Canine Cytokines Profile in an Endemic Region of L. infantum: Related Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Animals and Data Collection
2.3. Samples Collection and Cytokines Levels
2.4. Statistical Analysis
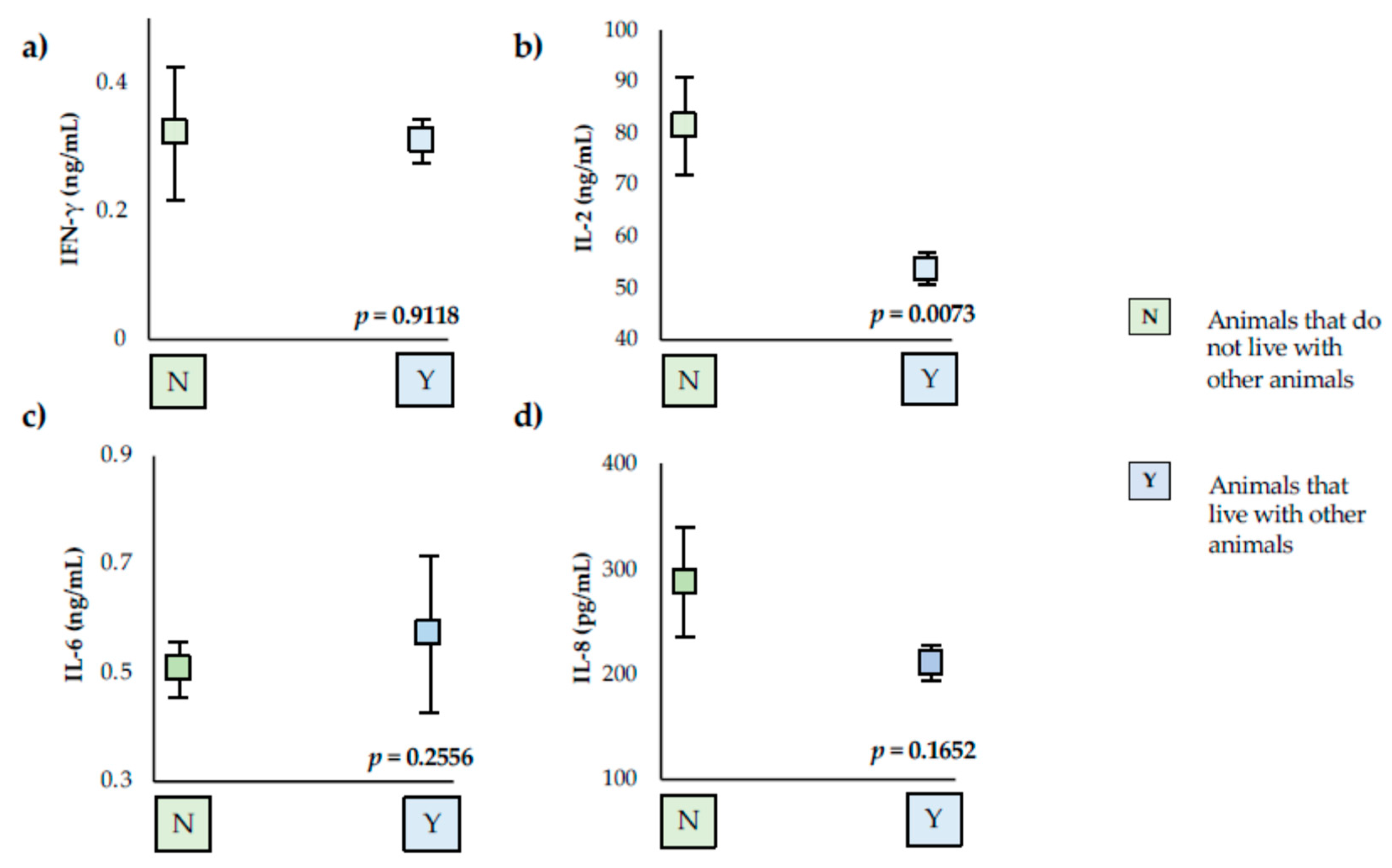
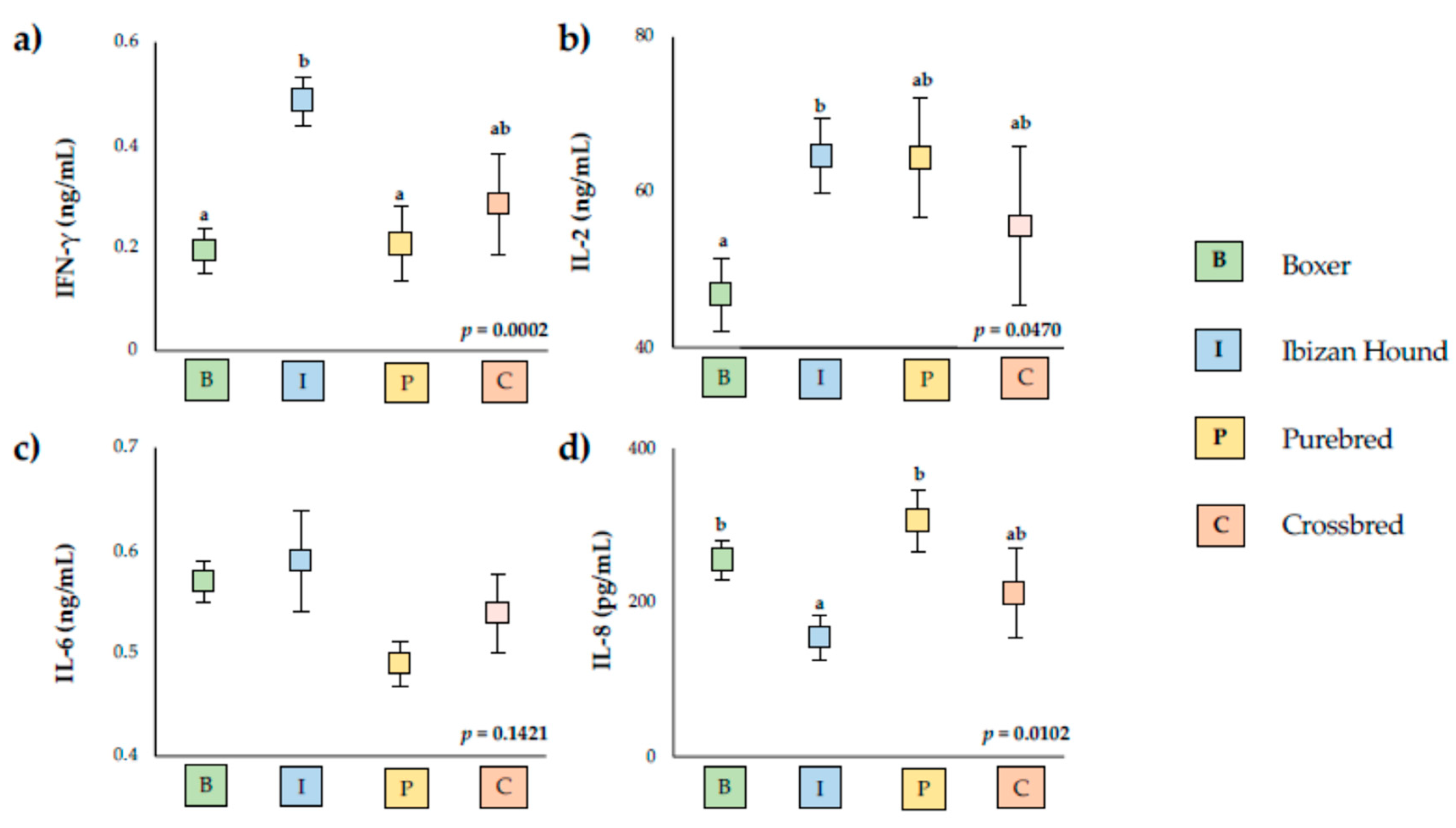
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gramiccia, M. Recent Advances in Leishmaniosis in Pet Animals: Epidemiology, Diagnostics and Anti-Vectorial Prophylaxis. Vet. Parasitol. 2011, 181, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Koutinas, A.F.; Solano-Gallego, L.; Bourdeau, P.; Ferrer, L. Canine Leishmaniosis—New Concepts and Insights on an Expanding Zoonosis: Part One. Trends Parasitol. 2008, 24, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Barbiéri, C.L. Immunology of Canine Leishmaniasis. Parasite Immunol. 2006, 28, 329–337. [Google Scholar] [CrossRef]
- Ready, P.D. Epidemiology of Visceral Leishmaniasis. Clin. Epidemiol. 2014, 6, 147–154. [Google Scholar] [CrossRef]
- Trájer, A.J.; Sebestyén, V. The Changing Distribution of Leishmania Infantum Nicolle, 1908 and Its Mediterranean Sandfly Vectors in the Last 140 Kys. Sci. Rep. 2019, 9, 11820. [Google Scholar] [CrossRef]
- Abbate, J.M.; Arfuso, F.; Napoli, E.; Gaglio, G.; Giannetto, S.; Latrofa, M.S.; Otranto, D.; Brianti, E. Leishmania Infantum in Wild Animals in Endemic Areas of Southern Italy. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101374. [Google Scholar] [CrossRef]
- Ahuir-Baraja, A.E.; Ruiz, M.P.; Garijo, M.M.; Llobat, L. Feline Leishmaniosis: An Emerging Public Health Problem. Vet. Sci. 2021, 8, 173. [Google Scholar] [CrossRef]
- Mancianti, F.; Gramiccia, M.; Gradoni, L.; Pieri, S. Studies on Canine Leishmaniasis Control. 1. Evolution of Infection of Different Clinical Forms of Canine Leishmaniasis Following Antimonial Treatment. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 566–567. [Google Scholar] [CrossRef]
- Gálvez, R.; Montoya, A.; Cruz, I.; Fernández, C.; Martín, O.; Checa, R.; Chicharro, C.; Migueláñez, S.; Marino, V.; Miró, G. Latest Trends in Leishmania Infantum Infection in Dogs in Spain, Part I: Mapped Seroprevalence and Sand Fly Distributions. Parasit Vectors 2020, 13, 204. [Google Scholar] [CrossRef]
- Miró, G.; Cardoso, L.; Pennisi, M.G.; Oliva, G.; Baneth, G. Canine Leishmaniosis--New Concepts and Insights on an Expanding Zoonosis: Part Two. Trends Parasitol. 2008, 24, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. The LeishVet Group, LeishVet Guidelines for the Practical Management of Canine Leishmaniosis. Parasit Vectors 2011, 4, 86. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Fasel, N. How to Master the Host Immune System? Leishmania Parasites Have the Solutions! Int. Immunol. 2018, 30, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Vanloubbeeck, Y.; Jones, D.E. The Immunology of Leishmania Infection and the Implications for Vaccine Development. Ann. N. Y. Acad. Sci. 2004, 1026, 267–272. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Koutinas, A.; Miró, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the Diagnosis, Clinical Staging, Treatment and Prevention of Canine Leishmaniosis. Vet. Parasitol. 2009, 165, 1–18. [Google Scholar] [CrossRef]
- Hosein, S.; Blake, D.P.; Solano-Gallego, L. Insights on Adaptive and Innate Immunity in Canine Leishmaniosis. Parasitology 2017, 144, 95–115. [Google Scholar] [CrossRef]
- Coffman, R.L.; Seymour, B.W.; Lebman, D.A.; Hiraki, D.D.; Christiansen, J.A.; Shrader, B.; Cherwinski, H.M.; Savelkoul, H.F.; Finkelman, F.D.; Bond, M.W. The Role of Helper T Cell Products in Mouse B Cell Differentiation and Isotype Regulation. Immunol. Rev. 1988, 102, 5–28. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two Types of Murine Helper T Cell Clone. I. Definition According to Profiles of Lymphokine Activities and Secreted Proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar]
- Pinelli, E.; Killick-Kendrick, R.; Wagenaar, J.; Bernadina, W.; del Real, G.; Ruitenberg, J. Cellular and Humoral Immune Responses in Dogs Experimentally and Naturally Infected with Leishmania Infantum. Infect. Immun. 1994, 62, 229–235. [Google Scholar] [CrossRef]
- Ordeix, L.; Montserrat-Sangrà, S.; Martínez-Orellana, P.; Solano-Gallego, L. Toll-Like Receptors 2, 4, and 7, Interferon-Gamma, Interleukin 10, and Programmed Death Ligand 1 Transcripts in Leishmanin Skin Test-Positive Reactions of Ibizan Hound Dogs. J. Immunol. Res. 2020, 2020, 9602576. [Google Scholar] [CrossRef]
- Priolo, V.; Martínez-Orellana, P.; Pennisi, M.G.; Masucci, M.; Prandi, D.; Ippolito, D.; Bruno, F.; Castelli, G.; Solano-Gallego, L. Leishmania Infantum-Specific IFN-γ Production in Stimulated Blood from Cats Living in Areas Where Canine Leishmaniosis Is Endemic. Parasit Vectors 2019, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Montserrrat-Sangrà, S.; Ordeix, L.; Martínez-Orellana, P. Leishmania infantum-Specific Production of IFN-γ and IL-10 in Stimulated Blood from Dogs with Clinical Leishmaniosis. Parasit Vectors 2016, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Abbehusen, M.M.C.; Almeida, V.D.A.; da S Solcà, M.; da Silva Pereira, L.; Costa, D.J.; Gil-Santana, L.; Bozza, P.T.; Fraga, D.B.M.; Veras, P.S.T.; Dos-Santos, W.L.C.; et al. Clinical and Immunopathological Findings during Long Term Follow-up in Leishmania Infantum Experimentally Infected Dogs. Sci. Rep. 2017, 7, 15914. [Google Scholar] [CrossRef] [PubMed]
- Cortese, L.; Annunziatella, M.; Palatucci, A.T.; Rubino, V.; Piantedosi, D.; Di Loria, A.; Ruggiero, G.; Ciaramella, P.; Terrazzano, G. Regulatory T Cells, Cytotoxic T Lymphocytes and a T(H)1 Cytokine Profile in Dogs Naturally Infected by Leishmania Infantum. Res. Vet. Sci. 2013, 95, 942–949. [Google Scholar] [CrossRef]
- Lombardi, P.; Palatucci, A.T.; Giovazzino, A.; Mastellone, V.; Ruggiero, G.; Rubino, V.; Musco, N.; Crupi, R.; Cutrignelli, M.I.; Britti, D.; et al. Clinical and Immunological Response in Dogs Naturally Infected by L. infantum Treated with a Nutritional Supplement. Animals 2019, 9, 501. [Google Scholar] [CrossRef]
- Castelo-Branco, C.; Soveral, I. The Immune System and Aging: A Review. Gynecol. Endocrinol. 2014, 30, 16–22. [Google Scholar] [CrossRef]
- Pinti, M.; Appay, V.; Campisi, J.; Frasca, D.; Fülöp, T.; Sauce, D.; Larbi, A.; Weinberger, B.; Cossarizza, A. Aging of the Immune System: Focus on Inflammation and Vaccination. Eur. J. Immunol. 2016, 46, 2286–2301. [Google Scholar] [CrossRef]
- Sadighi Akha, A.A. Aging and the Immune System: An Overview. J. Immunol. Methods 2018, 463, 21–26. [Google Scholar] [CrossRef]
- Alexander, J.E.; Colyer, A.; Haydock, R.M.; Hayek, M.G.; Park, J. Understanding How Dogs Age: Longitudinal Analysis of Markers of Inflammation, Immune Function, and Oxidative Stress. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 720–728. [Google Scholar] [CrossRef]
- HogenEsch, H.; Thompson, S.; Dunham, A.; Ceddia, M.; Hayek, M. Effect of Age on Immune Parameters and the Immune Response of Dogs to Vaccines: A Cross-Sectional Study. Vet. Immunol. Immunopathol. 2004, 97, 77–85. [Google Scholar] [CrossRef]
- Edo, M.; Marín-García, P.J.; Llobat, L. Is the Prevalence of Leishmania infantum Linked to Breeds in Dogs? Characterization of Seropositive Dogs in Ibiza. Animals 2021, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Orellana, P.; Marí-Martorell, D.; Montserrat-Sangrà, S.; Ordeix, L.; Baneth, G.; Solano-Gallego, L. Leishmania infantum-Specific IFN-γ Production in Stimulated Blood from Dogs with Clinical Leishmaniosis at Diagnosis and during Treatment. Vet. Parasitol. 2017, 248, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Robert, E.; Altet, L.; Sanchez, A.; Francino, O. Polymorphism of Slc11a1 (Nramp1) Gene and Canine Leishmaniasis in a Case-Control Study. J. Hered. 2005, 96, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Llull, J.; Ramos, G.; Riera, C.; Arboix, M.; Alberola, J.; Ferrer, L. The Ibizian Hound Presents a Predominantly Cellular Immune Response against Natural Leishmania Infection. Vet. Parasitol. 2000, 90, 37–45. [Google Scholar] [CrossRef]
- Sanchez-Robert, E.; Altet, L.; Utzet-Sadurni, M.; Giger, U.; Sanchez, A.; Francino, O. Slc11a1 (Formerly Nramp1) and Susceptibility to Canine Visceral Leishmaniasis. Vet. Res. 2008, 39, 36. [Google Scholar] [CrossRef]
- Olías-Molero, A.I.; Corral, M.J.; Jiménez-Antón, M.D.; Alunda, J.M. Early Antibody Response and Clinical Outcome in Experimental Canine Leishmaniasis. Sci. Rep. 2019, 9, 18606. [Google Scholar] [CrossRef]
- Miró, G.; Checa, R.; Montoya, A.; Hernández, L.; Dado, D.; Gálvez, R. Current Situation of Leishmania infantum Infection in Shelter Dogs in Northern Spain. Parasit Vectors 2012, 5, 60. [Google Scholar] [CrossRef]
- Morillas, F.; Sanchez Rabasco, F.; Ocaña, J.; Martin-Sanchez, J.; Ocaña-Wihelmi, J.; Acedo, C.; Sanchiz-Marin, M.C. Leishmaniosis in the Focus of the Axarquía Region, Malaga Province, Southern Spain: A Survey of the Human, Dog, and Vector. Parasitol. Res. 1996, 82, 569–570. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Ratzlaff, F.R.; Pötter, L.; Romão, P.R.T.; de Avila Botton, S.; Vogel, F.S.F.; Sangioni, L.A. Clinical and Pathological Aspects of Canine Cutaneous Leishmaniasis: A Meta-Analysis. Acta Parasitol. 2019, 64, 916–922. [Google Scholar] [CrossRef]
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine Sandflies and the Spreading of Leishmaniases and Other Diseases of Public Health Concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef]
- Galluzzi, L.; Ceccarelli, M.; Diotallevi, A.; Menotta, M.; Magnani, M. Real-Time PCR Applications for Diagnosis of Leishmaniasis. Parasit Vectors 2018, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Paz, G.F.; Rugani, J.M.N.; Marcelino, A.P.; Gontijo, C.M.F. Implications of the Use of Serological and Molecular Methods to Detect Infection by Leishmania spp. in Urban Pet Dogs. Acta Trop. 2018, 182, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.K.; Alcover, M.M.; Martínez-Orellana, P.; Montserrat-Sangrà, S.; Nachum-Biala, Y.; Fisa, R.; Riera, C.; Baneth, G.; Solano-Gallego, L. Serological and Molecular Survey of Leishmania Infection in Dogs from Venezuela. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100420. [Google Scholar] [CrossRef]
- de Arruda, M.M.; Figueiredo, F.B.; Marcelino, A.P.; Barbosa, J.R.; Werneck, G.L.; Noronha, E.F.; Romero, G.A.S. Sensitivity and Specificity of Parallel or Serial Serological Testing for Detection of Canine Leishmania Infection. Mem. Inst. Oswaldo Cruz. 2016, 111, 168–173. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, J.C.C.; Nunes-Pinheiro, D.C.S.; Neto, B.E.L.; Santos, G.J.L.; de Abreu, C.R.A.; Braga, R.R.; de Morais Campos, R.; de Oliveira, L.F. Clinical and Laboratory Alterations in Dogs Naturally Infected by Leishmania chagasi. Rev. Soc. Bras. Med. Trop. 2012, 45, 24–29. [Google Scholar] [CrossRef] [PubMed]
- de Ybáñez, R.R.; del Río, L.; Martínez-Carrasco, C.; Segovia, M.; Cox, J.; Davies, C.; Berriatua, E. Questionnaire Survey on Canine Leishmaniosis in Southeastern Spain. Vet. Parasitol. 2009, 164, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.F.; de Amorim, I.F.G.; Moura, E.P.; Ribeiro, R.R.; Alves, C.F.; Michalick, M.S.; Kalapothakis, E.; Bruna-Romero, O.; Tafuri, W.L.; Teixeira, M.M.; et al. Expression of IFN-Gamma, TNF-Alpha, IL-10 and TGF-Beta in Lymph Nodes Associates with Parasite Load and Clinical Form of Disease in Dogs Naturally Infected with Leishmania (Leishmania) chagasi. Vet. Immunol. Immunopathol. 2009, 128, 349–358. [Google Scholar] [CrossRef]
- DE F Michelin, A.; Perri, S.H.V.; De Lima, V.M.F. Evaluation of TNF-α, IL-4, and IL-10 and Parasite Density in Spleen and Liver of L. (L.) chagasi Naturally Infected Dogs. Ann. Trop. Med. Parasitol. 2011, 105, 373–383. [Google Scholar] [CrossRef]
- Pereira-Fonseca, D.C.M.; Oliveira-Rovai, F.M.; Rodas, L.A.C.; Beloti, C.A.C.; Torrecilha, R.B.P.; Ito, P.K.R.K.; Avanço, S.V.; Cipriano, R.S.; Utsunomiya, Y.T.; Hiramoto, R.M.; et al. Dog Skin Parasite Load, TLR-2, IL-10 and TNF-α Expression and Infectiousness. Parasite Immunol. 2017, 39. [Google Scholar] [CrossRef]
- Venturin, G.L.; Chiku, V.M.; Silva, K.L.O.; de Almeida, B.F.M.; de Lima, V.M.F. M1 Polarization and the Effect of PGE2 on TNF-α Production by Lymph Node Cells from Dogs with Visceral Leishmaniasis. Parasite Immunol. 2016, 38, 698–704. [Google Scholar] [CrossRef]
- do Nascimento, P.R.P.; Martins, D.R.A.; Monteiro, G.R.G.; Queiroz, P.V.; Freire-Neto, F.P.; Queiroz, J.W.; Morais Lima, A.L.; Jeronimo, S.M.B. Association of Pro-Inflammatory Cytokines and Iron Regulatory Protein 2 (IRP2) with Leishmania Burden in Canine Visceral Leishmaniasis. PLoS ONE 2013, 8, e73873. [Google Scholar] [CrossRef] [PubMed]
- de Lima, V.M.F.; Peiro, J.R.; de Oliveira Vasconcelos, R. IL-6 and TNF-Alpha Production during Active Canine Visceral Leishmaniasis. Vet. Immunol. Immunopathol. 2007, 115, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Bouman, A.; Schipper, M.; Heineman, M.J.; Faas, M.M. Gender Difference in the Non-Specific and Specific Immune Response in Humans. Am. J. Reprod. Immunol. 2004, 52, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Allison, L.N.; Jaffey, J.A.; Bradley-Siemens, N.; Tao, Z.; Thompson, M.; Backus, R.C. Immune Function and Serum Vitamin D in Shelter Dogs: A Case-Control Study. Vet. J. 2020, 261, 105477. [Google Scholar] [CrossRef] [PubMed]
- Calvalido, J.; Wood, G.A.; Mutsaers, A.J.; Wood, D.; Sears, W.; Woods, J.P. Comparison of Serum Cytokine Levels between Dogs with Multicentric Lymphoma and Healthy Dogs. Vet. Immunol. Immunopathol. 2016, 182, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.D.; Thiel, B.; Mukhtar, E.; Sharp, P.; Larson, L.J. Age and Long-Term Protective Immunity in Dogs and Cats. J. Comp. Pathol. 2010, 142 Suppl 1, S102–S108. [Google Scholar] [CrossRef]
- Park, H.S.; Park, J.Y.; Yu, R. Relationship of Obesity and Visceral Adiposity with Serum Concentrations of CRP, TNF-Alpha and IL-6. Diabetes Res. Clin. Pract. 2005, 69, 29–35. [Google Scholar] [CrossRef]
- Sewter, C.P.; Digby, J.E.; Blows, F.; Prins, J.; O’Rahilly, S. Regulation of Tumour Necrosis Factor-Alpha Release from Human Adipose Tissue in Vitro. J. Endocrinol. 1999, 163, 33–38. [Google Scholar] [CrossRef]
- La Cava, A.; Matarese, G. The Weight of Leptin in Immunity. Nat. Rev. Immunol. 2004, 4, 371–379. [Google Scholar] [CrossRef]
- Sarraf, P.; Frederich, R.C.; Turner, E.M.; Ma, G.; Jaskowiak, N.T.; Rivet, D.J.; Flier, J.S.; Lowell, B.B.; Fraker, D.L.; Alexander, H.R. Multiple Cytokines and Acute Inflammation Raise Mouse Leptin Levels: Potential Role in Inflammatory Anorexia. J. Exp. Med. 1997, 185, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Cortese, L.; Annunziatella, M.; Palatucci, A.T.; Lanzilli, S.; Rubino, V.; Di Cerbo, A.; Centenaro, S.; Guidetti, G.; Canello, S.; Terrazzano, G. An Immune-Modulating Diet Increases the Regulatory T Cells and Reduces T Helper 1 Inflammatory Response in Leishmaniosis Affected Dogs Treated with Standard Therapy. BMC Vet. Res. 2015, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Bendickova, K.; Fric, J. Roles of IL-2 in Bridging Adaptive and Innate Immunity, and as a Tool for Cellular Immunotherapy. J. Leukoc. Biol. 2020, 108, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Yu, A.; Dee, M.J.; Malek, T.R. IL-2R Signaling Is Essential for Functional Maturation of Regulatory T Cells during Thymic Development. J. Immunol. 2013, 190, 1567–1575. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, Q.; La Cava, A.; Campagnolo, D.I.; Van Kaer, L.; Shi, F.-D. Expansion of Regulatory T Cells via IL-2/Anti-IL-2 MAb Complexes Suppresses Experimental Myasthenia. Eur. J. Immunol. 2010, 40, 1577–1589. [Google Scholar] [CrossRef]
- Kalia, V.; Sarkar, S. Regulation of Effector and Memory CD8 T Cell Differentiation by IL-2-A Balancing Act. Front. Immunol. 2018, 9, 2987. [Google Scholar] [CrossRef]
- Abbehusen, M.M.C.; Cunha, J.; Suarez, M.S.; Teixeira, C.; Almeida, V.D.A.; da Silva Pereira, L.; Bordoni, M.; Gil-Santana, L.; da Silva Solcà, M.; Fraga, D.B.M.; et al. Immunization of Experimental Dogs With Salivary Proteins From Lutzomyia longipalpis, Using DNA and Recombinant Canarypox Virus Induces Immune Responses Consistent With Protection Against Leishmania infantum. Front. Immunol. 2018, 9, 2558. [Google Scholar] [CrossRef]
- Costa, A.S.A.; Costa, G.C.; de Aquino, D.M.C.; de Mendonça, V.R.R.; Barral, A.; Barral-Netto, M.; Caldas, A. de J.M. Cytokines and Visceral Leishmaniasis: A Comparison of Plasma Cytokine Profiles between the Clinical Forms of Visceral Leishmaniasis. Mem. Inst. Oswaldo Cruz. 2012, 107, 735–739. [Google Scholar] [CrossRef]
- Samant, M.; Sahu, U.; Pandey, S.C.; Khare, P. Role of Cytokines in Experimental and Human Visceral Leishmaniasis. Front. Cell Infect. Microbiol. 2021, 11, 624009. [Google Scholar] [CrossRef]
- Lima, M.L.; Abengózar, M.A.; Nácher-Vázquez, M.; Martínez-Alcázar, M.P.; Barbas, C.; Tempone, A.G.; López-Gonzálvez, Á.; Rivas, L. Molecular Basis of the Leishmanicidal Activity of the Antidepressant Sertraline as a Drug Repurposing Candidate. Antimicrob. Agents Chemother. 2018, 62, e01928-18. [Google Scholar] [CrossRef]
- Cabré, M.; Planellas, M.; Ordeix, L.; Solano-Gallego, L. Is Signalment Associated with Clinicopathological Findings in Dogs with Leishmaniosis? Vet. Rec. 2021, 189, e451. [Google Scholar] [CrossRef] [PubMed]
- Wardini, A.B.; Pinto-da-Silva, L.H.; Nadaes, N.R.; Nascimento, M.T.; Roatt, B.M.; Reis, A.B.; Viana, K.F.; Giunchetti, R.C.; Saraiva, E.M. Neutrophil Properties in Healthy and Leishmania infantum-Naturally Infected Dogs. Sci. Rep. 2019, 9, 6247. [Google Scholar] [CrossRef] [PubMed]
| Variable | Categories | No. of Dogs (%) |
|---|---|---|
| Dog population | Households | 71 (91.03) |
| Animal shelters | 7 (8.91) | |
| Gender | Male | 40 (51.28) |
| Female | 38 (48.72) | |
| Age | Puppy (<1 year) | 8 (10.26) |
| Young (1 to 5 years) | 21 (26.92) | |
| Adult (5 to 10 years) | 23 (29.49) | |
| Elder (>10 years) | 26 (33.33) | |
| Breed | Ibizan hound | 28 (35.90) |
| boxer | 31 (39.74) | |
| Purebred (no Ibizan hound and boxer) | 28 (35.90) | |
| Crossbred | 7 (8.97) | |
| Diet | Commercial | 69 (88.46) |
| Home prepared/raw food consumption | 9 (11.54) | |
| Lived with other animals | Yes | 69 (88.46) |
| No | 9 (11.54) | |
| Obligatory vaccination | Yes | 69 (88.46) |
| No | 9 (11.54) | |
| Anti-Leishmania vaccination | Yes | 8 (10.26) |
| No | 70 (89.74) | |
| External deworming | Yes | 71 (91.03) |
| No | 7 (8.91) | |
| Overall | 78 (100.00) |
| Clinical Feature | No. of Dogs (%) | Anti-Leishmania Antibodies Titer |
|---|---|---|
| Lymphadenomegaly | 5 (50) | 1:640 to 1:1280 |
| Weight loss | 2 (20) | 1:640 |
| Lethargy | 1 (10) | 1:1280 |
| Pale mucous membranes | 2 (20) | 1:640 |
| Splenomegaly | 1 (10) | 1:100 |
| Exfoliative dermatitis | 2 (20) | 1:640 |
| Onychogryphosis | 1 (10) | 1:100 |
| Blepharitis | 1 (10) | 1:640 |
| Conjunctivitis | 3 (30) | 1:200 |
| Thrombocytopenic anemia | 1 (10) | 1:100 |
| Leukocytosis/leukopenia | 1 (10) | 1:200 |
| Thrombocytopenia | 2 (20) | 1:200 |
| Hyperglobulinemia | 3 (30) | 1:200 to 1:1280 |
| Hypoalbuminemia | 2 (20) | 1:200 |
| Reduced ALB/GLOB ratio | 2 (20) | 1:200 |
| Proteinuria | 3 (30) | 1:200 |
| Renal azotemia | 1 (10) | 1:200 |
| Hepatic transaminase elevation | 2 (20) | 1:640 |
| Overall | 10 (100) | 1:100 to 1:1280 |
| Cytokine 1 | n | Range 2 | Mean ± SD 2 | CV (%) |
|---|---|---|---|---|
| IFN-γ | 72 | 0.1–1.29 | 0.31 ± 0.03 | 88 |
| IL-2 | 75 | 10.37–148.00 | 56.71 ± 3.01 | 46 |
| IL-6 | 70 | 0.57–0.01 | 0.37 ± 0.95 | 20 |
| IL-8 | 77 | 0–624.00 | 223.00 ± 17.15 | 67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-García, P.J.; Llobat, L. Canine Cytokines Profile in an Endemic Region of L. infantum: Related Factors. Vet. Sci. 2022, 9, 305. https://doi.org/10.3390/vetsci9060305
Marín-García PJ, Llobat L. Canine Cytokines Profile in an Endemic Region of L. infantum: Related Factors. Veterinary Sciences. 2022; 9(6):305. https://doi.org/10.3390/vetsci9060305
Chicago/Turabian StyleMarín-García, Pablo Jesús, and Lola Llobat. 2022. "Canine Cytokines Profile in an Endemic Region of L. infantum: Related Factors" Veterinary Sciences 9, no. 6: 305. https://doi.org/10.3390/vetsci9060305
APA StyleMarín-García, P. J., & Llobat, L. (2022). Canine Cytokines Profile in an Endemic Region of L. infantum: Related Factors. Veterinary Sciences, 9(6), 305. https://doi.org/10.3390/vetsci9060305







