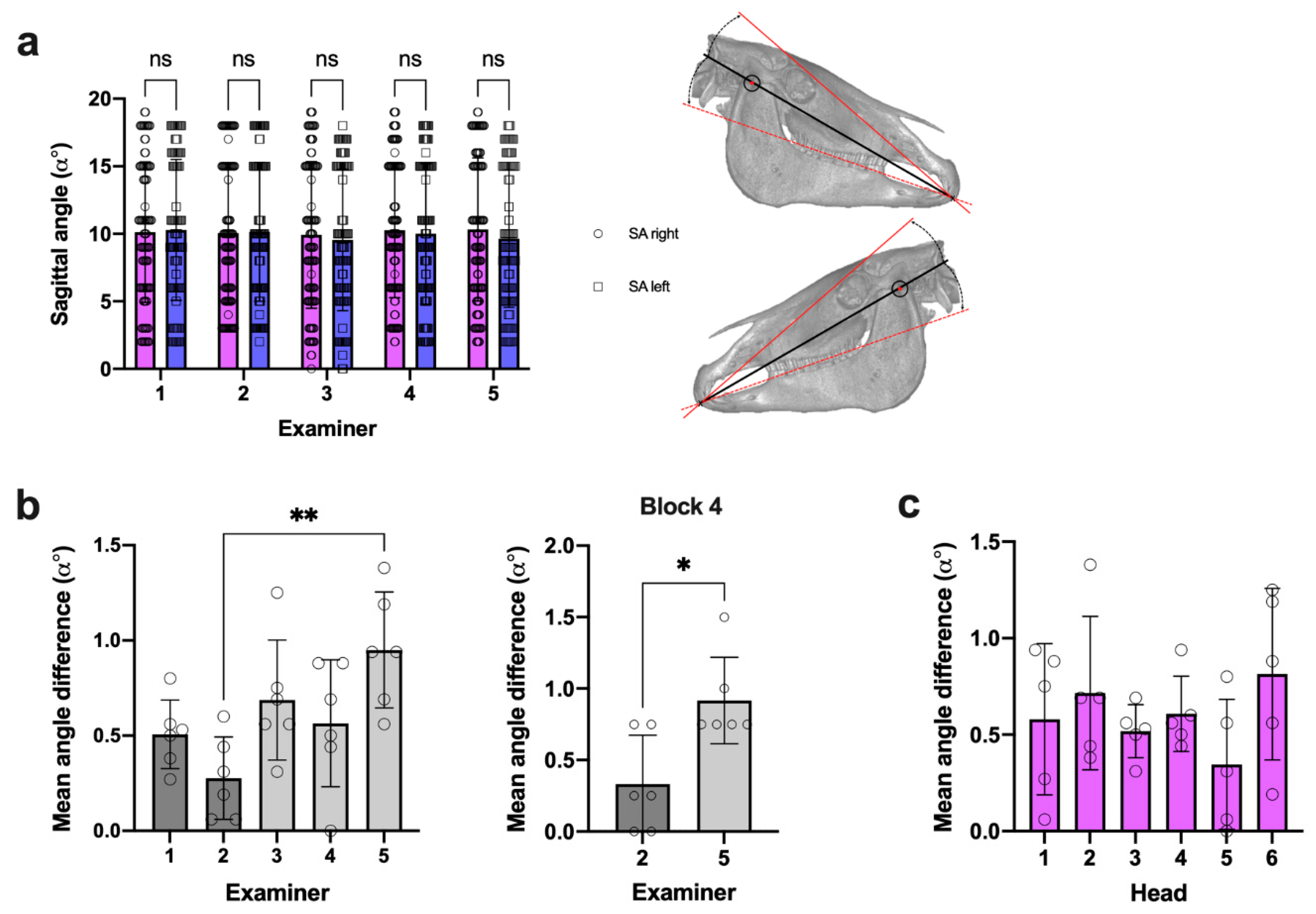
3.2. SA Measures Revealed Negligible Side Difference within All Examiners
3.3. Intraexaminer Data Repeatability Was Comparable in SA and TA Measures
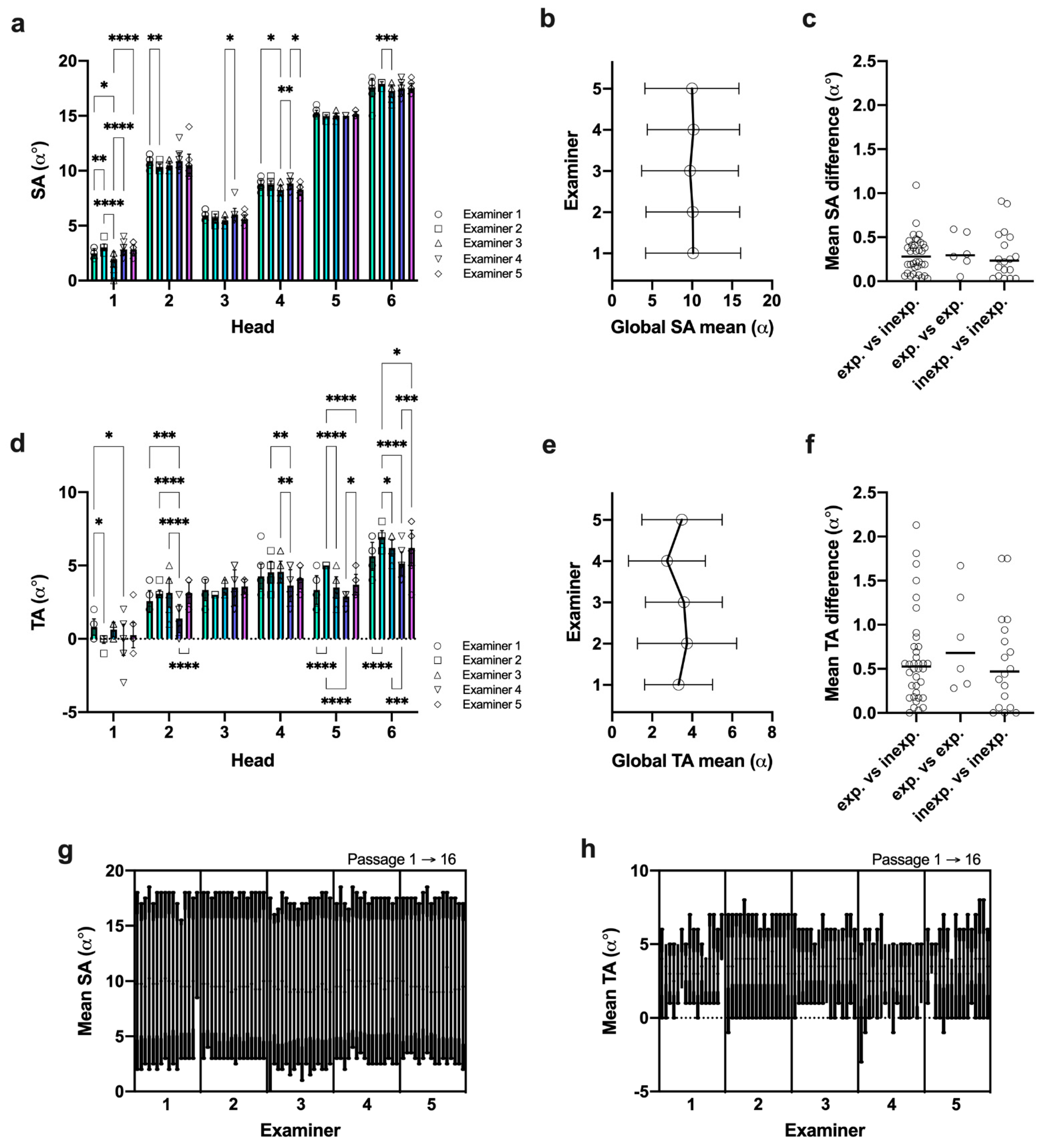
3.4. Data Reproducibility Was Higher in SA Measures under Given Reference Landmarks
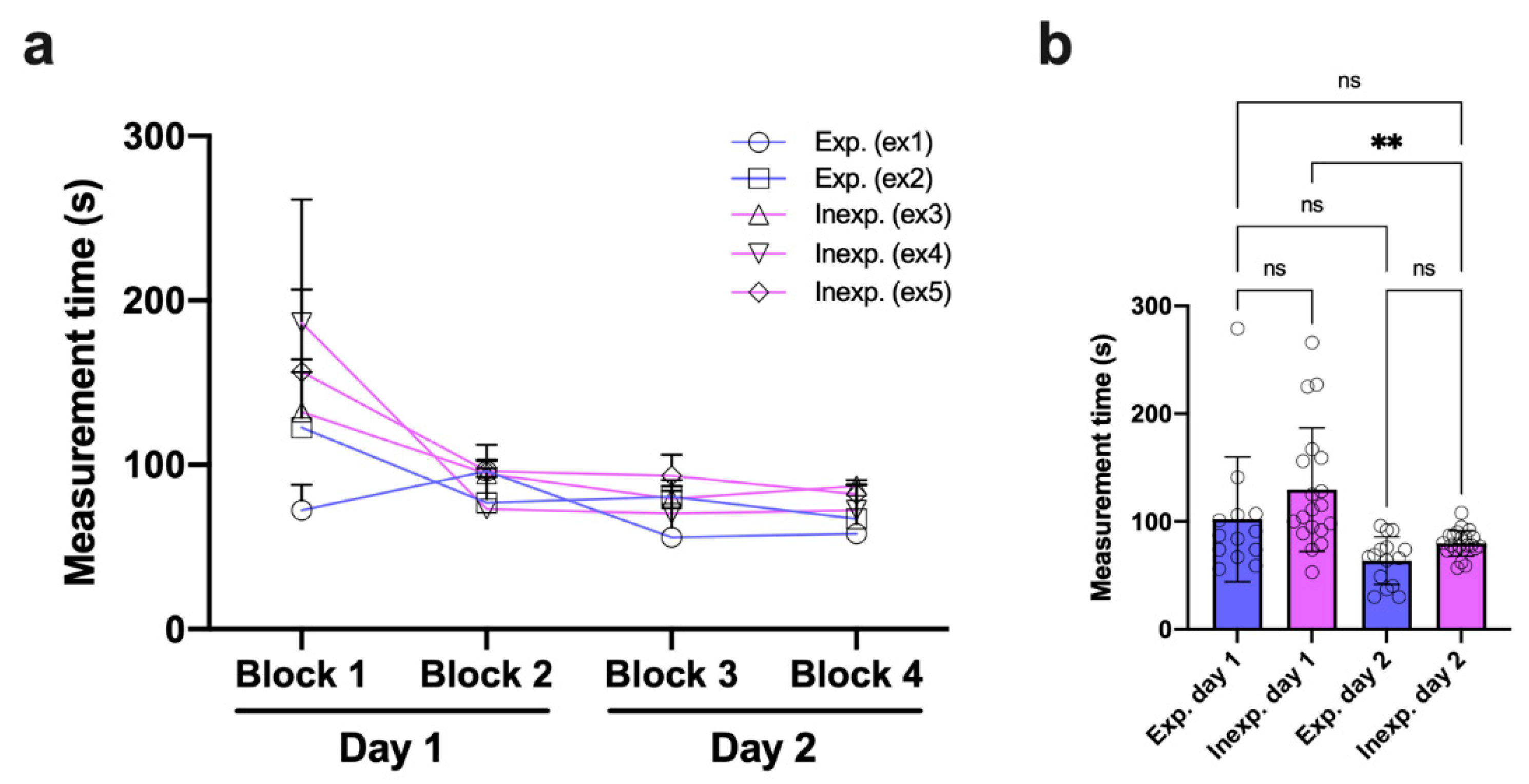
3.5. Inexperienced Examiners Rapidly Adapt to the Measurement Procedure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, S.L.; Arkins, S.; Shaw, D.J.; Dixon, P.M. Occlusal angles of cheek teeth in normal horses and horses with dental disease. Vet. Rec. 2008, 162, 807–810. [Google Scholar] [CrossRef]
- Listmann, L.; Schrock, P.; Failing, K.; Staszyk, C. Occlusal Angles of Equine Incisors. J. Vet. Dent. 2017, 34, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Listmann, L.; Schrock, P.; Failing, K.; Staszyk, C. Occlusal angles of equine cheek teeth. Livest. Sci. 2016, 186, 78–84. [Google Scholar] [CrossRef]
- DeLorey, M.S. A Retrospective Evaluation of 204 Diagonal Incisor Malocclusion Corrections in the Horse. J. Vet. Dent. 2007, 24, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Sterkenburgh, T.; Schulz-Kornas, E.; Nowak, M.; Staszyk, C. A Computerized Simulation of the Occlusal Surface in Equine Cheek Teeth: A Simplified Model. Front. Vet. Sci. 2022, 8, 1519. [Google Scholar] [CrossRef] [PubMed]
- Bonin, S.J.; Clayton, H.M.; Lanovaz, J.L.; Johnston, T. Comparison of mandibular motion in horses chewing hay and pellets. Equine Vet. J. 2007, 39, 258–262. [Google Scholar] [CrossRef]
- Allen, T. Examination. In Manual of Equine Dentistry, 1st ed.; Allen, T., Ed.; Muleicorn Press: St. Louis, MO, USA, 2008; pp. 67–88. [Google Scholar]
- Rucker, B.A. Incisor and molar occlusion: Normal ranges and indications for incisor reduction. In Proceedings of the 50th Annual Convention of the American Association of Equine Practitioners, Denver, CO, USA, 4–8 December 2004; pp. 7–12. [Google Scholar]
- Klugh, D.O. Anatomical characteristics of equine dentition. In Principles of Equine Dentistry, 1st ed.; Klugh, D.O., Ed.; Manson Publishing: London, UK, 2010; pp. 27–48. [Google Scholar]
- Klugh, D.O. Principles of Occlusal Equilibration. In Principles of Equine Dentistry, 1st ed.; Klugh, D.O., Ed.; Manson Publishing: London, UK, 2010; pp. 67–78. [Google Scholar]
- Schrock, P.; Lüpke, M.; Seifert, H.; Staszyk, C. Three-dimensional anatomy of equine incisors: Tooth length, enamel cover and age related changes. BMC Vet. Res. 2013, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Muylle, S.; Simoens, P.; Lauwers, H. Age-related Morphometry of Equine Incisors. J. Vet. Med. Ser. A 1999, 46, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Kau, S.; Failing, K.; Staszyk, C. Computed Tomography (CT)-Assisted 3D Cephalometry in Horses: Interincisal Angulation of Clinical Crowns. Front. Vet. Sci. 2020, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Muylle, S.; Simoens, P.; Lauwers, H. Ageing horses by an examination of their incisor teeth: An (im)possible task? Vet. Rec. 1996, 138, 295–301. [Google Scholar] [CrossRef]
- Damuth, J.; Janis, C.M. On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biol. Rev. 2011, 86, 733–758. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.M.; Tremaine, W.H.; Pickles, K.; Kuhns, L.; Hawe, C.; McCann, J.; McGorum, B.; Railton, D.I.; Brammer, S. Equine dental disease Part 1: A long-term study of 400 cases: Disorders of incisor, canine and first premolar teeth. Equine Vet. J. 1999, 31, 369–377. [Google Scholar] [CrossRef]
- de Oliveira Pimentel, L.F.R.; do Valle De Zoppa, A.L. Analysis of the relationship between occlusal and clinical parameters and the need for incisor reduction in confined horses—a retrospective study. Ciência Rural.-Clin. Surg. 2014, 44, 2052–2057. [Google Scholar] [CrossRef]
- Vogt, C. Anamnesis and Clinical Examination. In Textbook of Equine Dentistry, 1st ed.; Vogt, C., Ed.; Schattauer: Stuttgart, Germany, 2011; pp. 49–63. [Google Scholar]
- Easley, J. Corrective dental procedures. In Equine Dentistry, 3rd ed.; Easley, J., Dixon, P.M., Schumacher, J., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2011; pp. 261–278. [Google Scholar]
- Pellachin, M. Objective Measurements of occlusal angles. In Proceedings of the 11th IGFP Conference, Wiesbaden, Germany, 9–10 March 2013; pp. 45–57. [Google Scholar]
- Rodrigues, J.B.; Araújo, S.; Sanroman-Llorens, F.; Bastos, E.; San Roman, F.; Viegas, C. A Clinical Survey Evaluating the Prevalence of Incisor Disorders in Zamorano-Leonés and Mirandês Donkeys (Equus asinus). J. Equine Vet. Sci. 2013, 33, 710–718. [Google Scholar] [CrossRef]
- Kunz, J.R.; Granella, M.C.S.; Mendes, R.P.; Müller, T.R.; Kau, S.; Fonteque, J.H. High Prevalence of Orodental Disorders in South Brazilian Cart Horses: Walking a Tightrope Between Animal Welfare and Socioeconomic Inevitability. J. Vet. Dent. 2020, 37, 149–158. [Google Scholar] [CrossRef]
- Earley, E.; Rawlinson, J.T. A New Understanding of Oral and Dental Disorders of the Equine Incisor and Canine Teeth. Vet. Clin. N. Am. Equine Pract. 2013, 29, 273–300. [Google Scholar] [CrossRef]
- Walmsley, J.P. Some observations on the value of ageing 5–7-year-old horses by examination of their incisor teeth. Equine Vet. Educ. 1993, 5, 295–298. [Google Scholar] [CrossRef]
- Dixon, P.M. The Gross, Histological, and Ultrastructural Anatomy of Equine Teeth and Their Relationship to Disease. In Proceedings of the 48th Annual Convention of the American Association of Equine Practitioners, Orlando, FL, USA, 4–8 December 2002; pp. 421–437. [Google Scholar]
- Ros, K. Biomechanics. In Textbook of Equine Dentistry, 1st ed.; Vogt, C., Ed.; Schattauer: Stuttgart, Germany, 2011; pp. 31–48. [Google Scholar]
- Baratt, R.M. How to Recognize and Clinically Manage Class 1 Malocclusions in the Horse. In Proceedings of the 56th Annual Convention of the American Association of Equine Practitioners, Baltimore, MD, USA, 4–8 December 2010; pp. 458–464. [Google Scholar]
- Gieche, J.M. How to Assess Equine Oral Health. In Proceedings of the 53rd Annual Convention of the American Association of Equine Practitioners, Orlando, FL, USA, 1–5 December 2007; p. 499. [Google Scholar]
- Rawlinson, J.T.; Earley, E.T. Advances in the treatment of diseased equine incisor and canine teeth. Vet. Clin. N. Am.-Equine Pract. 2013, 29, 411–440. [Google Scholar] [CrossRef]
- Earley, E.T.; Reiswig, J.D. Equine Dental Floating (Crown Osontoplasty). Vet. Clin. Equine Pract. 2020, 36, 501–526. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Rahimah, A.K. Occlusion, malocclusion and method of measurements-an overview. Arch. Orofac. Sci. 2007, 2, 3–9. [Google Scholar]
- Pellachin, M. Device for Measuring the Angles of Inclination of the Incisor Occlusal Plane of Equidae and Other Animals. US Patent 8,793,888 B2, 5 August 2014. [Google Scholar]
- Brennan, P.; Silman, A. Statistical methods for assessing observer variability in clinical measures. BMJ Br. Med. J. 1992, 304, 1491–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, P.C.; Parks, E.T.; Eraso, F.; Hartsfield, J.K.; Roberts, W.E.; Ofner, S. Comparison of reliability in anatomical landmark identification using two-dimensional digital cephalometrics and three-dimensional cone beam computed tomography in vivo. Dentomaxillofacial Rad. 2014, 38, 262–273. [Google Scholar] [CrossRef]
- Zamora, N.; Llamas, J.M.; Cibrián, R.; Gandia, J.L.; Paredes, V. A study on the reproducibility of cephalometric landmarks when undertaking a three-dimensional (3D) cephalometric analysis. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e678. [Google Scholar] [CrossRef] [PubMed]
- Dot, G.; Rafflenbeul, F.; Arbotto, M.; Gajny, L.; Rouch, P.; Schouman, T. Accuracy and reliability of automatic three-dimensional cephalometric landmarking. Int. J. Oral Maxillofac. Surg. 2020, 49, 1367–1378. [Google Scholar] [CrossRef]
- Schrock, P.; Lüpke, M.; Seifert, H.; Staszyk, C. Finite element analysis of equine incisor teeth. Part 2: Investigation of stresses and strain energy densities in the periodontal ligament and surrounding bone during tooth movement. Vet. J. 2013, 198, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Domanska-Kruppa, N.; Venner, M.; Bienert-Zeit, A. Cephalometric Study of the Overjet Development in Warmblood Foals. Front. Vet. Sci. 2019, 6, 431. [Google Scholar] [CrossRef]
- Bartlett, S.P.; Grossman, R.; Whitaker, L.A. Age-related changes of the craniofacial skeleton: An anthropometric and histologic analysis. Plast. Reconstr. Surg. 1992, 90, 592–600. [Google Scholar] [CrossRef]
- Albert, A.M.; Ricanek, K.; Patterson, E. A review of the literature on the aging adult skull and face: Implications for forensic science research and applications. Forensic Sci. Int. 2007, 172, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pancherz, H.; Gökbuget, K. The reliability of the Frankfort Horizontal in roentgenographic cephalometry. Eur. J. Orthod. 1996, 18, 367–372. [Google Scholar] [CrossRef]
- Staszyk, C.; Carmalt, J. Equine mastication: Morphological, functional and biomechanical adaptations. In Equine Dentistry and Maxillofacial Surgery, 1st ed.; Easley, J., Dixon, P.M., du Toit, N., Eds.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2022; pp. 99–110. [Google Scholar]
- Phulari, B. An Atlas on Cephalometric Landmarks, 1st ed.; Phulari, B., Ed.; Jaypee Brothers Medical Publishers: New Delhi, India, 2013. [Google Scholar]
- Hilgers, M.L.; Scarfe, W.C.; Scheetz, J.P.; Farman, A.G. Accuracy of linear temporomandibular joint measurements with cone beam computed tomography and digital cephalometric radiography. Am. J. Orthod. Dentofac. Orthop. 2005, 128, 803–811. [Google Scholar] [CrossRef]
- De-Giorgio, F.; Grassi, S.; d’Aloja, E.; Pascali, V.L. Post-mortem ocular changes and time since death: Scoping review and future perspective. Leg. Med. 2021, 50, 101862. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, E.A. Diseases of the adnexa and nasolacrimal system. In Equine Ophthalmology, 3rd ed.; Gilger, B.C., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016; pp. 197–251. [Google Scholar]
- Schlicher, W.; Nielsen, I.; Huang, J.C.; Maki, K.; Hatcher, D.C.; Miller, A.J. Consistency and precision of landmark identification in three-dimensional cone beam computed tomography scans. Eur. J. Orthod. 2012, 34, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Lagravère, M.O.; Low, C.; Flores-Mir, C.; Chung, R.; Carey, J.P.; Heo, G.; Major, P.W. Intraexaminer and interexaminer reliabilities of landmark identification on digitized lateral cephalograms and formatted 3-dimensional cone-beam computerized tomography images. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Kau, S. Supplementary files to article “Intra- and interexaminer measurement variability analysis of an orthodontic gauge device to determine incisor occlusal surface angles in the horse” [Data set]. Zenodo 2022. [Google Scholar] [CrossRef]



| Horse No. | Breed | Sex | Age | Coat | Condition |
|---|---|---|---|---|---|
| 1 | Riding Pony | mare | 22 | black | frozen-thawed |
| 2 | WB 1 | gelding | 21 | brown | frozen-thawed |
| 3 | WB | gelding | 8 | brown | frozen-thawed |
| 4 | WB | mare | 12 | brown | frozen-thawed |
| 5 | WB | mare | 13 | black | frozen-thawed |
| 6 | WB | mare | 12 | brown | fresh |
| Examiner | ITA | Head 1 | Head 2 | Head 3 | Head 4 | Head 5 | Head 6 | Mean SD |
|---|---|---|---|---|---|---|---|---|
| 1 | SA | 0.52° | 0.49° | 0.55° | 0.50° | 0.53° | 0.95° | 0.59° |
| TA | 0.56° | 0.73° | 0.72° | 0.86° | 0.98° | 0.96° | 0.80° | |
| 2 | SA | 0.31° | 0.46° | 0.46° | 0.52° | 0.13° | 0.30° | 0.36° |
| TA | 0.25° | 0.25° | 0.00° | 0.74° | 0.00° | 0.44° | 0.28° | |
| 3 | SA | 0.83° | 0.52° | 0.51° | 0.61° | 0.40° | 0.79° | 0.61° |
| TA | 0.50° | 0.96° | 0.52° | 0.73° | 0.73° | 0.54° | 0.66° | |
| 4 | SA | 0.71° | 1.25° | 0.69° | 0.60° | 0.00° | 0.76° | 0.67° |
| TA | 1.06° | 0.96° | 1.21° | 1.09° | 0.34° | 0.81° | 0.91° | |
| 5 | SA | 0.72° | 1.12° | 0.52° | 0.65° | 0.50° | 0.51° | 0.67° |
| TA | 0.86° | 0.72° | 0.51° | 0.72° | 0.70° | 1.22° | 0.79° | |
| Mean | SA | 0.62° | 0.77° | 0.55° | 0.58° | 0.31° | 0.66° | 0.58° |
| TA | 0.65° | 0.72° | 0.59° | 0.83° | 0.55° | 0.79° | 0.69° |
| Examiner Combination | ITA | Head 1 | Head 2 | Head 3 | Head 4 | Head 5 | Head 6 | Mean |
|---|---|---|---|---|---|---|---|---|
| Ex 1 vs. Ex 2 | SA | 0.56° | 0.59° | 0.28° | 0.05° | 0.23° | 0.31° | 0.34° |
| TA | 0.86° | 0.50° | 0.33° | 0.28° | 1.67° | 1.31° | 0.83° | |
| Ex 1 vs. Ex 3 | SA | 0.53° | 0.41° | 0.46° | 0.53° | 0.17° | 0.34° | 0.41° |
| TA | 0.18° | 0.56° | 0.17° | 0.31° | 0.17° | 0.56° | 0.32° | |
| Ex 1 vs. Ex 4 | SA | 0.35° | 0.16° | 0.07° | 0.03° | 0.20° | 0.09° | 0.15° |
| TA | 0.86° | 1.19° | 0.17° | 0.63° | 0.46° | 0.50° | 0.63° | |
| Ex 1 vs. Ex 5 | SA | 0.38° | 0.38° | 0.34° | 0.47° | 0.04° | 0.06° | 0.28° |
| TA | 0.55° | 0.56° | 0.23° | 0.13° | 0.35° | 0.56° | 0.40° | |
| Ex 2 vs. Ex 3 | SA | 1.09° | 0.19° | 0.19° | 0.48° | 0.06° | 0.66° | 0.44° |
| TA | 0.69° | 0.06° | 0.50° | 0.03° | 1.50° | 0.75° | 0.59° | |
| Ex 2 vs. Ex 4 | SA | 0.22° | 0.44° | 0.34° | 0.08° | 0.03° | 0.41° | 0.25° |
| TA | 0.00° | 1.69° | 0.50° | 0.91° | 2.13° | 1.81° | 1.17° | |
| Ex 2 vs. Ex 5 | SA | 0.19° | 0.22° | 0.06° | 0.42° | 0.19° | 0.38° | 0.24° |
| TA | 0.31° | 0.06° | 0.56° | 0.41° | 1.31° | 0.75° | 0.57° | |
| Ex 3 vs. Ex 4 | SA | 0.88° | 0.25° | 0.53° | 0.56° | 0.03° | 0.25° | 0.42° |
| TA | 0.69° | 1.75° | 0.00° | 0.94° | 0.63° | 1.06° | 0.84° | |
| Ex 3 vs. Ex 5 | SA | 0.91° | 0.03° | 0.13° | 0.06° | 0.13° | 0.28° | 0.26° |
| TA | 0.38° | 0.00° | 0.06° | 0.44° | 0.19° | 0.00° | 0.18° | |
| Ex 4 vs. Ex 5 | SA | 0.03° | 0.22° | 0.41° | 0.50° | 0.16° | 0.03° | 0.22° |
| TA | 0.31° | 1.75° | 0.06° | 0.50° | 0.81° | 1.06° | 0.75° | |
| Mean | SA | 0.51° | 0.29° | 0.28° | 0.32° | 0.12° | 0.28° | 0.30° |
| TA | 0.42° | 0.63° | 0.26° | 0.51° | 0.57° | 0.59° | 0.64° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kau, S.; Motter, K.S.; Moser, V.J.; Kunz, J.R.; Pellachin, M.; Hartl, B. Intra- and Interexaminer Measurement Variability Analysis of an Orthodontic Gauge Device to Determine Incisor Occlusal Surface Angles in the Horse. Vet. Sci. 2022, 9, 481. https://doi.org/10.3390/vetsci9090481
Kau S, Motter KS, Moser VJ, Kunz JR, Pellachin M, Hartl B. Intra- and Interexaminer Measurement Variability Analysis of an Orthodontic Gauge Device to Determine Incisor Occlusal Surface Angles in the Horse. Veterinary Sciences. 2022; 9(9):481. https://doi.org/10.3390/vetsci9090481
Chicago/Turabian StyleKau, Silvio, Katharina S. Motter, Viktoria J. Moser, João R. Kunz, Matteo Pellachin, and Bettina Hartl. 2022. "Intra- and Interexaminer Measurement Variability Analysis of an Orthodontic Gauge Device to Determine Incisor Occlusal Surface Angles in the Horse" Veterinary Sciences 9, no. 9: 481. https://doi.org/10.3390/vetsci9090481
APA StyleKau, S., Motter, K. S., Moser, V. J., Kunz, J. R., Pellachin, M., & Hartl, B. (2022). Intra- and Interexaminer Measurement Variability Analysis of an Orthodontic Gauge Device to Determine Incisor Occlusal Surface Angles in the Horse. Veterinary Sciences, 9(9), 481. https://doi.org/10.3390/vetsci9090481







