Inhibiting Fatty Acid Amide Hydrolase Ameliorates Enteropathy in Diabetic Mice: A Cannabinoid 1 Receptor Mediated Mechanism
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Experimental Design
2.2. Diabetes Induction
2.3. Fecal Pellet Output Study
2.4. Drugs
2.5. Evans Blue Experiment
2.6. Data Analysis
3. Results
3.1. Induction of Diabetes Changed Blood Glucose Levels Not Body Weight
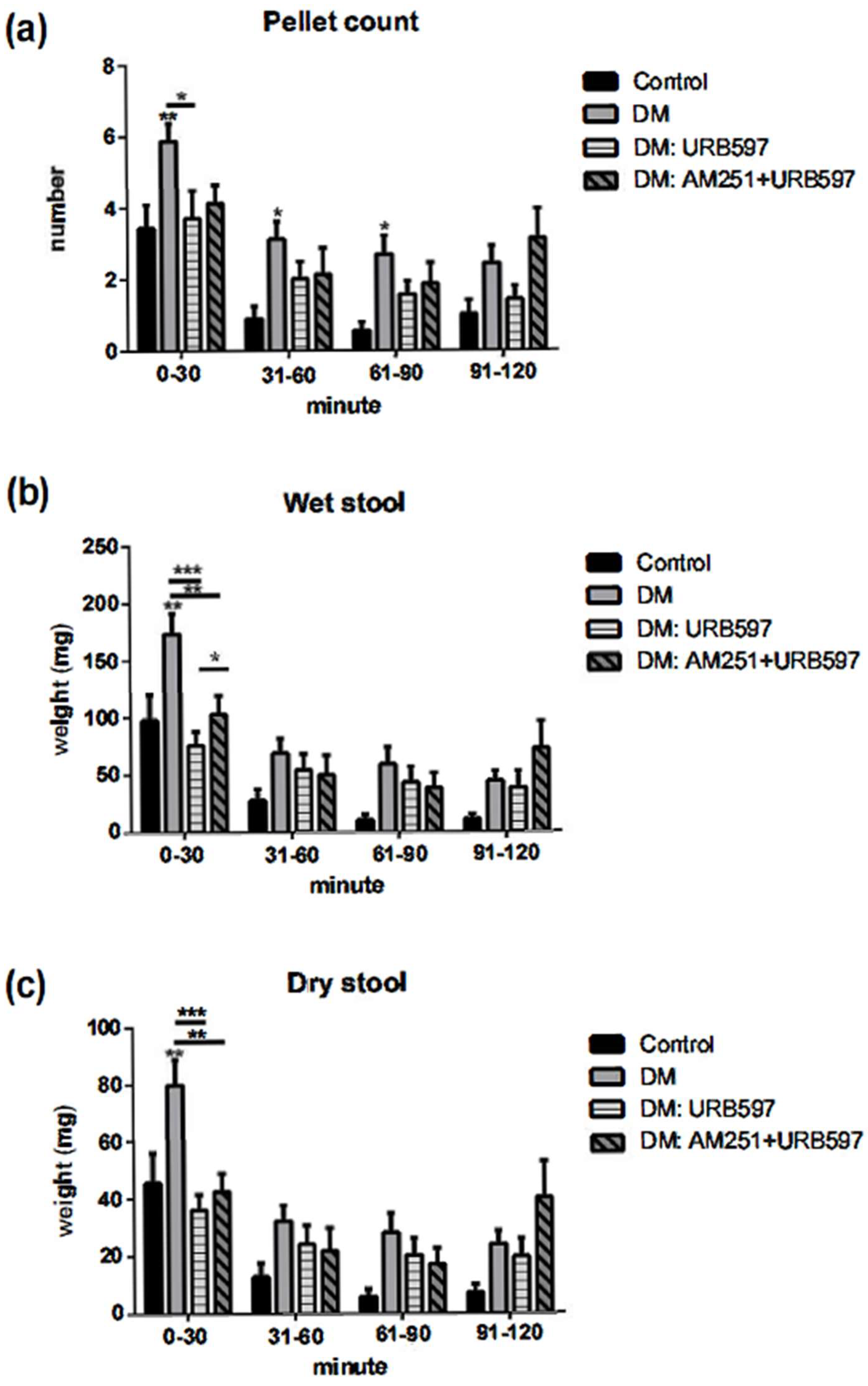
3.2. Increased Output in the Fecal Pellet Was Ameliorated by an FAAH Inhibitor in Diabetic Animals
3.3. Effects of FAAH Inhibitor on Wet and Dry Fecal Pellet Production
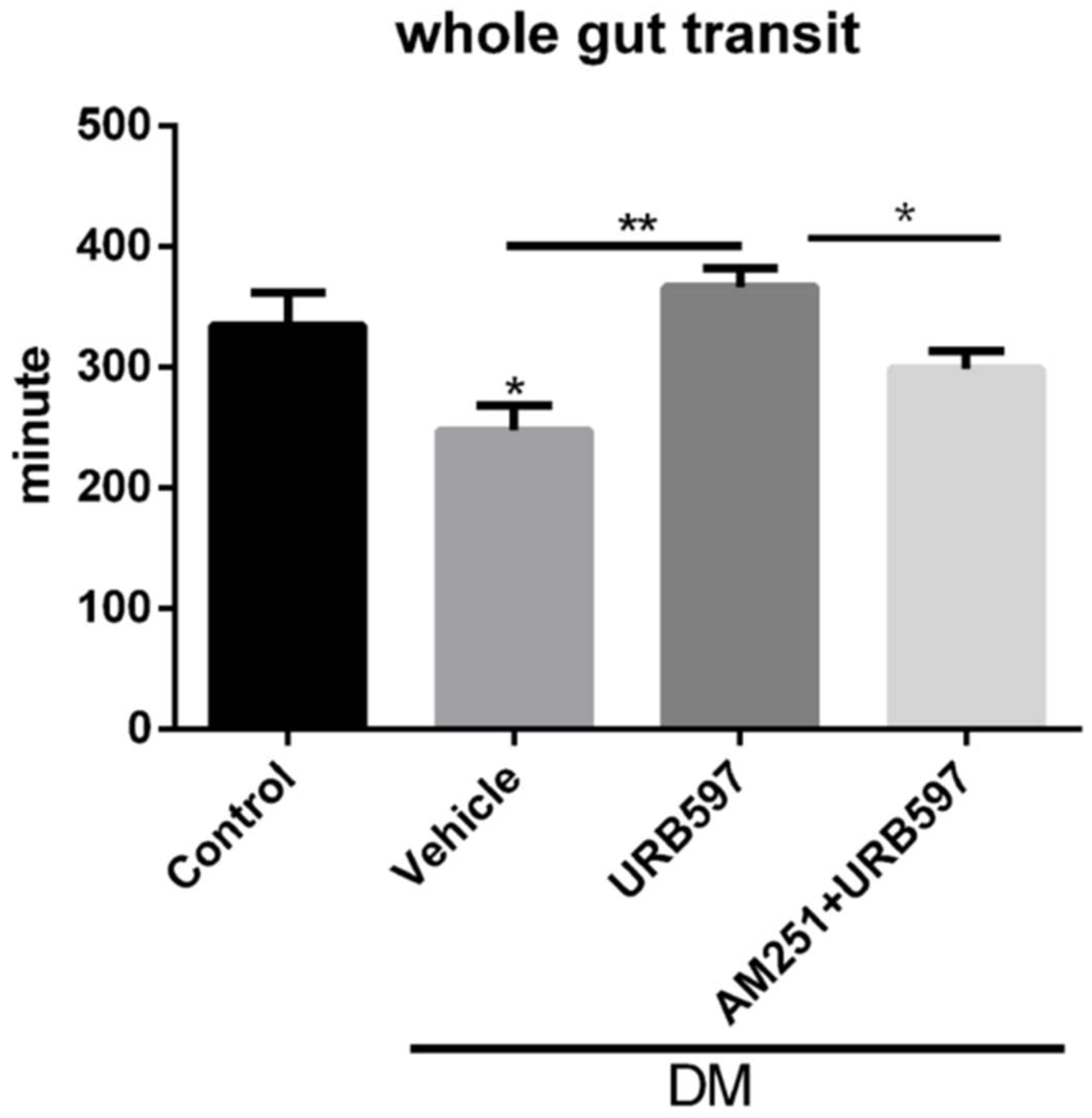
3.4. Whole Gut Transit Time
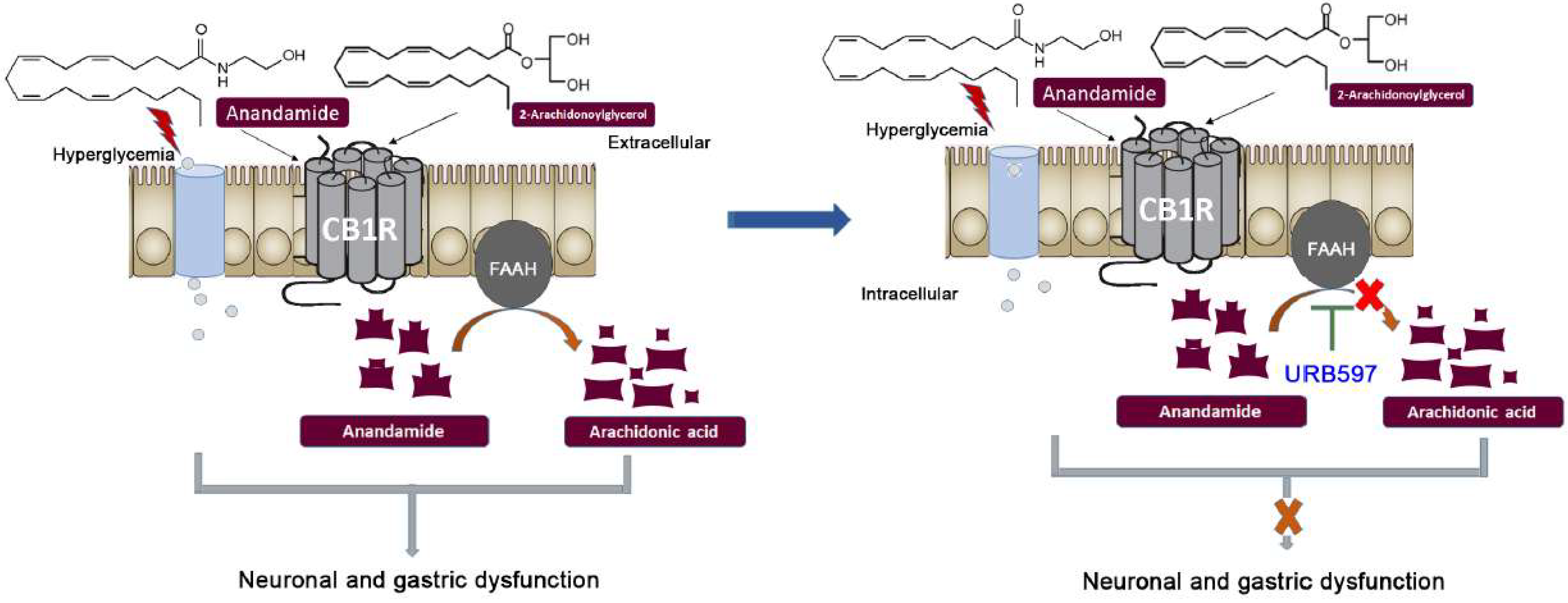
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maisey, A. A Practical Approach to Gastrointestinal Complications of Diabetes. Diabetes Ther. 2016, 7, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bytzer, P.; Talley, N.J.; Leemon, M.; Young, L.J.; Jones, M.P.; Horowitz, M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: A population-based survey of 15,000 adults. Arch. Intern. Med. 2001, 161, 1989–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, B.; Babu, S.; Walker, J.; Walker, A.B.; Pappachan, J.M. Gastrointestinal complications of diabetes mellitus. World J. Diabetes 2013, 4, 51–63. [Google Scholar] [CrossRef]
- Keller, J.; Layer, P. Intestinal and anorectal motility and functional disorders. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 407–423. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Anitha, M.; de Souza, S.M.; Jia, D.; Lu, X.; Kozlowski, M.; Olson, D.E.; Srinivasan, S.; Thule, P.M. Hepatic insulin gene therapy prevents diabetic enteropathy in STZ-treated CD-1 mice. Mol. Ther.-Methods Clin. Dev. 2015, 2, 15028. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.K.; Rayner, C.K.; Jones, K.L.; Horowitz, M. An update on autonomic neuropathy affecting the gastrointestinal tract. Curr. Diabetes Rep. 2006, 6, 417–423. [Google Scholar] [CrossRef]
- Wouters, M.M.; Vicario, M.; Santos, J. The role of mast cells in functional GI disorders. Gut 2016, 65, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Spiller, R. Role of motility in chronic diarrhoea. Neurogastroenterol. Motil. 2006, 18, 1045–1055. [Google Scholar] [CrossRef]
- Gruden, G.; Barutta, F.; Kunos, G.; Pacher, P. Role of the endocannabinoid system in diabetes and diabetic complications. Br. J. Pharm. 2016, 173, 1116–1127. [Google Scholar] [CrossRef]
- Fraser, R.J.; Horowitz, M.; Maddox, A.F.; Harding, P.E.; Chatterton, B.E.; Dent, J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1990, 33, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Bult, H.; Boeckxstaens, G.E.; Pelckmans, P.A.; Jordaens, F.H.; Van Maercke, Y.M.; Herman, A.G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 1990, 345, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.E.; Plurad, S.B.; Modert, C.W. Experimental diabetic autonomic neuropathy characterization in streptozotocin-diabetic Sprague-Dawley rats. Lab. Investig. 1983, 49, 538–552. [Google Scholar]
- Tougas, G.; Hunt, R.H.; Fitzpatrick, D.; Upton, A.R. Evidence of impaired afferent vagal function in patients with diabetes gastroparesis. Pacing Clin. Electrophysiol. 1992, 15, 1597–1602. [Google Scholar] [CrossRef]
- DiPatrizio, N.V. Endocannabinoids in the Gut. Cannabis Cannabinoid Res. 2016, 1, 67–77. [Google Scholar] [CrossRef]
- Aviello, G.; Romano, B.; Izzo, A.A. Cannabinoids and gastrointestinal motility: Animal and human studies. Eur. Rev. Med. Pharmacol. Sci. 2008, 12 (Suppl. S1), 81–93. [Google Scholar] [PubMed]
- Izzo, A.A.; Camilleri, M. Emerging role of cannabinoids in gastrointestinal and liver diseases: Basic and clinical aspects. Gut 2008, 57, 1140–1155. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Ross, R.A. Cannabinoid receptors and their ligands. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Abalo, R.; Vera, G.; Lopez-Perez, A.E.; Martinez-Villaluenga, M.; Martin-Fontelles, M.I. The gastrointestinal pharmacology of cannabinoids: Focus on motility. Pharmacology 2012, 90, 1–10. [Google Scholar] [CrossRef]
- Hornby, P.J.; Prouty, S.M. Involvement of cannabinoid receptors in gut motility and visceral perception. Br. J. Pharm. 2004, 141, 1335–1345. [Google Scholar] [CrossRef]
- Howlett, A.C.; Blume, L.C.; Dalton, G.D. CB(1) cannabinoid receptors and their associated proteins. Curr. Med. Chem. 2010, 17, 1382–1393. [Google Scholar] [CrossRef] [Green Version]
- Izzo, A.A.; Fezza, F.; Capasso, R.; Bisogno, T.; Pinto, L.; Iuvone, T.; Esposito, G.; Mascolo, N.; Di Marzo, V.; Capasso, F. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br. J. Pharm. 2001, 134, 563–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertwee, R.G. Cannabinoids and the gastrointestinal tract. Gut 2001, 48, 859–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasser, Y.; Bashashati, M.; Andrews, C.N. Toward modulation of the endocannabinoid system for treatment of gastrointestinal disease: FAAHster but not “higher”. Neurogastroenterol. Motil. 2014, 26, 447–454. [Google Scholar] [CrossRef]
- Storr, M.A.; Bashashati, M.; Hirota, C.; Vemuri, V.K.; Keenan, C.M.; Duncan, M.; Lutz, B.; Mackie, K.; Makriyannis, A.; Macnaughton, W.K.; et al. Differential effects of CB(1) neutral antagonists and inverse agonists on gastrointestinal motility in mice. Neurogastroenterol. Motil. 2010, 22, 787-e223. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, M.; Walter, C.; Mata, M.; Fink, D.J. Neuroprotective effect of herpes simplex virus-mediated gene transfer of erythropoietin in hyperglycemic dorsal root ganglion neurons. Brain 2008, 132, 879–888. [Google Scholar] [CrossRef] [Green Version]
- Bashashati, M.; Nasser, Y.; Keenan, C.M.; Ho, W.; Piscitelli, F.; Nalli, M.; Mackie, K.; Storr, M.A.; Di Marzo, V.; Sharkey, K.A. Inhibiting endocannabinoid biosynthesis: A novel approach to the treatment of constipation. Br. J. Pharm. 2015, 172, 3099–3111. [Google Scholar] [CrossRef] [PubMed]
- Bashashati, M.; Storr, M.A.; Nikas, S.P.; Wood, J.T.; Godlewski, G.; Liu, J.; Ho, W.; Keenan, C.M.; Zhang, H.; Alapafuja, S.O.; et al. Inhibiting fatty acid amide hydrolase normalizes endotoxin-induced enhanced gastrointestinal motility in mice. Br. J. Pharm. 2012, 165, 1556–1571. [Google Scholar] [CrossRef] [Green Version]
- Lupoli, R.; Creanza, A.; Griffo, E.; Nardone, G.; Rocco, A.; Bozzetto, L.; Annuzzi, G.; Riccardi, G.; Capaldo, B. Gastric Emptying Impacts the Timing of Meal Glucose Peak in Subjects With Uncomplicated Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 2269–2276. [Google Scholar] [CrossRef]
- Perano, S.J.; Rayner, C.K.; Kritas, S.; Horowitz, M.; Donaghue, K.; Mpundu-Kaambwa, C.; Giles, L.; Couper, J.J. Gastric Emptying Is More Rapid in Adolescents With Type 1 Diabetes and Impacts on Postprandial Glycemia. J. Clin. Endocrinol. Metab. 2015, 100, 2248–2253. [Google Scholar] [CrossRef]
- Rayner, C.K.; Samsom, M.; Jones, K.L.; Horowitz, M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 2001, 24, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Ebert, E.C. Gastrointestinal complications of diabetes mellitus. Dis. Mon. 2005, 51, 620–663. [Google Scholar] [CrossRef] [PubMed]
- Wrzos, H.F.; Cruz, A.; Polavarapu, R.; Shearer, D.; Ouyang, A. Nitric oxide synthase (NOS) expression in the myenteric plexus of streptozotocin-diabetic rats. Dig. Dis. Sci. 1997, 42, 2106–2110. [Google Scholar] [CrossRef] [PubMed]
- Kuznik, E.; Dudkowiak, R.; Adamiec, R.; Poniewierka, E. Diabetic autonomic neuropathy of the gastrointestinal tract. Prz. Gastroenterol. 2020, 15, 89–93. [Google Scholar] [PubMed]
- Storr, M.A.; Keenan, C.M.; Emmerdinger, D.; Zhang, H.; Yuce, B.; Sibaev, A.; Massa, F.; Buckley, N.E.; Lutz, B.; Goke, B.; et al. Targeting endocannabinoid degradation protects against experimental colitis in mice: Involvement of CB1 and CB2 receptors. J. Mol. Med. 2008, 86, 925–936. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, V.; Bashashati, M.; Enriquez, J.; Chattopadhyay, M. Inhibiting Fatty Acid Amide Hydrolase Ameliorates Enteropathy in Diabetic Mice: A Cannabinoid 1 Receptor Mediated Mechanism. Vet. Sci. 2022, 9, 364. https://doi.org/10.3390/vetsci9070364
Thakur V, Bashashati M, Enriquez J, Chattopadhyay M. Inhibiting Fatty Acid Amide Hydrolase Ameliorates Enteropathy in Diabetic Mice: A Cannabinoid 1 Receptor Mediated Mechanism. Veterinary Sciences. 2022; 9(7):364. https://doi.org/10.3390/vetsci9070364
Chicago/Turabian StyleThakur, Vikram, Mohammad Bashashati, Josue Enriquez, and Munmun Chattopadhyay. 2022. "Inhibiting Fatty Acid Amide Hydrolase Ameliorates Enteropathy in Diabetic Mice: A Cannabinoid 1 Receptor Mediated Mechanism" Veterinary Sciences 9, no. 7: 364. https://doi.org/10.3390/vetsci9070364
APA StyleThakur, V., Bashashati, M., Enriquez, J., & Chattopadhyay, M. (2022). Inhibiting Fatty Acid Amide Hydrolase Ameliorates Enteropathy in Diabetic Mice: A Cannabinoid 1 Receptor Mediated Mechanism. Veterinary Sciences, 9(7), 364. https://doi.org/10.3390/vetsci9070364






