Minute Cellular Nodules as Early Lesions in Rats with Silica Exposure via Inhalation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Histological Examination of Lung Tissue
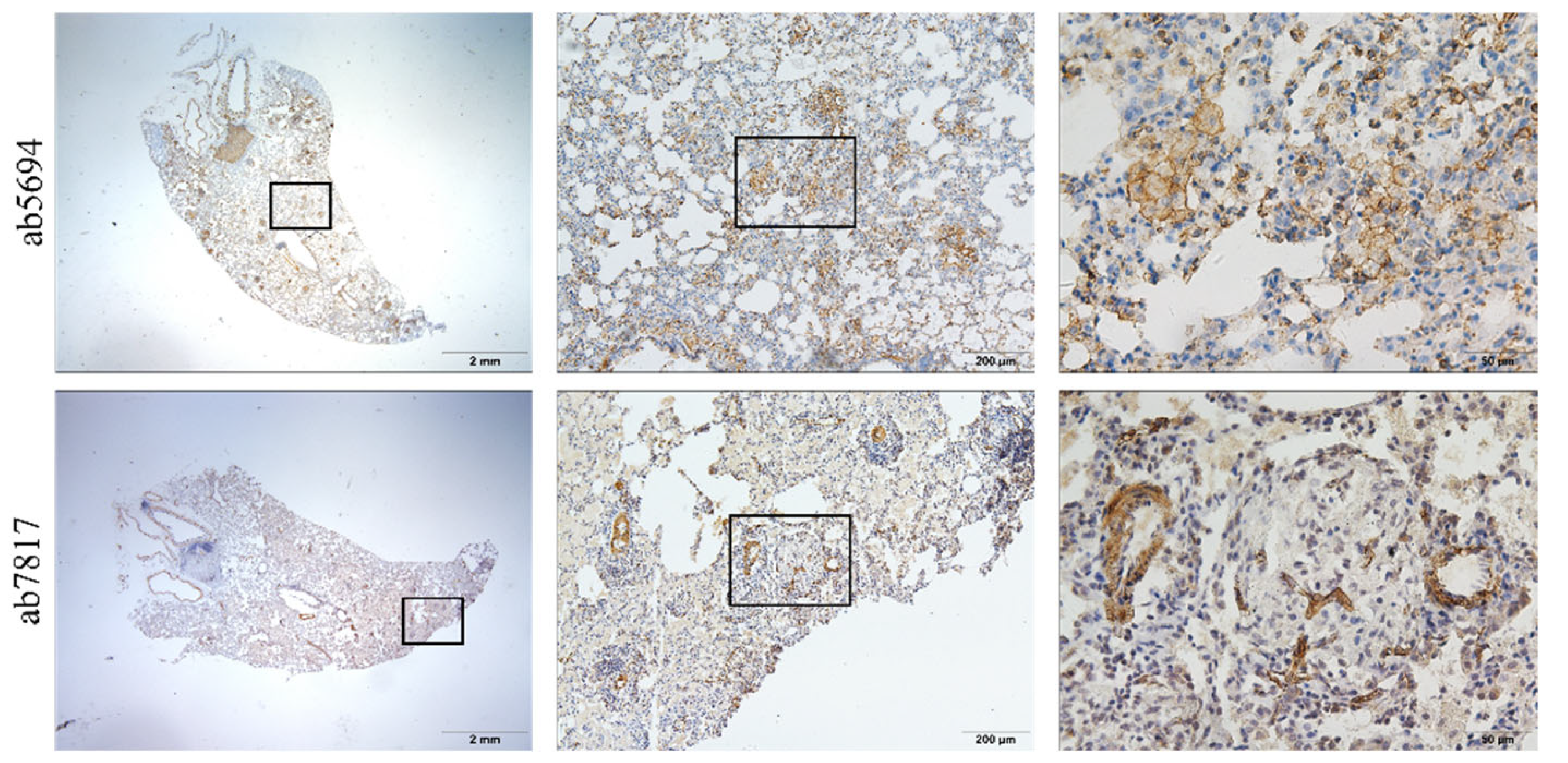
2.3. Immunohistochemistry and Immunofluorescence Staining
3. Results
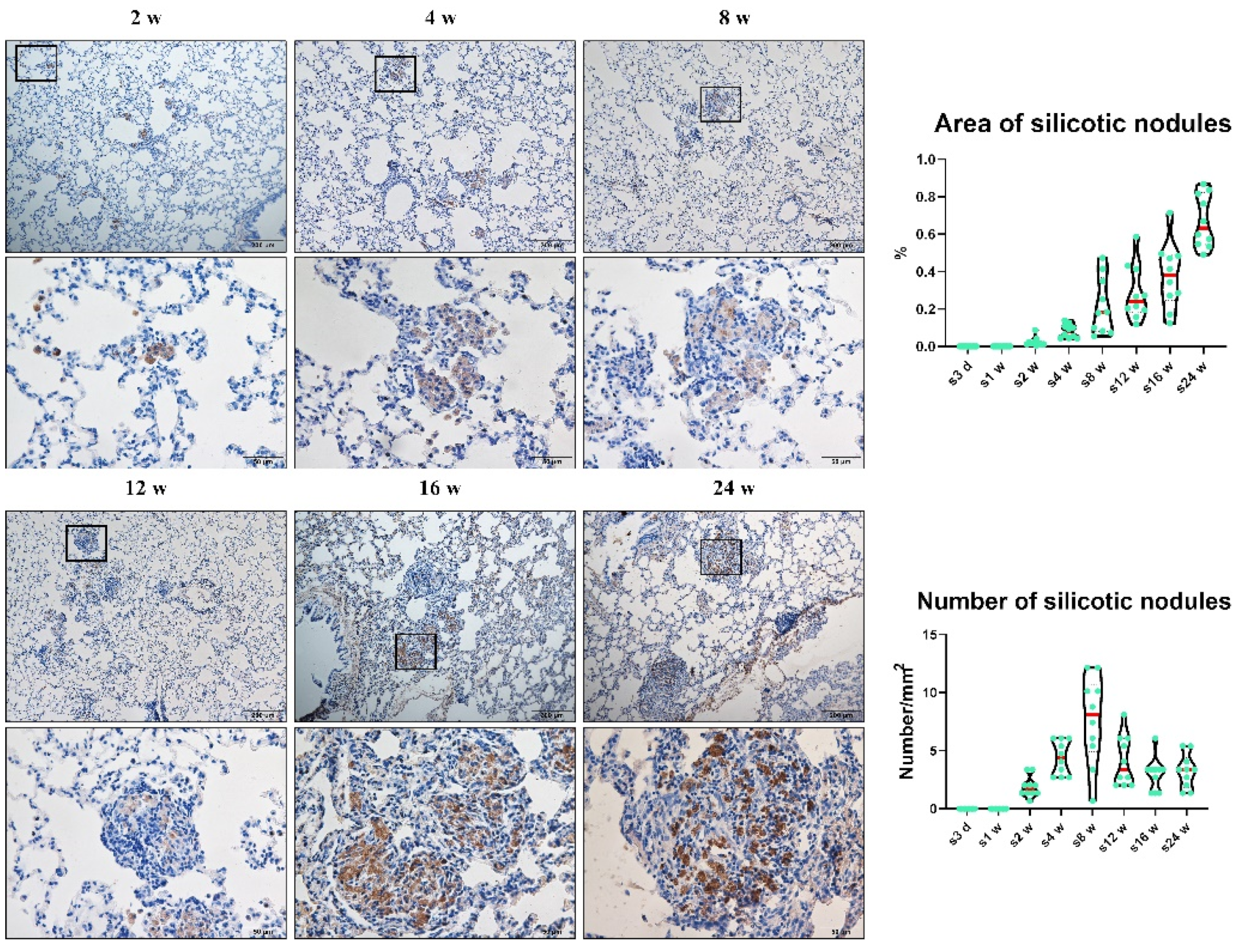
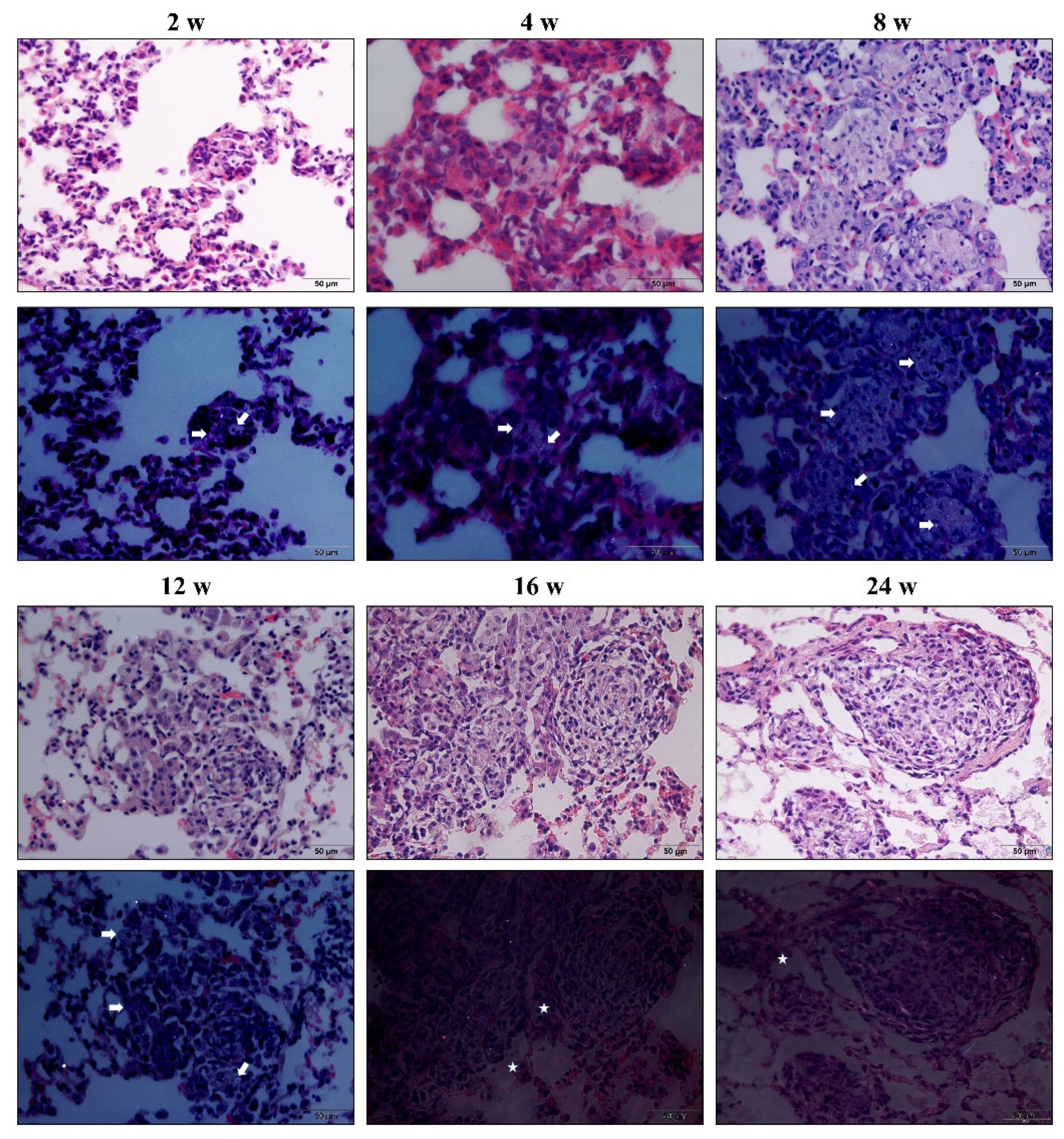
3.1. Chronic Inhalation of Silica Induced Progressive Pulmonary Fibrosis
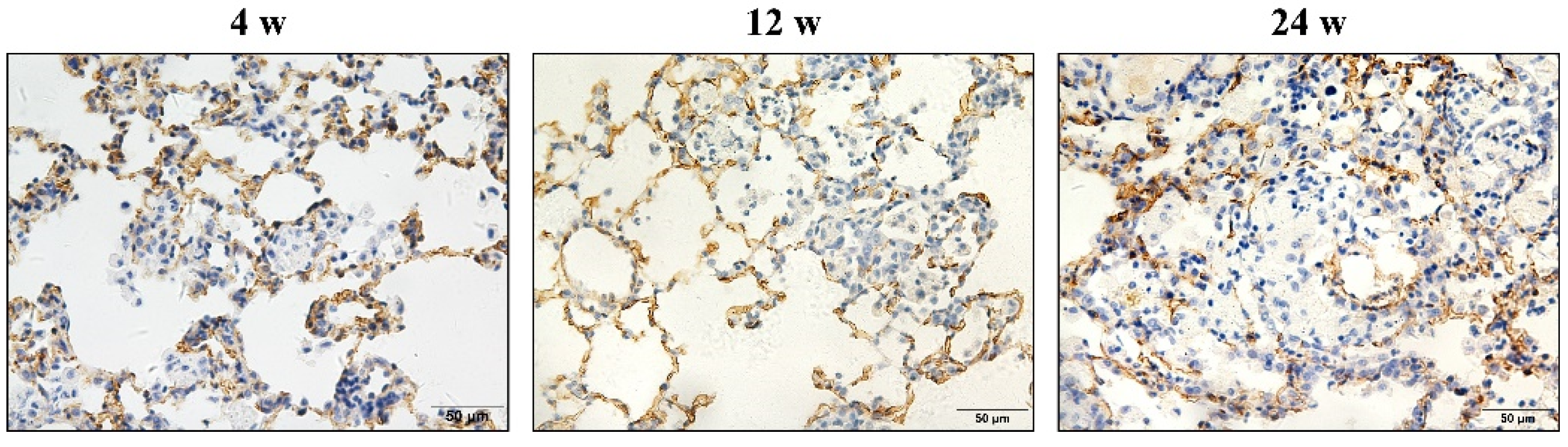
3.2. Hypertrophy and Hyperplasia of AT2 Cells
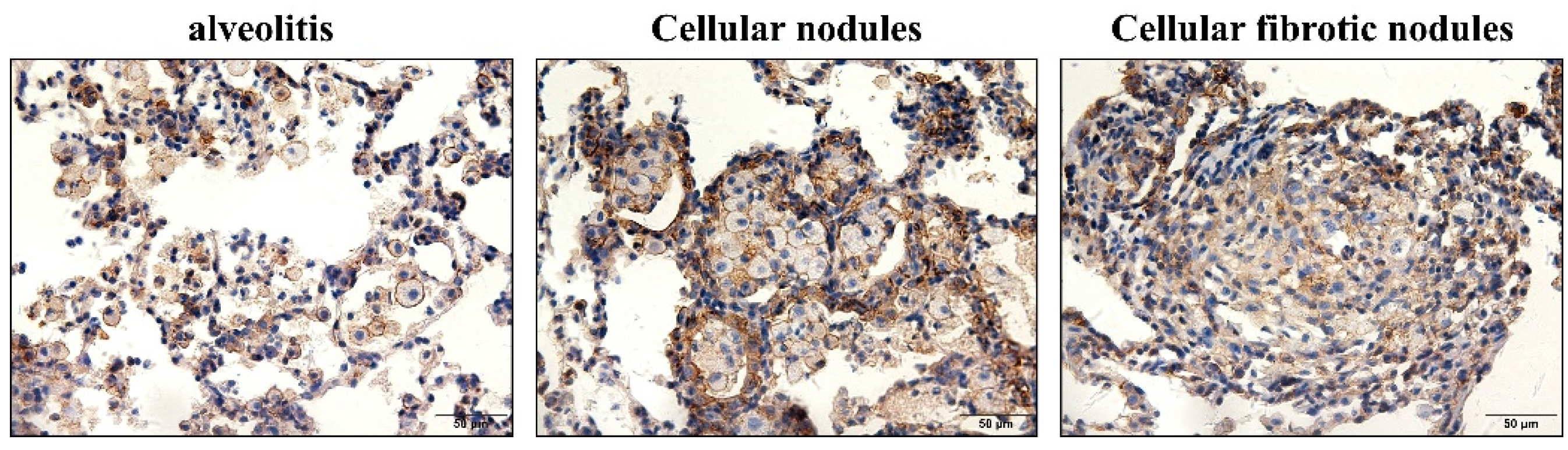
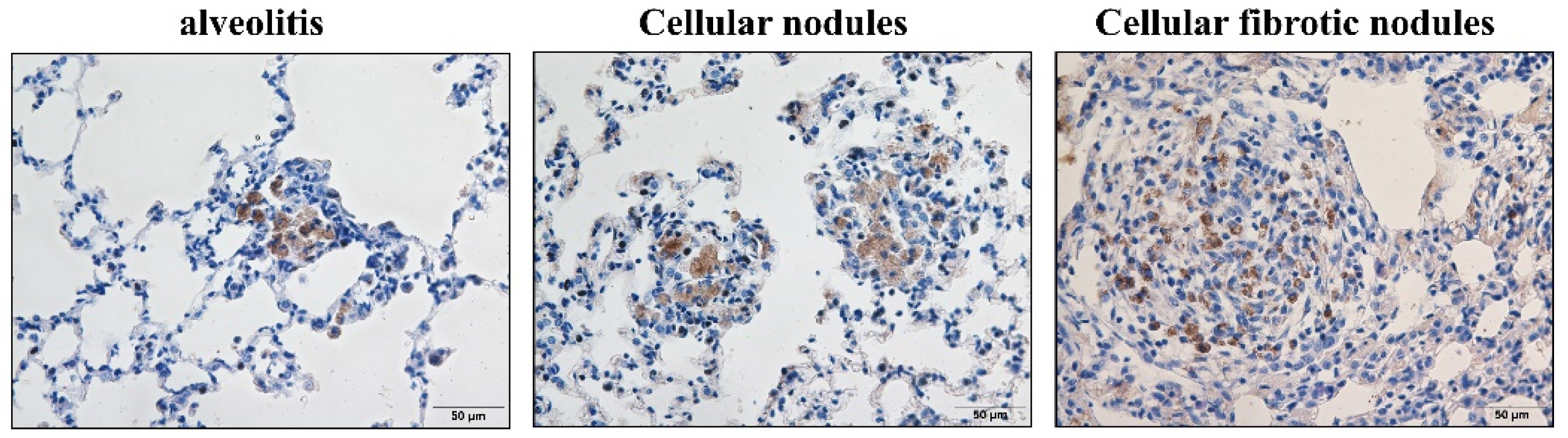
3.3. α-SMA Was Positively Expressed in Macrophages and Smooth Muscle Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoy, R.F.; Chambers, D.C. Silica-Related Diseases in the Modern World. Allergy 2020, 75, 2805–2817. [Google Scholar] [CrossRef] [Green Version]
- The Lancet Respiratory Medicine. The world is failing on silicosis. Lancet Respir. Med. 2019, 7, 283. [Google Scholar] [CrossRef] [Green Version]
- Pang, J.L.; Qi, X.M.; Luo, Y.; Li, X.N.; Shu, T.; Li, B.C.; Song, M.Y.; Liu, Y.; Wei, D.; Chen, J.Y.; et al. Multi-Omics Study of Silicosis Reveals the Potential Therapeutic Targets PGD2 and TXA2. Theranostics 2021, 11, 2381–2394. [Google Scholar] [CrossRef]
- Gao, X.; Xu, D.; Li, S.; Wei, Z.; Li, S.; Cai, W.; Mao, N.; Jin, F.; Li, Y.; Yi, X.; et al. Pulmonary Silicosis Alters MicroRNA Expression in Rat Lung and miR-411-3p Exerts Anti-fibrotic Effects by Inhibiting MRTF-A/SRF Signaling. Mol. Ther. Nucleic. Acids. 2020, 20, 851–865. [Google Scholar] [CrossRef]
- Cai, W.; Xu, H.; Zhang, B.; Gao, X.; Li, S.; Wei, Z.; Li, S.; Mao, N.; Jin, F.; Li, Y.; et al. Differential expression of lncRNAs during silicosis and the role of LOC103691771 in myofibroblast differentiation induced by TGF-β1. Biomed. Pharmacother. 2020, 125, 109980. [Google Scholar] [CrossRef]
- Cao, Z.J.; Song, M.Y.; Liu, Y.; Pang, J.L.; Li, Z.G.; Qi, X.M.; Shu, T.; Li, B.C.; Wei, D.; Chen, J.Y.; et al. A novel pathophysiological classification of silicosis models provides some new insights into the progression of the disease. Ecotoxicol. Environ. Saf. 2020, 202, 110834. [Google Scholar] [CrossRef]
- Cao, Z.J.; Liu, Y.; Zhang, Z.; Yang, P.R.; Song, M.Y.; Qi, X.M.; Han, Z.F.; Pang, J.L.; Li, B.C.; Zhang, X.R.; et al. Pirfenidone Ameliorates Silica-Induced Lung Inflammation and Fibrosis in Mice by Inhibiting the Secretion of Interleukin-17A. Acta Pharmacol. Sin. 2021, 43, 908–918. [Google Scholar] [CrossRef]
- Wollin, L.; Maillet, I.; Quesniaux, V.; Holweg, A.; Ryffel, B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 2014, 349, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Y.C.; Zhang, S.J.; Li, J.; Fang, H. Effects of tetrandrine combined with acetylcysteine on exercise tolerance, pulmonary function and serum TNF-β1 and MMP-7 in silicosis patients. Exp. Ther. Med. 2020, 19, 2195–2201. [Google Scholar] [CrossRef] [Green Version]
- Song, M.Y.; Wang, J.X.; Sun, Y.L.; Han, Z.F.; Zhou, Y.T.; Liu, Y.; Fan, T.H.; Li, Z.G.; Qi, X.M.; Luo, Y.; et al. Tetrandrine alleviates silicosis by inhibiting canonical and non-canonical NLRP3 inflammasome activation in lung macrophages. Acta Pharmacol. Sin. 2021, 43, 1274–1284. [Google Scholar] [CrossRef]
- Walters, E.H.; Shukla, S.D. Silicosis: Pathogenesis and utility of animal models of disease. Allergy 2021, 76, 3241–3242. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Geng, Y.C.; Xu, D.J.; Zhang, L.J.; Yang, Y.; Wei, Z.Q.; Zhang, B.N.; Li, S.F.; Gao, X.M.; et al. Dibutyryl-cAMP attenuates pulmonary fibrosis by blocking myofibroblast differentiation via PKA/CREB/CBP signaling in rats with silicosis. Respir. Res. 2017, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zang, H.; Xu, D.J.; Yuan, Y.; Wei, Z.Q.; Mao, N.; Bei, M.J.; Gou, Y.; Liu, G.Y.; Gao, X.M.; Li, S.F.; et al. Silicosis decreases bone mineral density in rats. Toxicol. Appl. Pharmacol. 2018, 348, 117–122. [Google Scholar] [CrossRef]
- Li, S.F.; Xu, H.; Yi, X.; Niu, S.Y.; Zhang, Q.D.; Xu, D.J.; Zhang, L.J.; Wei, Z.Q.; Gao, X.M.; Cai, W.C.; et al. Ac-SDKP increases α-TAT 1 and promotes the apoptosis in lung fibroblasts and epithelial cells double-stimulated with TGF-β1 and silica. Toxicol. Appl. Pharmacol. 2019, 369, 17–29. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.; Xu, D.J.; Gao, Z.Y.; Gao, Y.B.; Jin, F.Y.; Li, Y.Q.; Liu, S.P.; Li, S.F.; Gao, X.M.; et al. OC-STAMP Overexpression Drives Lung Alveolar Epithelial Cell Type II Senescence in Silicosis. Oxid. Med. Cell. Longev. 2021, 2021, 17–29. [Google Scholar] [CrossRef]
- Porter, D.W.; Hubbs, A.F.; Mercer, R.; Robinson, V.A.; Ramsey, D.; McLaurin, J.; Khan, A.; Battelli, L.; Brumbaugh, K.; Teass, A.; et al. Progression of Lung Inflammation and Damage in Rats After Cessation of Silica Inhalation. Toxicol. Sci. 2004, 79, 370–380. [Google Scholar] [CrossRef] [Green Version]
- Castranova, V.; Porter, D.; Millecchia, L.; Ma, J.Y.C.; Hubbs, A.F.; Teass, A. Effect of inhaled crystalline silica in a rat model: Time course of pulmonary reactions. Mol. Cell. Biochem. 2002, 37, 177–184. [Google Scholar] [CrossRef]
- Porter, D.W.; Ramsey, D.; Hubbs, A.F.; Battelli, L.; Ma, J.; Barger, M.; Landsittel, D.; Robinson, V.A.; McLaurin, J.; Khan, A.; et al. Time course of pulmonary response of rats to inhalation of crystalline silica: Histological results and biochemical indices of damage, lipidosis, and fibrosis. J. Environ. Pathol. Toxicol. Oncol. 2001, 20, 1–14. [Google Scholar] [CrossRef]
- Carey, B.; Trapnell, B.C. The molecular basis of pulmonary alveolar proteinosis. Clin. Immunol. 2010, 135, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.J.; Xu, H.; Zhang, X.H.; Sun, Y.; Wang, R.M.; Brann, D.; Yang, F. Protective effect of Ac-SDKP on alveolar epithelial cells through inhibition of EMT via TGF-β1/ROCK1 pathway in silicosis in rat. Toxicol. Appl. Pharmacol. 2016, 294, 1–10. [Google Scholar] [CrossRef]
- Zhang, L.J.; Xu, D.J.; Li, Q.; Yang, Y.; Xu, H.; Wei, Z.Q.; Wang, R.M.; Zhang, W.L.; Liu, Y.; Geng, Y.C.; et al. N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) attenuates silicotic fibrosis by suppressing apoptosis of alveolar type II epithelial cells via mediation of endoplasmic reticulum stress. Toxicol. Appl. Pharmacol. 2018, 350, 1–10. [Google Scholar] [CrossRef]
- Komai, M.; Mihira, K.; Shimada, A.; Miyamoto, I.; Ogihara, K.; Naya, Y.; Morita, T.; Inoue, K.; Takano, H. Pathological Study on Epithelial-Mesenchymal Transition in Silicotic Lung Lesions in Rat. Vet. Sci. 2019, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Pradere, J.-P.; Kluwe, J.; De Minicis, S.; Jiao, J.-J.; Gwak, G.-Y.; Dapito, D.H.; Jang, M.-K.; Guenther, N.D.; Mederacke, I.; Friedman, R.; et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology 2013, 58, 1461–1473. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Yang, F.; Sun, Y.; Yuan, Y.; Cheng, H.; Wei, Z.Q.; Li, S.Y.; Cheng, T.; Brann, D.; Wang, R.M. A new antifibrotic target of Ac-SDKP: Inhibition of myofibroblast differentiation in rat lung with silicosis. PLoS ONE 2012, 7, e40301. [Google Scholar] [CrossRef] [Green Version]
- Mariani, T.J.; Arikan, M.C.; Pierce, R.A. Fibroblast tropoelastin and alpha-smooth-muscle actin expression are repressed by particulate-activated macrophage-derived tumor necrosis factor-alpha in experimental silicosis. Am. J. Respir. Cell Mol. Biol. 1999, 21, 185–192. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, W.H.; Cui, G.B.; Yan, L.F.; Zhang, S. Potential role of the Jagged1/Notch1 signaling pathway in the endothelial-myofibroblast transition during BLM-induced pulmonary fibrosis. J. Cell. Physiol. 2017, 233, 2451–2463. [Google Scholar] [CrossRef]
- Porter, D.W.; Ye, J.; Ma, J.; Barger, M.; Robinson, V.A.; Ramsey, D.; McLaurin, J.; Khan, A.; Landsittel, D.; Teass, A.; et al. Time course of pulmonary response of rats to inhalation of crystalline silica: NF-kappa B activation, inflammation, cytokine production, and damage. Inhal. Toxicol. 2002, 14, 349–367. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Dong, J.; Liu, W.Y. Antifibrotic effect of interferon gamma in silicosis model of rat. Toxicol. Lett. 2005, 155, 353–360. [Google Scholar] [CrossRef]
- Jiao, J.; Li, L.; Yao, W.; Qin, W.D.; Hao, C.F.; Lu, L.G. Influence of Silica Exposure for Lung Silicosis Rat. Dis. Markers. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Peeters, P.M.; Eurlings, I.M.J.; Perkins, T.N.; Wouters, E.F.; Schins, R.P.F.; Borm, P.J.A.; Drommer, W.; Reynaert, N.L.; Albrecht, C. Silica-induced NLRP3 inflammasome activation in vitro and in rat lungs. Part. Fibre Toxicol. 2014, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kambouchner, M.; Bernaudin, J.-F. The pathologist’s view of silicosis in 1930 and in 2015. The Johannesburg Conference legacy. Am. J. Ind. Med. 2015, 58, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissonnette, E.Y.; Lauzon-Joset, J.-F.; Debley, J.S.; Ziegler, S.F. Cross-Talk between Alveolar Macrophages and Lung Epithelial Cells is Essential to Maintain Lung Homeostasis. Front. Immunol. 2020, 11, 583042. [Google Scholar] [CrossRef] [PubMed]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.-I.; Ren, Z.; et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care. Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.Y.; Geng, F.; Xu, D.J.; Li, Y.Q.; Li, T.; Yang, X.Y.; Liu, S.P.; Zhang, H.; Wei, Z.Q.; Li, S.F.; et al. Ac-SDKP Attenuates Ac-tivation of Lung Macrophages and Bone Osteoclasts in Rats Exposed to Silica by Inhibition of TLR4 and RANKL Signaling Pathways. J. Inflamm. Res. 2021, 14, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Mao, N.; Yang, H.H.; Yin, J.; Li, Y.Q.; Jin, F.Y.; Li, T.; Yang, X.Y.; Sun, Y.; Liu, H.L.; Xu, H.; et al. Glycolytic Reprogramming in Silica-Induced Lung Macrophages and Silicosis Reversed by Ac-SDKP Treatment. Int. J. Mol. Sci. 2021, 22, 10063. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kuwano, K.; Maeyama, T.; Hamada, N.; Yoshimi, M.; Nakanishi, Y.; Kasper, M. Dual-immunohistochemistry provides little evidence for epithelial-mesenchymal transition in pulmonary fibrosis. Histochem. Cell. Biol. 2008, 129, 453–462. [Google Scholar] [CrossRef]
- Kierdorf, K.; Prinz, M.; Geissmann, F.; Perdiguero, E.G. Development and function of tissue resident macrophages in mice. Semin. Immunol. 2015, 27, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Happle, C.; Lachmann, N.; Ackermann, M.; Mirenska, A.; Göhring, G.; Thomay, K.; Mucci, A.; Hetzel, M.; Glomb, T.; Suzuki, T.; et al. Pulmonary Transplantation of Human Induced Pluripotent Stem Cell-derived Macrophages Ameliorates Pulmonary Alveolar Proteinosis. Am. J. Respir. Crit. Care. Med. 2018, 198, 350–360. [Google Scholar] [CrossRef]
- Lenti, M.V.; Di Sabatino, A. Intestinal fibrosis. Mol. Asp. Med. 2019, 65, 100–109. [Google Scholar] [CrossRef]
- Langley, R.J.; Mishra, N.C.; Peña-Philippides, J.C.; Hutt, J.A.; Sopori, M.L. Granuloma formation induced by low-dose chronic silica inhalation is associated with an anti-apoptotic response in Lewis rats. J. Toxicol. Environ. Health Part A 2010, 73, 669–683. [Google Scholar] [CrossRef] [Green Version]
- Nardi, J.; Nascimento, S.; Göethel, G.; Gauer, B.; Sauer, E.; Fão, N.; Cestonaro, L.V.; Peruzzi, C.; Souza, J.; Garcia, S.C. Inflammatory and oxidative stress parameters as potential early biomarkers for silicosis. Clin. Chim. Acta. 2018, 484, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Hoy, R.F.; Glass, D.C.; Dimitriadis, C.; Hansen, J.; Hore-Lacy, F.; Sim, M.R. Identification of early-stage silicosis through health screening of stone benchtop industry workers in Victoria, Australia. Occup. Environ. Med. 2020, 78, 296–302. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Jin, F.; Li, T.; Yang, X.; Cai, W.; Li, S.; Gao, X.; Mao, N.; Liu, H.; Xu, H.; et al. Minute Cellular Nodules as Early Lesions in Rats with Silica Exposure via Inhalation. Vet. Sci. 2022, 9, 251. https://doi.org/10.3390/vetsci9060251
Li Y, Jin F, Li T, Yang X, Cai W, Li S, Gao X, Mao N, Liu H, Xu H, et al. Minute Cellular Nodules as Early Lesions in Rats with Silica Exposure via Inhalation. Veterinary Sciences. 2022; 9(6):251. https://doi.org/10.3390/vetsci9060251
Chicago/Turabian StyleLi, Yaqian, Fuyu Jin, Tian Li, Xinyu Yang, Wenchen Cai, Shifeng Li, Xuemin Gao, Na Mao, Heliang Liu, Hong Xu, and et al. 2022. "Minute Cellular Nodules as Early Lesions in Rats with Silica Exposure via Inhalation" Veterinary Sciences 9, no. 6: 251. https://doi.org/10.3390/vetsci9060251
APA StyleLi, Y., Jin, F., Li, T., Yang, X., Cai, W., Li, S., Gao, X., Mao, N., Liu, H., Xu, H., & Yang, F. (2022). Minute Cellular Nodules as Early Lesions in Rats with Silica Exposure via Inhalation. Veterinary Sciences, 9(6), 251. https://doi.org/10.3390/vetsci9060251





