Simple Summary
Gastrointestinal stromal tumors represent the most common mesenchymal tumor of the canine gastrointestinal tract. Activating mutations in c-KIT and PDGFRA genes are considered the key molecular drivers of human gastrointestinal stromal tumors pathogenesis and are used to predict the response to Receptor tyrosine kinase inhibitors. However, in veterinary medicine, the significance of these mutations in canine gastrointestinal stromal tumors has not been sufficiently explored yet. The aim of this study is to investigate the mutational status of c-KIT and PDGFRA, by PCR and sequencing, in 17 canine gastrointestinal stromal tumors. Mutations of c-KIT were detected in 47% of cases; in one case, PDGFRA mutation was also identified. Although follow-up data were not available for all specimens, based on the information collected, we observed at the time of diagnosis the presence of metastases in cases with c-KIT mutation. In conclusion, this study provides evidence for the presence of c-KIT and PDGFRA mutations in canine gastrointestinal stromal tumors and suggests a potential association of c-KIT mutation with the more aggressive biological behavior of the tumor.
Abstract
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the canine gastrointestinal tract and are diagnosed by the immunohistochemical expression of the receptor tyrosine kinase (RTK) KIT. Activating mutations of the proto-oncogenes c-KIT and PDGFRA drive GIST oncogenesis and are used to predict the response to RTK-inhibitors in human oncology. Currently, the frequency and significance of these mutations in canine GIST have not been adequately explored. Therefore, we investigated the mutational status of c-KIT (exons 9, 11 and 13) and PDGFRA (exons 12 and 18) genes by PCR followed by fragment analysis for c-KIT deletions and PCR followed by screening with DHPLC and direct sequencing confirmation for single nucleotide variations in 17 formalin-fixed paraffin-embedded canine GISTs confirmed by KIT immunopositivity. c-KIT mutations were detected in 47% of cases, with a mutation detection rate significantly higher (p = 0.0004, Fisher’s exact test) and always involving exon 11. A PDGFRA gene mutation (exon 18) was identified in one case. Even if follow-up data were not available for all cases, four cases with documented abdominal metastases displayed c-KIT mutations. These data confirm that c-KIT exon 11 mutations occur frequently in canine GISTs, and identify the presence of a PDGFRA mutation similar to human GISTs. This study also suggests a potential association of c-KIT mutation with more aggressive biological behavior.
1. Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms affecting the gastrointestinal (GI) tract wall in humans [1,2] as well as dogs [3] and arise from the neoplastic transformation of interstitial cells of Cajal (ICC) [1,2,4], which are ‘pacemaker’ cells interposed between the plexuses myenteric and muscular tunic of the GI tract, with the function of coordinating peristalsis. Most GISTs are diagnosed on the basis of the immunohistochemical demonstration of the expression of KIT (CD117, stem cell factor receptor), a type-III tyrosine kinase receptor encoded by the protoncogene c-KIT, which, therefore, has evidential value in the diagnosis of GIST, which is otherwise difficult to distinguish from other GI mesenchymal neoplasms. In fact, retrospective studies regarding dogs and humans have shown that most of the gastrointestinal mesenchymal neoplasms, previously classified as leiomyomas or leiomyosarcomas, express CD117 and are then to be diagnosed as GISTs [5,6,7,8,9,10,11,12,13,14,15,16].
Mutations in the proto-oncogene c-KIT in exon 11, 9, 13 and 17 of the c-KIT gene have been observed in human GISTs. The mutation with the highest frequency (of more than 65%) is located in exon 11, in the juxtamembrane domain [17,18,19]. The frequent finding of these mutations has demonstrated their important pathogenetic role, which is responsible for the constitutive dimerization and activation of the KIT receptor regardless of the interaction with the ligand. In canine GISTs, activating mutations have been found and all of them in exon 11, as recorded in humans [7,8,12,15].
PDGFRA encodes a transmembrane type-II tyrosine kinase receptor that is normally activated by platelet-derived growth factors (PDGFs), mitogens for cells of mesenchymal origin [20]. PDGFRA mutations were identified in 35% of human GISTs with wild-type c-KIT [20] and mostly (about 90%) localized in exon 18. In canine GISTs, only Gregory-Bryson et al. (2010) [12] found the presence of synonymous single nucleotide polymorphism (SNPs) in exon 12 and 14 of PDGFRA.
Two Tyrosine kinase inhibitors (TKIs), toceranib and masitinib, have recently entered into veterinary clinical practice [21,22] and are currently changing the therapeutic approach for malignancies in dogs and cats. For the treatment of human GISTs, imatinib mesylate, an anticancer agent targeting several protein tyrosine kinases including c-KIT and PDGFR [23], is the first-line therapy for GISTs carrying the c-KIT [24] and PDGFR mutations [20,25]. A favorable response to imatinib mesylate has also been reported in dogs with unresected or metastatic GISTs carrying c-KIT mutations, suggesting the possibility of the effectiveness of the treatment [7,15]. Strikingly, a metastatic GIST reported by Irie et al. (2015) [7] showed complete remission after imatinib mesylate treatment. A recent study has shown the biological activity of toceranib in canine GISTs with shorter progression-free intervals in GISTs with metastasis and high tumor mitotic index [26]. Therefore, it is important to identify c-KIT and PDGFR mutations in canine GISTs so that appropriate therapeutic options can be identified.
In this study, the type and frequency of c-KIT exon 8, 9 and 11 and PDGFRA exon 12 and 18 mutations, and the potential relationship between mutational status and histotype and biological behavior, were investigated.
2. Materials and Methods
2.1. Samples Collection
The study was conducted at Department of Veterinary Medical Sciences (DIMEVET) University of Bologna (Ozzano Emilia, Bologna, Italy) and was carried out on formalin-fixed paraffin-embedded histological samples of canine GISTs received at the Pathological Service for histological diagnosis over a period of 16 years (2000–2016). All specimens previously diagnosed as GISTs by histomorphology and CD117 immunopositivity were included in this study. From records, anamnestic information about the caseload, including breed, gender and age of the patient, tumors localization, and the presence of metastasis, when detectable, were extracted.
2.2. Histology
The classification was based on histopathological observations according to the criteria established by the WHO specific for the tumors of gastrointestinal tract [3], and on the basis of the veterinary literature [5,6,7,8,9,10,12,13,14,15,16]. During the histological evaluations, the morphological type of each case was identified, and they were classified according to their histological pattern (storiform, epithelioid, myxoid and fascicular). Malignant tumors were classified using criteria based on cell differentiation, presence and extension of necrosis within the neoplastic mass, and mitotic index [27,28]. For the evaluation of the histological grade, the samples were scored from 1 to 3 for each of the three previous parameters: overall cell differentiation (1, neoplasms that show close resemblance to the physiological tissue of the adult animal and do not show cellular atypia; 2, neoplasms showing a defined histological subtype or moderate atypia; 3, neoplasms with poor differentiation and marked atypia); mitotic activity (1, range from 0 to 9 of mitotic figures for ten fields at high magnification; 2, range from 10 to 19 of mitotic figures for ten fields at high magnification; 3, 20 or more mitotic figures for ten fields at high magnification) and necrosis (1, absence of necrosis; 2, necrosis ≤ 50% of the tumor surface of the histological section; 3, necrosis > 50% of the tumor surface of the histological section). Grade I was assigned to a final score of 3 or 4; Grade II was assigned a score of 5 or 6 and Grade III was assigned with a score of 7, 8 or 9.
2.3. Immunohistochemistry
All the GISTs were subjected to immunohistochemical (IHC) evaluations with CD117, PDGFRα, SMA and Desmin.
The immunohistochemical staining was performed following the streptavidin-biotin-peroxidase technique (BIO SPA, Milan, Italy). The antibodies and their dilutions are as follows: CD117-protein c-KIT (1:500, polyclonal; Dako, Glostrup, Denmark), desmin (1:100, monoclonal; Dako), actin smooth muscle (1:100, monoclonal; Dako), and PDGFRα (1:300, polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation with 0.3% hydrogen peroxide in methanol for 20 min (to block the activity of endogenous peroxidases), and treatment in a microwave oven (750 W) for the unmasking of the antigen in citrate buffer at pH 6.0 (one cycle of 5 min and 5 cycles of 2 and a half minutes each, topping up the evaporated buffer after each session), the sections were then incubated overnight at 4 °C in a humid chamber with the primary antibody diluted, according to the appropriate dilutions, in PBS (0.01 M, pH 7.4). The sections, subsequently washed in PBS, were first incubated with the secondary antibody (anti-rabbit IgG conjugated with biotin) for 30 min at room temperature, then incubated with the streptavidin–peroxidase complex for 25 min at room temperature. After a 12-minute passage in the DAB chromogen solution (diaminobenzidine 0.02%, and H2O2 0.001% in PBS), the sections were immediately rinsed in PBS, then in running water, stained with a counterstain (hematoxylin; Histo-Line Laboratories, Pantigliate, MI, Italy), dehydrated and mounted with DPX (Fluka, Riedel-de Häen, Germany).
The results were graded as follows: −, negative reaction; +, foci of positivity (<50% of neoplastic cells show a positive reaction); ++, widespread cellular positivity (>50% and <75% of cells immunoreactive); +++, most (>75%) of neoplastic cells are positive. Appropriate positive controls were used in order to evaluate the specificity of the reactions and ascertain the appropriate cross-reactivity in the dog tissue. Normal skeletal muscle and normal dog intestine were used as positive controls for mesenchymal markers (SMA, Desmin and PDGFRα). Purkinje cells from cerebellar tissue and mast cell tumors were used as a positive control for CD117 [29,30]. As a negative control for the immunohistochemical procedure, 10% normal goat serum was used on the replicated sections instead of the primary antibody.
2.4. Analysis of c-KIT and PDGFRA Mutations
Tissue sample from each case was analyzed to determine the c-KIT and PDGFRA mutations status in genomic DNA.
Extraction of the DNA from a 6–8 µm section of formalin-fixed paraffin-embedded tissue was performed using the NucleoSpin® FFPE DNA kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. Different primers for c-KIT and PDGFRA (Table 1) were used for PCR amplification.

Table 1.
Primers and their respective annealing temperature used in the study.
The PCR products were subjected to the following post PCR analyses:
Exon 8 c-KIT: Genescanning analysis, DHPLC and direct sequencing;
Exon 9 c-KIT: DHPLC and direct sequencing;
Exon 11 c-KIT: Genescanning and fragment analysis on DHPLC;
Exon 12 PDGFRA: DHPLC and direct sequencing;
Exon 18 PDGFRA: DHPLC and direct sequencing (see Supplementary Materials for details).
2.5. Statistical Analysis
Statistical analysis was performed using the Fisher’s exact test to examine the associations between the presence of c-KIT mutations and histotype, histological grade and the presence of metastases.
3. Results
3.1. Sample Collection
Seventeen cases of canine gastrointestinal stromal tumors were included in this study. Nine of these patients were males and 6 were females, and two were of an unknown gender; half of them were sterilized (Table 2). Two of the affected dogs were German Shepherd (2/17; 12%) and two Setter (2/17; 12%), while all the other breeds are represented with only one case; finally, 5 out of 17 dogs were Mixed breed (29%). The age of onset of the primary neoplastic mass ranged from 5 to 14 years, with a mean age of 11 years (Table 2). The localization of the tumor was at the gastric level in two cases (2/17; 12%), one of which was near the pylorus. In nine subjects (9/17; 53%), the GISTs were found in the small intestine. Of these, three involve the duodenum (30%), two the jejunum, two the ileum and two had unspecified location. The remaining six cases (35%) mainly involved the large intestine with two cases at the level of the ileocecal valve, three at the level of the cecum and one at the colon (Table 1) (Figure 1). Metastatic involvement is reported in 4 out of 17 subjects (24%) affecting the tributary lymph nodes and the mesentery in case #13; liver, diaphragm and mesentery in case #7; spleen in case #9 and mesentery in case #10 (Table 2).

Table 2.
Anamnestic information and histological results of the 17 canine GISTs. Abbreviations: F: female; FS: spayed female; M: male; MN: neutered male; u: unknown; y: years; a: absent (at the time of diagnosis).
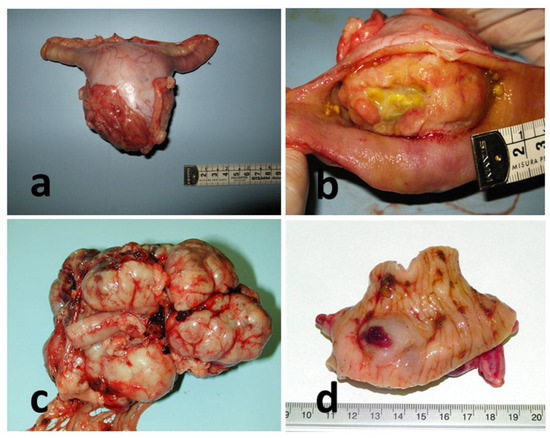
Figure 1.
Gross features of GISTs cases. (a) Case #11, intestine, jejunum. (b) Case #14, intestine, duodenum. (c) Case #10, intestine, ileum; (d) Case #16, intestine, cecum.
3.2. Histology
Four out of seventeen GISTs (24%) showed high cellularity, with a storiform pattern. Upon histological observation, they appear highly cellular, consisting of densely packed sheets of cells, with a markedly spindle morphology, arranged in palisades or intertwined and/or intercalated bundles or structured in a ‘storiform’ arrangement, with shaped nuclei oval and eosinophilic cytoplasm. The cell limits appear indistinct (Figure 2a). In three cases (#2, 6, 16) it was possible to observe an abundant presence of necrosis (>50%), hemorrhagic foci, marked neoangiogenesis and extremely dilated and blood-filled blood vessels.
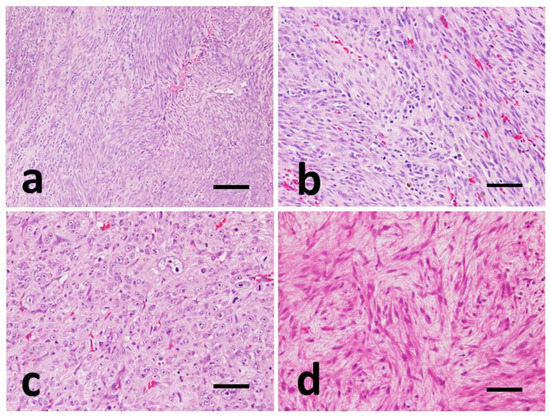
Figure 2.
Representative images of histomorphological pattern of GISTs, Hematoxylin-Eosin (HE). (a) Case #8, storiform pattern. Spindle cells forming whirls and palisades. (b) Case #16, fascicular pattern. Spindle cells are organized into interlacing and crisscrossing bundles. (c) Case #9, epithelioid pattern. Nests and sheets of round to polygonal cells. (d) Case #12, myxoid pattern. Scattered, non-cohesive, spindle cells with myxoid matrix. (a) Scale bar 300 µm and (b–d) scale bar 100 µm.
In 7 cases out of 17 (41%), the neoplasm shows a fasciculate pattern with less marked cellularity than the previous ones, and on histological observation it appears to consist of cells with fusiform morphology, which are less densely packed and arranged in loosely intertwined palisades and of smaller extension (Figure 2b).
In 5 out of 17 samples (29%), the neoplastic cells showed an epithelioid-like appearance, and appear arranged in trabecular-like structures or, more frequently, in a solid-compact carpet. The cells, with a polygonal to rounded shape, have an often-vacuolated cytoplasm and high pleomorphism (Figure 2c).
In one case (#12), it is possible to observe a myxoid pattern, characterized by poorly structured cells, spindle-shaped and separated by an abundant myxoid matrix. The nuclei are very elongated with clearly evident nucleoli. Interspersed with these areas it is possible to observe more or less extensive areas with a fascicular or storiform pattern (Figure 2d).
At the evaluation of the histological grade, 10/17 cases (59%) showed a Grade I; 6/17 cases (35%) have Grade II, and only one case (n° 11) is Grade III (Table 2). Evaluation of the presence and extent of intratumoral hemorrhage gave the following results: 7/17 (41%) grade 0, 3/17 (18%) grade 1 and 7/17 (41%) grade 2 (Table 2).
3.3. Immunohistochemistry
In all 17 GISTs, the cells show a strong and widespread cytoplasmic immunopositivity of the granular type for the KIT protein (CD117) with a percentage of immunopositive cells greater than about 90%. In only one case (#4) immunoreactivity occurs mainly in the cell membrane (Figure 3a) and in another (#8) immunoreactivity aggregated at the perinuclear area with focal distribution (Figure 3b).
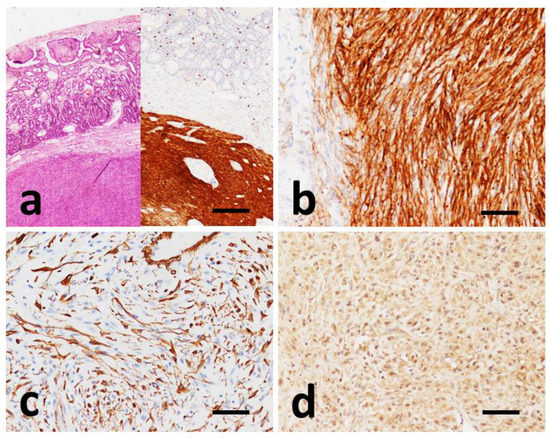
Figure 3.
Immunohistochemical findings in GISTs. (a) Case #6, HE and IHC with CD117 of consecutive slides showing strong and uniform cytoplasmic CD117 positivity of the tumor mass. (b) Case #8, prevalent strong perinuclear dots and membranous staining of CD117. (c) Case #6, SMA positivity of neoplastic cells. (d) Case #4, cytoplasmic positivity to PDGFRα. (a) Scale bar 500 µm, (b–d) scale bar 100 µm.
Ten samples were tested with SMA antibody; only one of these (#6) was found to be diffusely immunopositive (Figure 3c), while the remaining nine cases showed focal and weak immunopositivity, with a predominantly cytoplasmic pattern.
Eight samples were tested for Desmin antibody. Of these, seven were negative, while only one sample (#1) was focally positive at the cytoplasmic level, in about 30% of the cells.
Of all samples tested for PDGFRα antibody, three cases were completely negative, eight samples were weakly positive and six samples moderately positive. The immunopositivity pattern for this marker is localized at the cytoplasmic level with variable intensity, from weak to moderate, with a percentage of positive cells greater than 70% (Figure 3d).
3.4. Analysis of c-KIT and PDGFRA Mutations
c-KIT mutations were detected in 8/17 cases (47%) and always involved exon 11 (deletion of 3–46 bp), while exons 8 and 9 were wild-type in all cases. PDGFRA gene mutation was identified only in one case in the exon 18 (ENSCAFT00000003270.5 c.2597 G > A, SNP activating mutation) (Table 3). The mutation in the dog results in the substitution of Arginine with Lysine in position 866 and it was identified by DHPLC screening and confirmed by sequencing, which allowed definition of the mutation. The mutation is predicted to be cancer causative with a score of −2.10 (standard threshold at −0.75) using FATHMM [31].

Table 3.
c-KIT and PDGFRA gene status of the 17 canine GISTs.
Although follow-up information was not available for all cases, abdominal metastases were documented in four cases, and all showed a mutation in c-KIT exon-11.
3.5. Statistical Analysis
No significant correlations were found between the presence of metastases and histological pattern (p = 0.2605), histological grade and histotype (p = 1.0000), histological grade and presence of mutations (p = 0.5840). The presence of mutations, on the other hand, appears to be significantly (p < 0.05) correlated with the presence of metastases (p = 0.0004).
4. Discussion
Gastrointestinal stromal tumors (GISTs) represent a distinctive diagnostic neoplastic entity that, although quite uncommon and not yet fully characterized in dogs, has acquired over the years a well-defined clinical and histopathological identity and generates considerable interest for several reasons. Indeed, canine GISTs bear a very close resemblance to their human counterparts, representing an excellent spontaneous comparative model. This similarity paves the way for significant therapeutic perspectives in dogs, since features and the mutational aspect are almost completely superimposable [6,7,8,12,19,32,33,34,35].
In our caseload, collected over a long period (16 years), GISTs are scarcely represented. However, these data follow epidemiological data from the literature [5,6,10,11,12,13,14,16], which considers GISTs a neoplasm whose incidence is rather difficult to determine but still relatively rare, although it represents an important share of gastrointestinal mesenchymal neoplasms (from 39% to 50%).
CD117 positivity in over 70% of neoplastic cells is the prerequisite for diagnosing these neoplasms as GISTs [3]. In all our tumors, CD117 revealed an intense and widespread positivity to over 90% of the neoplastic cells. For most of the cases, this positivity was of a finely granular type, and in one case a paranuclear positivity was expressed in the form of small rounded and intensely colored clusters near the nucleus. This result reflects literature data [16] in which paranuclear positivity has been reported in a small number of cases.
Some attempts have been made to correlate the CD117 immunohistochemical pattern to the presence of mutations in canine GISTs, similar to what has already been extensively analyzed for mast cell tumors in dogs [36], but without significant results [8]. Furthermore, in our cases, no association was found between the IHC pattern and the mutational status of c-KIT.
Eight distinct mutations of the same type (deletions) were identified in this study, all involving exon 11 of the c-KIT gene in the juxtamembrane domain. On the other hand, no mutation was recognized in the other exons investigated (exons 8, 9, 13 and 17). These results confirm what has already been reported in canine cases [6,7,12] in which the mutations are localized in exon 11 in almost all of them. Only Irie et al. (2015) [7] reports a mutation in exon 9 of c-KIT, which is found in humans, but not in our series.
Almost half of the cases analyzed have this KIT mutation (47%; 8/17), in a percentage that falls within the range of the few studies that have evaluated the frequency of this mutation in canine GISTs (from 35% by Gregory-Bryson et al., 2010 [12] to 50% by Kumagai et al., 2003 [9] up to 74% by Takanosu et al., 2016 [8]). The different results might be the consequence of the employment of different methodological approaches (conventional PCR vs RT-PCR). The presence of KIT mutations does not appear to be related either to the GISTs localization, nor to the histotype and to the histological grade of the GI tumors. Nonetheless, most cases with mutations showed metastases at the time of diagnosis and, thus, exhibited a more biologically aggressive behavior (p = 0.0004).
One case showed the presence of an activating point mutation at the level of exon 18 of the PDGFRA oncogene (case #4) in correspondence to a human equivalent mutational hotspot. Until now, no significant activating mutation affecting PDGFRA had ever been reported in the dog, unlike what had been found for some time in humans, in which activating mutations of this gene represent a share ranging from 10 to 35% [16,19,34]. Interestingly, case #4 also presented an activating mutation in KIT Exon 11. This finding, both mutations in the same tumor, has never been observed in human GISTs.
In humans, PDGFRA-mutated GISTs are frequently found in the stomach, presented an epithelioid histotype and are often associated with a PDGFRα strong immunopositivity [37]. Of note is that case # 4, although localized in the intestine and with a moderate PDGFRα immunopositivity, shows the same epithelioid histotype.
There are several ‘inhibitory’ molecules that target tyrosine kinase receptors (TKIs), including imatinib mesylate, which has long been successfully used in the treatment of human GISTs with KIT mutations. To date, due to its effective therapeutic response, this TKI has recently been considered the treatment of choice for patients with advanced stage GISTs. In a study conducted on two dogs with documented exon-11 c-KIT mutations, imatinib mesylate (Gleevec) has been used for its ability to inhibit protein kinases and results in a marked tumor response and an increase in survival time [7,15]. The use of toceranib phosphate in seven dogs with GIST had recently shown a shorter disease-free interval in dogs with metastasis at diagnosis and a high tumor mitotic index [26]. Therefore, the role of c-KIT and PDGRFA mutations in canine GISTs must be extensively studied to understand their potential therapeutic efficacy.
5. Conclusions
c-KIT mutations were detected in half of the cases included in this study, and their presence appears to be associated with malignancy. An activating mutation of PDGFRA, which in humans is represented in a significant fraction of GISTs, had not yet been reported in canine GISTs. Unfortunately, follow-up data were not available and, therefore, no meaningful prognostic information can be provided from this study.
In conclusion, the study confirms that activating mutations of c-KIT play an important role in canine GISTs, are related to a malignant biological behavior and, therefore, have strong therapeutic implications. Further studies are needed involving a larger number of cases with follow-up data in order to demonstrate the prognostic and predictive role of KIT or PDGFRA mutations in canine GISTs.
Our results indicate, confirming what has already been stated in the literature, to what extent KIT mutations can have a prognostic and therapeutic significance in canine GISTs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci9070376/s1, PCR methodologic procedures.
Author Contributions
Conceptualization, M.M., F.G. (Fabio Gentilini) and G.B.; methodology, F.G. (Fabio Gentilini), M.E.T. and M.M.; validation, F.G. (Fabio Gentilini), M.E.T., L.M. and M.M.; data curation, F.G. (Francesca Gobbo), M.M. and G.B.; writing—original draft preparation, M.M., F.G. (Fabio Gentilini) and M.M.; writing—review and editing, F.G. (Francesca Gobbo), L.M. and G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because this was a retrospective study based on clinical data derived from the authors’ hospital’s database and on analyses performed on tissue samples taken for diagnostic procedures and stored in the pathological anatomy laboratory. Therefore, according to European and Italian laws, this study did not require authorization.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Authors thank Federica Turci for the data collection and statistical processing, and Michele Roncarati for the English revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Strickland, L.; Letson, G.D.; Muro-Cacho, C.A. Gastrointestinal Stromal Tumors. Cancer Control 2001, 8, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, A.A.; Bridge, J.A. Updates on the Cytogenetics and Molecular Genetics of Bone and Soft Tissue Tumors. Gastrointestinal Stromal Tumors. Cancer Genet. Cytogenet. 2002, 135, 1–23. [Google Scholar] [CrossRef]
- Head, K.W.; Cullen, J.M.; Dubielzig, R.R.; Else, R.W.; Misdorp, W.; Patnaik, A.K.; Tateyama, S.; Van der Gaag, I. Histological classification of tumors of the alimentary system of domestic animals. In World Health Organization International Histological Classification of Tumors of Domestic Animals; Armed Forces Institute of Pathology: Washington, DC, USA, 2003; Volume 10, pp. 58–72. [Google Scholar]
- Miettinen, M.; Lasota, J. Gastrointestinal Stromal Tumors—Definition, Clinical, Histological, Immunohistochemical and Molecular Genetic Features and Differential Diagnosis. Virchows Arch 2001, 438, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bettini, G.; Morini, M.; Marcato, P.S. Gastrointestinal Spindle Cell Tumours of the Dog: Histological and Immunohistochemical Study. J. Comp. Pathol. 2003, 129, 283–293. [Google Scholar] [CrossRef]
- Frost, D.; Lasota, J.; Miettinen, M. Gastrointestinal Stromal Tumors and Leiomyomas in the Dog: A Histopathologic, Immunohistochemical, and Molecular Genetic Study of 50 Cases. Vet. Pathol. 2003, 40, 42–54. [Google Scholar] [CrossRef]
- Irie, M.; Takeuchi, Y.; Ohtake, Y.; Suzuki, H.; Nagata, N.; Miyoshi, T.; Kagawa, Y.; Yamagami, T. Imatinib Mesylate Treatment in a Dog with Gastrointestinal Stromal Tumors with a C-Kit Mutation. J. Vet. Med. Sci. 2015, 77, 1535–1539. [Google Scholar] [CrossRef][Green Version]
- Takanosu, M.; Amano, S.; Kagawa, Y. Analysis of C-KIT Exon 11 Mutations in Canine Gastrointestinal Stromal Tumours. Vet. J. 2016, 207, 118–123. [Google Scholar] [CrossRef]
- Kumagai, K.; Uchida, K.; Miyamoto, T.; Ushigusa, T.; Shinohara, S.; Yamaguchi, R.; Tateyama, S. Three Cases of Canine Gastrointestinal Stromal Tumors with Multiple Differentiations and C-Kit-Expression. J. Vet. Med. Sci. 2003, 65, 1119–1122. [Google Scholar] [CrossRef][Green Version]
- Russell, K.N.; Mehler, S.J.; Skorupski, K.A.; Baez, J.L.; Shofer, F.S.; Goldschmidt, M.H. Clinical and Immunohistochemical Differentiation of Gastrointestinal Stromal Tumors from Leiomyosarcomas in Dogs: 42 Cases (1990–2003). J. Am. Vet. Med. Assoc. 2007, 230, 1329–1333. [Google Scholar] [CrossRef]
- Maas, C.P.H.J.; ter Haar, G.; van der Gaag, I.; Kirpensteijn, J. Reclassification of Small Intestinal and Cecal Smooth Muscle Tumors in 72 Dogs: Clinical, Histologic, and Immunohistochemical Evaluation. Vet. Surg. 2007, 36, 302–313. [Google Scholar] [CrossRef]
- Gregory-Bryson, E.; Bartlett, E.; Kiupel, M.; Hayes, S.; Yuzbasiyan-Gurkan, V. Canine and Human Gastrointestinal Stromal Tumors Display Similar Mutations in C-KIT Exon 11. BMC Cancer 2010, 10, 559. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, V.; Baer, K.; Farrelly, J.; Craft, D.; Luong, R. Canine Gastrointestinal Stromal Tumors: Immunohistochemical Expression of CD34 and Examination of Prognostic Indicators Including Proliferation Markers Ki67 and AgNOR. Vet. Pathol. 2011, 48, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Yuzbasiyan-Gurkan, V.; Gregory-Bryson, E.; Kiupel, M. Classification of Canine Nonangiogenic, Nonlymphogenic, Gastrointestinal Sarcomas Based on Microscopic, Immunohistochemical, and Molecular Characteristics. Vet. Pathol. 2013, 50, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kuroki, S.; Ito, K.; Yasuda, A.; Sawada, H.; Ono, K.; Washizu, T.; Bonkobara, M. Imatinib-Associated Tumour Response in a Dog with a Non-Resectable Gastrointestinal Stromal Tumour Harbouring a c-Kit Exon 11 Deletion Mutation. Vet. J. 2013, 198, 271–274. [Google Scholar] [CrossRef]
- Dailey, D.D.; Ehrhart, E.J.; Duval, D.L.; Bass, T.; Powers, B.E. DOG1 Is a Sensitive and Specific Immunohistochemical Marker for Diagnosis of Canine Gastrointestinal Stromal Tumors. J. Vet. Diagn. Investig. 2015, 27, 268–277. [Google Scholar] [CrossRef]
- Hirota, S.; Isozaki, K.; Moriyama, Y.; Hashimoto, K.; Nishida, T.; Ishiguro, S.; Kawano, K.; Hanada, M.; Kurata, A.; Takeda, M.; et al. Gain-of-Function Mutations of c-Kit in Human Gastrointestinal Stromal Tumors. Science 1998, 279, 577–580. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Maki, R.G.; Corless, C.L.; Antonescu, C.R.; Harlow, A.; Griffith, D.; Town, A.; McKinley, A.; Ou, W.-B.; Fletcher, J.A.; et al. Primary and Secondary Kinase Genotypes Correlate with the Biological and Clinical Activity of Sunitinib in Imatinib-Resistant Gastrointestinal Stromal Tumor. J. Clin. Oncol. 2008, 26, 5352–5359. [Google Scholar] [CrossRef]
- Corless, C.L.; Barnett, C.M.; Heinrich, M.C. Gastrointestinal Stromal Tumours: Origin and Molecular Oncology. Nat. Rev. Cancer 2011, 11, 865–878. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Gastrointestinal Stromal Tumors: Review on Morphology, Molecular Pathology, Prognosis, and Differential Diagnosis. Arch. Pathol. Lab. Med. 2006, 130, 1466–1478. [Google Scholar] [CrossRef]
- Bonkobara, M. Dysregulation of Tyrosine Kinases and Use of Imatinib in Small Animal Practice. Vet. J. 2015, 205, 180–188. [Google Scholar] [CrossRef]
- London, C.A. Tyrosine Kinase Inhibitors in Veterinary Medicine. Top. Companion Anim. Med. 2009, 24, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.F. Imatinib Mesylate. Small Mol. Oncol. 2010, 184, 3–20. [Google Scholar] [CrossRef]
- Rubin, B.P.; Heinrich, M.C.; Corless, C.L. Gastrointestinal Stromal Tumour. Lancet 2007, 369, 1731–1741. [Google Scholar] [CrossRef]
- Agaimy, A.; Otto, C.; Braun, A.; Geddert, H.; Schaefer, I.-M.; Haller, F. Value of Epithelioid Morphology and PDGFRA Immunostaining Pattern for Prediction of PDGFRA Mutated Genotype in Gastrointestinal Stromal Tumors (GISTs). Int. J. Clin. Exp. Pathol. 2013, 6, 1839–1846. [Google Scholar] [PubMed]
- Berger, E.P.; Johannes, C.M.; Jergens, A.E.; Allenspach, K.; Powers, B.E.; Du, Y.; Mochel, J.P.; Fox, L.E.; Musser, M.L. Retrospective Evaluation of Toceranib Phosphate (Palladia®) Use in the Treatment of Gastrointestinal Stromal Tumors of Dogs. J. Vet. Intern. Med. 2018, 32, 2045–2053. [Google Scholar] [CrossRef]
- Powers, B.E.; Hoopes, P.J.; Ehrhart, E.J. Tumor Diagnosis, Grading, and Staging. Semin. Vet. Med. Surg. 1995, 10, 158–167. [Google Scholar]
- Oliveira, A.M.; Nascimento, A.G. Grading in Soft Tissue Tumors: Principles and Problems. Skeletal Radiol. 2001, 30, 543–559. [Google Scholar] [CrossRef]
- Morini, M.; Bettini, G.; Preziosi, R.; Mandrioli, L. C-Kit Gene Product (CD117) Immunoreactivity in Canine and Feline Paraffin Sections. J. Histochem. Cytochem. 2004, 52, 705–708. [Google Scholar] [CrossRef]
- London, C.A.; Kisseberth, W.C.; Galli, S.J.; Geissler, E.N.; Helfand, S.C. Expression of Stem Cell Factor Receptor (c-Kit) by the Malignant Mast Cells from Spontaneous Canine Mast Cell Tumours. J. Comp. Pathol. 1996, 115, 399–414. [Google Scholar] [CrossRef]
- Shihab, H.A.; Gough, J.; Cooper, D.N.; Day, I.N.M.; Gaunt, T.R. Predicting the Functional Consequences of Cancer-Associated Amino Acid Substitutions. Bioinformatics 2013, 29, 1504–1510. [Google Scholar] [CrossRef]
- Steigen, S.E.; Eide, T.J. Gastrointestinal Stromal Tumors (GISTs): A Review. APMIS 2009, 117, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.A.C.S. Gastrointestinal Stromal Tumours--an Update for Histopathologists. Histopathology 2011, 59, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Lasota, J. Gastrointestinal Stromal Tumors. Gastroenterol. Clin. N. Am. 2013, 42, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Ricci, R.; Tos, A.P.D.; Rindi, G. GISTogram: A Graphic Presentation of the Growing GIST Complexity. Virchows Arch. 2013, 463, 481–487. [Google Scholar] [CrossRef]
- Thompson, J.J.; Morrison, J.A.; Pearl, D.L.; Boston, S.E.; Wood, G.A.; Foster, R.A.; Coomber, B.L. Receptor Tyrosine Kinase Expression Profiles in Canine Cutaneous and Subcutaneous Mast Cell Tumors. Vet. Pathol. 2016, 53, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Papke, D.J.; Forgó, E.; Charville, G.W.; Hornick, J.L. PDGFRA Immunohistochemistry Predicts PDGFRA Mutations in Gastrointestinal Stromal Tumors. Am. J. Surg. Pathol. 2022, 46, 3–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).